ABSTRACT
A keloid is defined as an overgrowth of the dense fibrous tissues that form around a wound. Since they destroy the vascular network, keloid tissues often exhibit anoxic conditions. Hypoxia-inducible factor-1α (HIF-1α) is a core factor that mediates hypoxia stress responses and regulates the hypoxic cellular and biological behaviors. In this study, we found that the expression level of HIF-1α in keloid tissue was significantly higher than that in the normal skin tissue. Hypoxia-induced HIF-1α expression significantly inhibited cellular apoptosis and promoted cellular proliferation in keloid fibroblasts but not in normal fibroblasts. Specifically, HIF-1α activated the TGF-β/Smad and TLR4/MyD88/NF-κB pathways, and the interaction of these two pathways may promote the development of keloids. Moreover, in vivo experiments showed that the inhibition of HIF-1α significantly reduced the growth of keloids.
Introduction
A keloid is a human-specific pathological healing of skin lesions that exhibits racial and family tendencies. In China, the incidence of keloids is up to 4.6–16% [Citation1]. The primary clinical manifestations include persistent tumor-like proliferation beyond the margins of the wound and invasion of adjacent tissues [Citation2]. The keloid is self-degenerating and can relapse easily with simple surgical resection, and the pathogenesis of keloids is not yet fully understood [Citation3,Citation4].
During wound healing, the skin vascular network is destroyed, leading to hypoxia in the local tissue [Citation5]. The high metabolic state during tissue injury-induced inflammation or repair also increases oxygen consumption and exacerbates the hypoxic state [Citation6]. Hypoxia-inducible factor-1 (HIF-1) is considered as the core factor mediating the hypoxia stress response and regulating the physiological behavior of hypoxic cells [Citation7]. Under normoxic conditions, the structure of the HIF-lα protein is regulated by the oxygen-dependent degradation domain (ODDD) that is rapidly degraded through the ubiquitin-proteasome pathway [Citation8]. Under hypoxic conditions, the ubiquitination and degradation processes of HIF-lα are inhibited, and the expression of the HIF-1α protein increases, resulting in an intracellular accumulation of HIF-1α protein and dimerization with HIF-1β, forming an active HIF-1 protein and thereby activating the expression of various hypoxia-responsive genes [Citation9].
The mechanisms underlying the formation of keloids are complex and based on several influencing factors. Transforming growth factor-beta (TGF-β) is secreted and released by injured tissues and plays a decisive role in pathological fibrosis [Citation10]. Additional evidence demonstrated that the ability to express TGF-β was significantly enhanced in keloid fibroblasts (KFs) [Citation11]. TGF-β, when bound to TGF-β receptor (TβR) on the target cell membrane, initiated the intracellular corresponding signal transduction (Smad pathway) to trigger a variety of physiological effects. With respect to the downstream target proteins in the TGF-β-regulated Smad pathway, upregulation of connective tissue growth factor (CTGF) is a critical factor in the formation of the keloid. CTGF plays a critical role in promoting the proliferation and activation of KFB to secrete extracellular matrix (ECM). Toll-like receptor (TLR) is a family of transmembrane glycoprotein receptors in the type I autoimmune system, which initiates the intracellular signaling transduction through the adaptor protein myeloid differentiation factor 88 (MyD88) and TIR-domain-containing adaptor-inducing interferon-β (TRIF) after interacting with ligands that mediate various biological effects. Seki et al. and Campbell et al. were the first to find that TLR4 mediates and promotes hepatic and renal fibrosis [Citation12,Citation13]. The subsequent studies confirmed that TLR4 was also highly expressed in keloids [Citation14,Citation15]. The expression of TLR4 was supposed to be regulated by HIF-1α[Citation16], and TLR4 promoted fibroblast proliferation and collagen synthesis during wound healing, leading to the over-healing of wounds and keloid formation [Citation14]. Previous studies on keloids have shown that TGF-β and HIF-1α could induce and stimulate the expression of vascular endothelial growth factor (VEGF) [Citation17,Citation18], which in turn lead to abnormal vascular hyperplasia that results in pathological wound healing [Citation19,Citation20]. We recently found that hypoxia promoted TGF-β1/Smad signaling via HIF-1α and promoted collagen deposition in fibroblasts, which is an important mechanism of keloid formation [Citation21]. These study suggested that hypoxia-induced activation of TGF-β pathway and TLR4 pathway might be dependently or independently implicate in the formation and development of keloids.
In the current study, we found that KFs were much more responsive to hypoxia than normal fibroblasts (NFs). Under the same hypoxic conditions, KFs expressed higher level of HIF-1α than that in NFs. Of note, the expression of HIF-1α induced by hypoxia in KFs could activate the TGF-β/Smad and TLR4-MyD88-NF-κB pathways at the same time. These two signaling pathways might have a crosstalk effect and synergistically promoted the development of keloids. Additionally, HIF-1α also promoted the ERK/IL-8 pathways in contributing to keloid development (Supplemental ). Moreover, in vivo experiments showed that the inhibition of HIF-1α significantly reduced the growth of keloids. As a conclusion, targeting inhibition of HIF-1α could be an promising treatment for keloid.
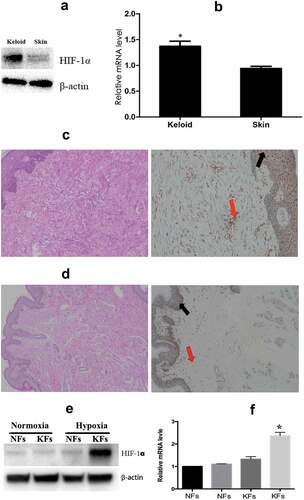
Figure 1. Keloid tissues expressed a higher level of HIF-1α than normal skin tissues. The expression of HIF-1α at both the protein (a) and mRNA (b) level was significantly higher in the keloid tissues than in normal skin tissues. Immunohistochemical staining of HIF-1α in keloid tissues (c) and normal skin tissues (d) for analysis of the expression and localization of HIF-1α. The transcription and protein levels of HIF-1α were also significantly higher in KFs (e, left) than those in NFs (f). Additionally, the level of HIF-1α protein in KFs was further increased under hypoxia (1e, right). (*p < 0.05)
Results
Expression of HIF-1α in keloid tissues was significantly higher than that in normal skin tissues
The hypoxic microenvironment in keloid tissues induced an increase in HIF-1α expression that was significantly higher in keloid tissues than that in the normal skin tissues (). RT-PCR showed that the mRNA expression of HIF-1α in keloid tissues was 1.47 ± 0.12-fold higher than that in the normal skin tissues (p < 0.05, ). Immunohistochemistry was used to analyze the expression and localization of HIF-1α. The results showed that in keloid tissues, HIF-1α was widely distributed in the dermis, basal lamina of the epidermis, and a small number of cells in the stratum corneum, primarily around the nucleus (), while almost no HIF-1α was detected in the NFs (). In addition, we isolated and cultured the fibroblasts from keloid tissues and normal tissues in normoxic conditions, and found that the transcription and protein levels of HIF-1α were not significantly different between in KFs and NFs (). Next, we cultured the cells under hypoxic conditions for 24 h and found an increased level of HIF-1α protein in KFs (), suggesting that KFs were much more sensitive to hypoxia stimulation than NFs.
HIF-1α promoted the proliferation of KFs and reduced the occurrence of apoptosis
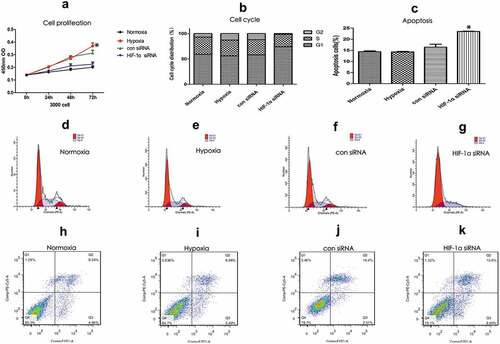
Based on the different treatments, the KFs were divided into four groups: 1. Hypoxia group (hypoxia-stimulated KFs); 2. Normoxia group (KFs that had been cultured in a normal oxygen concentration); 3. HIF-1α siRNA group (silencing of HIF-1α by siRNA in the KFs and hypoxic stimulation); 4. Con-siRNA group (siContr-treated KFs and hypoxic stimulation). The CCK8 assay results showed that the proliferation of KFs was significantly increased after hypoxic stimulation (p < 0.05, )), and this effect could be apparently abrogated by knockdown of HIF-1α ()). However, hypoxia stimulation for 72 hours would inhibit cell growth of NFs, but not KFs (Supplemental )). Moreover, we found that the percentage of cells in G1 phase (74.08% vs. 56.11%) was significantly higher in the HIF-1α-siRNA group than that in the hypoxia group (p < 0.05, )), while the percentage of KF cells in S phase was apparently decreased after silencing the HIF-1α expression (24.50% vs. 31.26%) (–)). The above results indicated that hypoxia-stimulated HIF-1α expression promotes the proliferation of KFs. In addition, we also found that the number of apoptotic KFs that were stimulated by appropriate hypoxia was not significantly increased (–)). However, the number of apoptotic KFs was significantly increased after the downregulation of HIF-1α expression ()), indicating a critical role of HF-1α in maintaining the escape from cellular apoptosis in hypoxia.
Figure 2. HIF-1α promoted the proliferation of KFs and reduced the occurrence of apoptosis. KFs were divided into four groups: 1. Hypoxia group; 2. Normoxia group; 3. HIF-1α siRNA group; and 4. Con-siRNA group. The CCK8 assay showed that the proliferation of KFs was significantly increased after hypoxic stimulation but reduced after knockdown of HIF-1α by siRNA treatment (a). After silencing of HIF-1α in KFs, the number of G1 phase cells was increased, and the number of S phase cells was decreased (b). The number of apoptotic KFs was increased after knockdown of HIF-1 expression (c). FACS analysis of the cell cycle distribution (d–g) and apoptosis (g–k) of the four groups. (*p < 0.05)
HIF-1α activates the TGF-β/smad signaling pathway to promote keloid formation
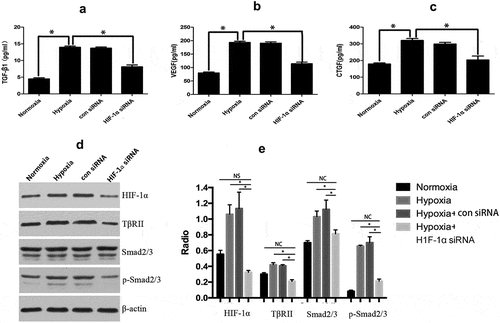
Analysis by ELISA showed that KFs could secrete TGF-β, CTGF, and VEGF, and the secretion was increased after hypoxic stimulation. However, hypoxia did not significantly increase the secretion of TGF-β, CTGF, and VEGF in KFs in the event of HIF-1α silencing (p < 0.05, –)). In addition, hypoxia stimulation induced the expression of TGF-βII receptor on KFs and increased the p-Smad2/3 protein levels, but the expression of the Smad2/3 protein was not significantly increased ()). However, the levels of TGF-βII receptors and p-Smad2/3 expression were significantly decreased in hypoxic conditions after knockdown of HIF-1α gene expression (p < 0.05, )). We also confirmed the results by densitometric quantified analysis ()). These results indicated that hypoxia-induced HIF-1α expression in KFs promoted the expression of TGF-β, whose receptors activated the TGF-β/Smad signaling pathway and increased the secretion of factors such as CTGF and VEGF in order to promote the progression of keloids.
Figure 3. HIF-1α activates the TGF-β/Smad signaling pathway. Hypoxic stimulation increased the secretion of TGF-β, CTGF, and VEGF; after silencing HIF-1α, hypoxia did not significantly increase the secretion of TGF-β, CTGF, and VEGF (a, b, and c). Hypoxic stimulation induced the KFs to express TGF-βII and p-Smad2/3; their expression levels were decreased in hypoxic conditions after knockdown of HIF-1α gene expression (d), and the results were confirmed by densitometric quantified analysis (e). (*p < 0.05)
HIF-1α activated the TLR/MyD88/NF-κB pathway and the downstream expression of IL-6
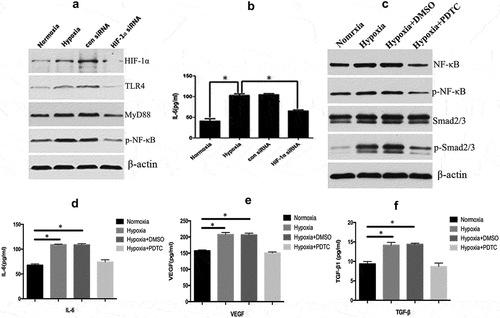
TLR/MyD88/NF-κB pathway is frequently activated and plays a critical role in the formation of keloids [Citation22,Citation23]. Our previous study suggested that TLR4 expression was significantly increased in keloid tissues where HIF-1α expression was high. We, therefore, speculated that HIF-1α is associated with TLR/MyD88/NF-κB pathway in the development of keloids. The expression levels of TLR-4, MyD88, and NF-κB in KFs were significantly increased after hypoxic stimulation ()). However, the expression levels of TLR-4, MyD88, and phosphorylated NF-κB protein were significantly decreased when HIF-1α was silenced upon hypoxia stimulation ()), and the results were confirmed by densitometric quantified analysis (Supplement Figure 1(a)). Analysis by ELISA found that KFs secreted the inflammatory factor IL-6, which was induced by TLR/MyD88/NF-κB pathway [Citation24] after hypoxic stimulation ()). Moreover, the secretion of IL-6 was reduced significantly after silencing of HIF-1α (p < 0.05, )). The above results indicated that the hypoxia-induced expression of HIF-1α in fibroblasts stimulated the TLR/MyD88/NF-κB signaling pathway and promoted the expression of some inflammatory factors such as IL-6. The secretion of IL-6 was significantly inhibited after KF cells were treated with the NF-κB blocker PDTC ()), indicating that the secretion of IL-6 was primarily regulated by the TLR-MyD88-NF-κB pathway. Previous studies found that TLR4 promoted hepatic fibrosis by enhancing TGF-β signaling transduction [Citation12]. We also found that the expression of NF-κB, p-NF-κB, and p-Smad2/3 (), Supplement )) and the secretion of IL-6, VEGF and TGF-β () were significantly enhanced by hypoxic stimulation and inhibited after PDTC treatment. We further treated both of KFs and NFs with 40 ng/mL of TNF-α to induce NF-κB activation as previous described [Citation25], and found that the expression of p-Smad2/3 were increased even when KFs were in normoxia condition (Supplemental )), thus suggesting that NF-κB was likely to crosstalk with Smad2/3 pathway. It has been reported that NF-κB could induce miR-148a to sustain TGF-β/Smad signaling activation in cancer cells [Citation26]. Consistently, we here revealed that miR-148a was upregulated in hypoxia-stimulated or TNF-a treated KFs (Supplemental )), which suggested that HIF-1α-induced TLR4/MyD88/NF-κB pathways could affect the TGF-β1/Smad signaling by inducing miR-148a expression.
Figure 4. HIF-1α activated the TLR/MyD88/NF-κB pathway and downstream IL-6 expression. The expression of TLR-4, MyD88, and NF-κB in KFs was increased significantly after hypoxic stimulation in KFs but decreased when HIF-1α was silenced (a). ELISA measurements revealed that KFs secreted an increased level of IL-6 after hypoxic stimulation that was decreased after silencing HIF-1α (b). The expression of NF-κB, p-NF-κB, and p-Smad2/3 was significantly inhibited by PDTC treatment (c). The secretion of IL-6, TGF-β, and VEGF was significantly inhibited by PDTC treatment (Fig. d, e, and f). (*p < 0.05)
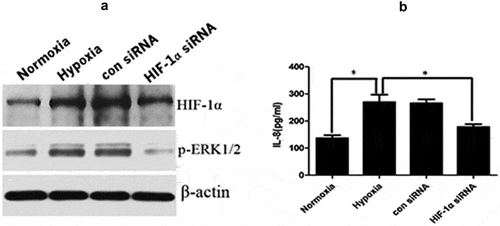
HIF-1α promoted ERK1/2 phosphorylation and downstream IL-8 expression
Extracellular signal-regulated kinases (ERKs) are a class of protein kinases that are distributed in the cytoplasm [Citation27], and their signaling pathways play a major role in fibrotic diseases [Citation28]. The stimulation of HIF-1α expression in KFs activated ERK1/2 [Citation29]. We also found that the expression of phosphorylated ERK1/2 was increased in KFs after hypoxic stimulation, while their expression was significantly decreased in KFs in the hypoxia group when HIF-1α was silenced (), Supplement Figure 1(c)). In addition, we found that the secretion of IL-8 was increased in KFs after hypoxic stimulation, while the secretion of IL-8 was decreased after knockdown of HIF-1α (p < 0.05, )).
Figure 5. HIF-1α promoted ERK1/2 phosphorylation and downstream IL-8 expression. The expression of phosphorylated ERK1/2 was increased in KFs after hypoxic stimulation and decreased after HIF-1α silencing (a). The secretion of IL-8 was increased in KFs after hypoxic stimulation and decreased as a result of HIF-1α knockdown (b). (*p < 0.05)
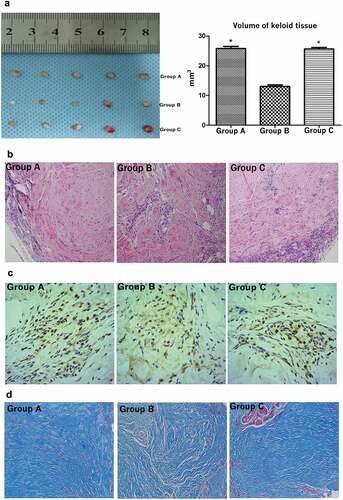
The HIF-1α inhibitor suppressed the growth of keloid tissues in nude mice
In vitro experiments proved that knockdown of HIF-1α significantly inhibited the growth of KFs. Herein, the keloid transplantation in the nude mouse model showed that HIF-1α inhibitors, 2-ME2 suppressed the growth of keloid tissues in vivo. Specifically, keloid tissues with a size of 5 × 4 × 3 mm3 were subcutaneously transplanted into the scapular area of nude mice. All nude mice survived and showed no rejection responses. After 21 days of treatment with a blank control (Group A), drug (Group B), or solvent control (Group C), the keloid tissue samples from the three groups of nude mice were collected. The volume of keloid tissue in the nude mice that had been treated with the HIF-1α inhibitor was significantly smaller than that in the control and blank groups (p < 0.05, )). H&E staining showed a large number of fibroblasts and collagen fibers in the dermal layer of Groups A and C; however, the collagen nodules did not display loose collagen fibers in Group B ()). In addition, immunohistochemistry staining of keloid tissue showed that HIF-1α expression in the keloid tissue of Groups C and A occurred primarily in the cytoplasm surrounding the nucleus, while the expression in the experimental Group B was significantly less than that of Groups A and C ()). We then counted the number of cells in the keloid tissues that showed HIF-1α expression, and the results confirmed that HIF-1α expression in Group B was obviously lower than that in Groups A and C (Supplement )). On the other hand, Masson staining showed that the collagen fibers of the keloid tissues of nude mice that had been treated with the HIF-1α inhibitor were looser and contained fewer long fiber bundles arranged in an orderly manner, compared to the collagen of the control group. As observed under the microscope, the overall blue staining was also less intense in the mice treated with the HIF-1α inhibitor ()). In order to validate the link to the pathway analysis presented for cultured fibroblasts, we tested the respective signaling components changes in this model tissues. We found that the expression of p-Smd/2/3 and p- NF-κB were significantly reduced in the keloid tissue of Group B. While the expression levels of p-Smd/2/3 and p-NF-κB were consistent with that of HIF-1α in keloid tissues of group A and C (Supplemental Figure 2(c)).
Figure 6. Treatment with the HIF-1α inhibitor suppressed the growth of keloid tissues in nude mice. The volume of keloid tissue in nude mice that had been treated with the HIF-1α inhibitor was significantly smaller than that in the control groups (a). Hematoxylin and eosin stain (a), immunohistochemistry staining (b) and Masson staining (c) of keloid tissues from nude mice. (*p < 0.05)
Discussion
The excessive proliferation of fibrous tissues, the formation of ECM, and the possibility of easy recurrence are the major challenges in the clinical treatment of keloids. The current strategies for the treatment of keloids, including surgical resection, laser therapy, and radiation therapy, do not provide optimal effects; thus, the ideal therapeutic methods for treating keloids are still awaiting discovery [Citation30,Citation31]. Here, we provide evidences that HIF-1α plays a important role in the development of keloids by activating the TGF-β/Smad pathway and TLR/MyD88/NF-κB pathway. HIF-1α, therefore, can be a potential treatment targets of keloid.
TGF-β/Smad pathway is suggested to play a decisive role in pathological fibrosis [Citation10]. Additionally, increase the ability of expressing TGF-β is always observed in keloid fibroblasts, where HIF-1α expression is really high [Citation11]. In this way, TGF-β/Smad pathway and HIF-1α can synergistically induce and stimulate the expression of VEGF [Citation17,Citation18] in fibroblasts, which in turn, led to abnormal proliferation of blood vessels and pathological healing of the wound [Citation19,Citation20]. Such overactivity of VEGF promotes the formation of keloids [Citation32]. Furthermore, with the rapid proliferation of fibroblasts, excessive secretion, and deposition of collagen in keloids that lead to local tissue hypoxia, the expression of HIF-1α increases further. On other hand, HIF-1α promotes the TLR pathway-induced inflammation that can contribute to keloid formation. Most importantly, the TLR/NF-κB and TGF-β/Smad pathways are mutually activated in the inflammatory response, thereby promoting inflammation and fibrosis [Citation33]. These two pathways are also simultaneously activated in the tissues of multiple myositis to promote autoimmunity [Citation34]. Furthermore, we also support that the TGF-β/Smad pathway can be significantly inhibited by the blockade of NF-κB, which indicated that HIF-1α-induced TLR/MyD88/NF-κB pathway also promotes the activation of TGF-β/Smad pathway. As a result, a cross-talk may exist between the TLR/MyD88/NF-κB and TGF-β/Smad pathways in the development of keloid. In head and neck cancers, TGF-β1 treatment induced phosphorylation TGF-β-activated kinase 1 (TAK1) to promote NF-κB activation [Citation35], while NF-κB could induce miR-148a to sustain TGF-β/Smad signaling activation in glioblastoma [Citation26], suggesting that miR-148a functions as a bridge to link these two pathways. We therefore revealed that miR-148a was significantly upregulated in TNF-a treated KFs, indicating that HIF-1α-induced activation of NF-κB pathways could affect the TGF-β1/Smad signaling by inducing miR-148a.
In some sense, HIF-1α acts a key factor in the mechanisms underlying the development of keloids and plays a crucial role in the development of keloids. The inhibition of HIF-1α expression, therefore, can efficiently suppresses the development of KFs. In this way, the development of a HIF-1α inhibitors is essential in the field of cancer treatment. Presently, more than 20 different HIF-1α inhibitors have been developed. However, very few are currently undergoing relevant preclinical studies [Citation36,Citation37]. One of these compounds, 2-ME2, is a natural estrogen metabolite that binds to the colchicine binding site of tubulin and inhibits tubulin polymerization, which in turn inhibits cell division. Reportedly, 2-ME2 also inhibits the translation and nuclear translocation of HIF-1α, eventually significantly decreasing the HIF-1α expression [Citation38]. After treatment with the 2-ME2 for 3 weeks, the keloid volume in the nude mouse keloid models was significantly reduced, which might be attributed to the inhibition of KF growth and collagen fiber secretion. Although a wide range of HIF-1α inhibitors are currently available, they are mainly used for the treatment of cancer, and only a small number are undergoing small-scale clinical trials. Therefore, extensive experimental studies and clinical trials are essential for investigating the role of HIF-1α inhibitors in the treatment of keloids.
Materials and methods
Clinical samples
Ten cases were pathologically confirmed as keloid specimens (without the epidermis), and the corresponding adjacent normal skin tissues were collected from the first affiliated hospital of Zhejiang University from October 2015 to January 2017. This study was approved by the audit department of the hospital when the requirements of the Ethics Committee were fulfilled. All subjects signed the informed consent forms. The patient’s medical history was recorded, and the clinical data registration form was completed. All experimental protocols were approved by the Ethics Committee of the first affiliated hospital of Zhejiang University. The methods were carried out in accordance with the relevant guidelines and regulations
Primary cell culture
The surgically excised keloid tissue and normal skin tissues were immediately placed in cold PBS supplemented with double antibiotics. The tissue was rinsed 3 times with 1× gentamycin in PBS and soaked in 75% alcohol for 30 s before being placed on a clean bench. The epidermis and fat were cut manually on the clean bench. Only the dermis layer of the fibrous tissue was preserved and washed again with PBS. Then, the sample was divided into small tissues of approximately 1 mm [Citation3] size; these pieces were placed in DMEM lysis buffer with 1× collagenase I and shocked for digestion at room temperature for 2 h. The residual tissue blocks were squeezed slightly on the clean bench. The liquid was aspirated after 5 blows. The precipitate was collected for culture after centrifugation at 800 × g.
Hypoxia culture model
Hypoxic conditions of 1% oxygen concentration (37°C, 1% O2, 5% CO2, 94% N2) were established by mixed gas hypoxia. Cells were detected after they were cultured in the hypoxic incubator for 24 h.
Transfection
Transfections of plasmids or siRNAs (synthesized by Dharmacom, Lafayette, CO) were performed using Lipofectamine 2000 (Invitrogen), following the manufacturer’s instruction. The sequence of the siRNA targeting HIF-1α was 5′-CAGAAGGCTCATGGTAAAACATC-3′.
RNA isolation and real-time quantitative reverse transcription PCR assay
RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and the RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The cDNA was obtained by reverse transcription of total RNA using a TaqMan reverse transcription kit (Applied Biosystems Inc., Foster City, CA). The amplification and analysis were performed on the ABI 7900 sequence detection system. The data were analyzed using the comparative Ct method.
Western blotting
Tissue protein extractions were performed as described previously [Citation39]. In brief, frozen tissues were lysed in an ice-cold radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS) supplemented with protease inhibitors and phosphatase inhibitors (Roche Applied Science). The lysates were centrifuged at 12,000 × g for 15 min at 4°C. The final supernatants were collected and stored at −80°C. For western blot analysis, protein extracts were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were incubated with specific primary antibodies and subsequently incubated with peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA, USA). Then, the signal was detected using a chemiluminescence detection system (Pierce, Rockford, IL, USA). (Antibody: HIF-1α, TβRII, β-actin, TLR4, and MyD88 were purchased from Abcam; Smad2/3, p-Smad2/3, and p-NF-κB were purchased from proteintech.)
Enzyme-linked immunosorbent assay (ELISA)
KFs or normal skin fibroblasts (NFs) (2 × 105 cells) in 6-cm culture dishes were treated with or without 20 μM pyrrolidine dithiocarbamate (PDTC), a metal chelating compound that can inhibit the NF-κB. At 2 days post-treatment, the supernatants were collected by centrifugation at 15,000 × g for 10 min at 4°C, and the levels of secreted TGF-β1, VEGF, CTGF, IL-6, and IL-8 were assessed using an ELISA kit (R&D Systems, Minneapolis, MN, USA).
Immunohistochemistry
The paraffin-embedded samples were sliced into 4-μm sections and incubated with a primary antibody HIF-1α (Santa Cruz Biotechnology. Dilution 1:250) after dewaxing, hydration, antigen retrieval, and blocking. The expression of HIF-1α was detected using the immunoperoxidase technique (Vectastain ABC Elite kit, UK.).
CCK8 assay for cell growth curve
The cell suspension was seeded in a 96-well plate (3000 cells/100 µL/well). The culture plate was preincubated for 24 h to allow for cell adherence to the wall. Subsequently, the cells were cultured in the normal and hypoxic incubator for 24 h and detected once every 24 h. After this, 10 µL of CCK8 solution was added to each well and the plates were incubated for 1–2 h. Finally, the absorbance was measured at 450 nm using a microplate reader.
Cell cycle testing
Six-well plates were used for cell transfection. The samples were divided into the Con-siRNA control group and HIF-1α siRNA experimental group. The resuspended cells were added to the precooled 75% absolute ethanol for more than 24 h, and then 1 mL of cell cycle staining solution was added and maintained in the dark at room temperature for 30 min. Flow cytometry was used to detect and analyze the results.
Cell apoptosis detection
Cells were collected and resuspended in 1× binding buffer at a concentration of 1.0 × 106 cells/mL, and 100 µL of sample was mixed with 5 µL of FITC Annexin V and 5 µL of propidium iodide (PI). The sample was mixed and shielded from light at room temperature for 15 min. Then, 400 µL of 1× binding buffer was added. The sample was detected and analyzed within 1 h by flow cytometry.
Animal experiments of keloids in nude mice
The animal experimental protocols were approved by the Ethics Committee of the first affiliated hospital of Zhejiang University. The methods were carried out in accordance with the relevant guidelines and regulations. Fifteen healthy male BALB/c nude mice, aged 4–6 weeks, were selected. A 5-mm incision was made in the scapular area of the nude mice after successful anesthesia. The keloid tissues that were collected in surgery were incised into blocks of approximately 5 × 4 × 3 mm3 in size and implanted in the subcutaneous space of the nude mice, and then the skin was sutured. After 21 days, the mice were randomly divided into 3 groups: blank group (Group A) with no intraperitoneal injection, experimental group (Group B) with an intraperitoneal injection of the HIF-1α inhibitor [2-methoxyestradiol (2-ME2)], and blank control group (Group C) with an intraperitoneal injection of the drug-free DMSO solvent. The gross morphology and histopathology of keloids were observed after 21 days of continuous injection.
Statistical analysis
All of the experiments were performed in triplicate, at a minimum. A repeated measures two-way analysis of variance (mixed model) and Bonferroni post-tests were performed to determine the significance of the grouped analyses. Data are presented as the mean±SD from more than two independent experiments. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA).
Author Contributions
Conceived and designed the experiments: RL and JX. Performed the experiments: RL, JL and FL. Analyzed the data: SZ, WL and YW. Contributed reagents/materials/analytical tools: RL, SZ and XC. Wrote the paper: RL. All authors reviewed the manuscript.
Data Availability
All data that were generated or analyzed during this study are included in this published article.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download Zip (5.2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental matrial
Supplemental data can be accessed here.
Additional information
Funding
References
- Lu WS, Zheng XD, Yao XH, et al. Clinical and epidemiological analysis of keloids in Chinese patients. Arch Dermatol Res. 2015;307:109–114.
- Ud-Din S, Bayat A. New insights on keloids, hypertrophic scars, and striae. Dermatol Clin. 2014;32:193–209.
- Mofikoya BO, Adeyemo WL, Abdus-salam AA. Keloid and hypertrophic scars: a review of recent developments in pathogenesis and management. Nig Q J Hosp Med. 2007;17:134–139.
- Mari W, Alsabri SG, Tabal N, et al. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec. 2015;7:1–7.
- Celeste CJ, Deschene K, Riley CB, et al. Regional differences in wound oxygenation during normal healing in an equine model of cutaneous fibroproliferative disorder. Wound Repair Regen. 2011;19:89–97.
- Schreml S, Szeimies RM, Prantl L, et al. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–268.
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443.
- Freedman SJ, Sun Z-YJ, Poy F, et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci U S A. 2002;99:5367–5372.
- Kaluz S, Kaluzova M, Stanbridge EJ. Regulation of gene expression by hypoxia: integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin Chim Acta. 2008;395:6–13.
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228.
- Naim R, Naumann A, Barnes J, et al. Transforming growth factor-beta1-antisense modulates the expression of hepatocyte growth factor/scatter factor in keloid fibroblast cell culture. Aesthetic Plast Surg. 2008;32:346–352.
- Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332.
- Campbell MT, Hile KL, Zhang H, et al. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res. 2011;168:e61–69.
- Chen J, Zeng B, Yao H, et al. The effect of TLR4/7 on the TGF-beta-induced Smad signal transduction pathway in human keloid. Burns. 2013;39:465–472.
- Tan WQ, Gao ZJ, Xu JH, et al. Inhibiting scar formation in vitro and in vivo by adenovirus-mediated mutant Smad4: a preliminary report. Exp Dermatol. 2011;20:119–124.
- Distler JH, Jüngel A, Pileckyte M, et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheumatism. 2007;56:4203–4215.
- Bozova S, Elpek GO. Hypoxia-inducible factor-1alpha expression in experimental cirrhosis: correlation with vascular endothelial growth factor expression and angiogenesis. APMIS. 2007;115:795–801.
- Moon JO, Welch TP, Gonzalez FJ, et al. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G582–592.
- Pakyari M, Farrokhi A, Maharlooei MK, et al. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle). 2013;2:215–224.
- Berse B, Hunt JA, Diegel RJ, et al. Hypoxia augments cytokine (transforming growth factor-beta (TGF-beta) and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts. Clin Exp Immunol. 1999;115:176–182.
- Mingyuan X, Qianqian P, Shengquan X, et al. Hypoxia-inducible factor-1alpha activates transforming growth factor-beta1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget. 2018;9:3188–3197.
- Xue H, McCauley RL, Zhang W. Elevated interleukin-6 expression in keloid fibroblasts. J Surg Res. 2000;89:74–77.
- Ghazizadeh M, Tosa M, Shimizu H, et al. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol. 2007;127:98–105.
- Barcelos RP, Bresciani G, Cuevas MJ, et al. Diclofenac pretreatment modulates exercise-induced inflammation in skeletal muscle of rats through the TLR4/NF-kappaB pathway. Appl Physiol Nutr Metab = Physiologie Appliquee, Nutrition Et Metabolisme. 2017;42:757–764.
- Ren K, Yong C, Yuan H, et al. TNF-alpha inhibits SCF, ghrelin, and substance P expressions through the NF-kappaB pathway activation in interstitial cells of Cajal. Brazilian J Med Biolog Res [Revista brasileira de pesquisas medicas e biologicas]. 2018;51:e7065 doi:10.1590/1414-431x20187065.
- Wang H, Pan JQ, Luo L, et al. NF-kappaB induces miR-148a to sustain TGF-beta/Smad signaling activation in glioblastoma. Mol Cancer. 2015;14:2.
- Yao Z, Seger R. The ERK signaling cascade–views from different subcellular compartments. Biofactors. 2009;35,:407–416.
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226.
- Zhang Q, Oh CK, Messadi DV, et al. Hypoxia-induced HIF-1 alpha accumulation is augmented in a co-culture of keloid fibroblasts and human mast cells: involvement of ERK1/2 and PI-3K/Akt. Exp Cell Res. 2006;312:145–155.
- Wolfram D, Tzankov A, Pulzl P, et al. Hypertrophic scars and keloids–a review of their pathophysiology, risk factors, and therapeutic management. Dermatologic Surg. 2009;35:171–181.
- Gupta S, Sharma VK. Standard guidelines of care: keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol. 2011;77:94–100.
- Kontochristopoulos G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathologic study. J Am Acad Dermatol. 2005;52:474–479.
- Zhao S, Gao Y, Xia X, et al. TGF-beta1 promotes Staphylococcus aureus adhesion to and invasion into bovine mammary fibroblasts via the ERK pathway. Microb Pathog. 2017;106:25–29.
- Zhang H, He F, Shi M, et al. Toll-like receptor 4-myeloid differentiation primary response gene 88 pathway is involved in the inflammatory development of polymyositis by mediating interferon-gamma and interleukin-17A in humans and experimental autoimmune myositis mouse model. Front Neurol. 2017;8:132.
- Freudlsperger C, Bian Y, Contag Wise S, et al. TGF-beta and NF-kappaB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32:1549–1559.
- Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40.
- Wang R, Zhou S, Li S. Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr Med Chem. 2011;18:3168–3189.
- Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375.
- Zhang Q, Karnak D, Tan M, et al. FBXW7 facilitates nonhomologous end-joining via K63-linked polyubiquitylation of XRCC4. Mol Cell. 2016;61:419–433.
