ABSTRACT
Chromatin Assembly Factor I (CAF-I) plays a central role in the reassembly of H3/H4 histones during DNA replication. In S. cerevisiae CAF-I is not essential and its loss is associated with reduced gene silencing at telomeres and increased sensitivity to DNA damage. Two kinases, Cyclin Dependent Kinase (CDK) and Dbf4-Dependent Kinase (DDK), are known to phosphorylate the Cac1p subunit of CAF-I, but their role in the regulation of CAF-I activity is not well understood. In this study we systematically mutated the phosphorylation target sites of these kinases. We show that concomitant mutations of the CDK and DDK target sites of Cac1p lead to growth retardation and significant cell cycle defects, altered cell morphology and increased sensitivity to DNA damage. Surprisingly, some mutations also produced flocculation, a phenotype that is lost in most laboratory strains, and displayed elevated expression of FLO genes. None of these effects is observed upon the destruction of CAF-I. In contrast, the mutations that caused flocculation did not affect gene silencing at the mating type and subtelomeric loci.
We conclude that dysfunctional CAF-I produces severe phenotypes, which reveal a possible role of CAF-I in the coordination of DNA replication, chromatin reassembly and cell cycle progression. Our study highlights the role of phosphorylation of Cac1p by CDK and a putative role for DDK in the transmission and re-assembly of chromatin during DNA replication.
Introduction
Chromatin Assembly Factor I (CAF-I) is a histone chaperone, which reassembles H3/H4 tetramers in the wake of the replication forks [Citation1,Citation2]. It is composed of three subunits (Cac1p, Cac2p and Cac3p) and is highly conserved across eukaryotes [Citation3,Citation4]. Recent studies have shown that the Cac1p subunit is required for the interaction with histones and the tetramerization of H3/H4 as well as for their deposition on DNA [Citation5–Citation7]. CAF-I associates with the replication fork via an interaction of Cac1p with the replication sliding clamp, PCNA (Proliferating Cell Nuclear Antigen, Pol30p) [Citation3] and cooperates with other chaperones such as Asf1p, Rtt106p and the replicative helicase CMG (Cdc45-MCM-GINS) [Citation1,Citation2]. It is believed that CAF-I, together with other histone chaperones and histone modifying enzymes, plays a central role in the transmission of the epigenetic states after the passage of the replication fork [Citation2]. However, mechanistic insights into the action of CAF-I at different loci are limited [Citation1,Citation2].
In mammalian cells CAF-I is essential [Citation8], yet in budding yeast the deletion of its primary subunit, CAC1 leads to only marginal loss of fitness, de-repression of subtelomeric genes and transient loss of silencing at the mating type loci [Citation9–Citation11]. Loss of CAF-I has also been linked to lower frequency of naturally occurring epigenetic switches at the telomeres [Citation11,Citation12] and to increased sensitivity to UV light and other mutagens [Citation13]. It is not clear what factors and mechanisms regulate these diverse phenotypes.
It is well established that in both mammalian and yeast cells CAF-I is phosphorylated by CDK (Cyclin Dependent Kinase, Cdc28p) and most likely by DDK (Dbf4-Dependent Kinase, Cdc7p-Dbf4p) [Citation14–Citation17]. Both kinases are essential and regulate key events at the onset of DNA replication [Citation18,Citation19]. The phosphorylation of Cac1p by CDK has been linked to the loading of CAF-I on chromatin; however, the role of the phosphorylation by DDK remains enigmatic [Citation14,Citation15]. To better understand the role of phosphorylation of CAF-I by CDK and DDK, we introduced multiple point mutations at the phosphorylation sites of Cac1p. Unlike the complete deletion of CAC1, some of these mutants displayed severe loss of fitness and altered morphology. The follow-up characterization of these mutants suggest that the phosphorylation of Cac1p by the CDK and DDK affects cell cycle progression, sensitivity to DNA damage and the expression of the FLO genes.
Materials and methods
Yeast strains, growth conditions and site directed mutagenesis
All experiments were performed with a cac1∆ (MATa cac1::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and an isogenic BY4742 strains obtained from Open Biosystems. ZGY450 (MATa cac1::LEU2 hmr::GFP) and ZGY450 (MATa cac1::LEU2 asf1::KanMX6 hmr::GFP) strains were used for the analysis of the expression of GFP from a recombinant HMR and were a gift from Dr. Z. Zhang [Citation20]. The strain JRY0803 [Citation10] was used in the CRASH assay. All strains were routinely grown at 30°C in SC and SC drop-out media, as appropriate. Analyses of growth rates were performed by serially diluting exponentially growing cultures in a 96-well plate and measuring optical density every 15 min for a 7 hour period using a ThermoScientific Multiskan G0 instrument with continuous shaking.
All mutations were introduced by site-directed mutagenesis in the pRS315-CAC1 plasmid, which harbors a 2.1 kb fragment containing the CAC1 promoter and the open reading frame C-terminally fused to three FLAG epitopes [Citation14]. Primer sequences are available upon request. All mutations have been confirmed by DNA sequencing.
Flocculation-like phenotypes were determined by visual observation of sedimentation and clustering of cells in liquid cultures and confirmed by light microscopy.
Plasmid stability assay
BY4742 or isogenic cac1∆ cells harboring the pRS315-CAC1 plasmid with the mutations shown on the horizontal axis were selected in SC-leu medium, then transferred to nonselective SC medium and grown for 20 generations. The cell suspensions were serially diluted and spotted on SC-leu and SC to measure the proportion of cells that have maintained the plasmid. Calculations of loss per generation were as in [Citation21].
Co-immunoprecipitation experiments were performed as in [Citation14], except that immunoprecipitation was performed using Rat anti-DYKDDDDK antibody from SinoBiological and the precipitated proteins were eluted by FLAG peptide .
CRASH assay was performed as in [Citation10]. Briefly, cac1∆ cells (strain JRY803 [Citation10]) with a CRE expression cassette in HML mating type locus and an RFP/GFP switch reporter harboring CAC1 wild type or mutant plasmids were grown on SC-leu plates containing 300μg/mL hygromycin to select for the initial 100% RFP positive state. A single colony from this plate was grown overnight in SC-leu liquid medium, then serially diluted and plated on SC-Leu-Ade-Trp plates at less than 100 colonies per plate. Colonies were imaged after 5–7 days growth using a Zeiss AxioZoom V16 microscope equipped with a 1X objective lens at 3X continuous magnification using a Hamamatsu Camera and Zen software. The number of de-repression events for each mutant was quantified by counting the number of green segments for 4–5 individual colonies from each strain, with the exception of the cac1Δ with pRS315 where the extent of the GFP signal made accurate counts impossible. Graphs and statistical analyses were produced using Microsoft Excel®; error bars show standard deviation, as determined using Excel®.
Analysis of sub-telomeric silencing
CAC1 plasmids were expressed in a cac1Δ strain with a URA3 reporter integrated at the VII-L telomere, as described previously [Citation14]. Three colonies from each strain were selected SC-leu-ura plates and transferred to liquid SC-leu for 20 generations to allow for switches between active and silenced URA3. Cultures were serially diluted and spotted onto SC-leu, SC-leu-ura and SC-leu+ 1 mg/mL 5-FOA then allowed to grow for 5 days. The %FOAR and %URA3+ colonies were calculated as a ratio of the number of colonies growing in the presence of FOA, or absence of Ura, relative to the number of colonies on SC-leu, respectively. Graphs and statistics were completed using MS Excel®. Bars show the average of three independent biological replicates from a representative experiment. Error bars show standard error.
Analysis of FLO gene length variation
DNA was isolated from saturated cultures and analyzed by PCR with primers flanking FLO1, FLO5, FLO9, FLO10 and FLO11 as in [Citation22]. Control reactions used primers binding interior to the yeast ACT1 gene. Primer sequences and the coordinates in the S. cerevisiae genome are available upon request.
Methyl Methanesulfonate (MMS) sensitivity assay was conducted by serial dilution of exponential cultures cells and spotting 5 μl aliquots on plates containing 0.005%, 0.01% and 0.02% MMS (Sigma).
Canavanine resistance assays were conducted by serially diluting liquid cultures in a 96 well plate. Four wells containing isolated single colonies were chosen for each strain; colonies (about 107 cells) were suspended into 200 μl liquid medium and spread onto SC-Arg plates containing 60 µg/ml canavanine (Sigma-Aldrich). The plates were incubated for three days and the number of CanR colonies were counted and plotted using “stock” graph by MSExcel©.
Flow cytometry was performed using FC500 flow cytometer (Beckman-Colter) with MXP software. Exponentially replicating cells were fixed with 70% ethanol and stained using propidium iodide as described previously [Citation14]. Analysis was performed using FCS Express 6 Plus software.
RT-PCR
Cells were grown to OD600 = 0.8 and total RNA was isolated with TRIzolTM solution according to manufacturer’s directions, except that samples were vortexed for 5 minutes (30 seconds on, 30 seconds off) in the presence of equal volume of glass beads and precipitated with Ethanol. Total RNA concentration and purity was determined by ThermoScientific NanoDrop 8000. cDNA synthesis was performed using Applied Biosystems High-capacity cDNA Reverse Transcription Kit, according to manufacturer’s instructions. Quantitative PCR was carried out using Applied Biosystems StepOneTM Plus thermocycler and PowerUp SYBR Green 2X Master Mix. 6.25ng of cDNA was added to each reaction. Relative expression values were determined using the ΔΔCq method, wherein the average Cq for each FLO gene was normalized to Cq values obtained from ACT1 primers. These ΔCq values were then normalized to ΔCq obtained for cells expressing wildtype CAC1, and fold expression was calculated as 2−ΔΔCq and shown as a bar graph using Microsoft Excel®.
Results
Cac1p with mutations at multiple phosphorylation sites
In order to understand the functional significance of the phosphorylation of CAF-I by CDK and DDK we produced combinations of S→A mutations in the phosphorylation sites in Cac1p as outlined in ,b). The S94A and S515A mutations are confirmed target sites of CDK while the S238A, S501A and S503A mutations alter putative targets of DDK [Citation14,Citation17]. These CAC1 mutants were expressed in cac1∆ cells as C-terminally FLAG-tagged proteins under the control of the native CAC1 promoter from a low copy plasmid (pRS315).
Figure 1. Mutations of the phosphorylation sites of CAC1.
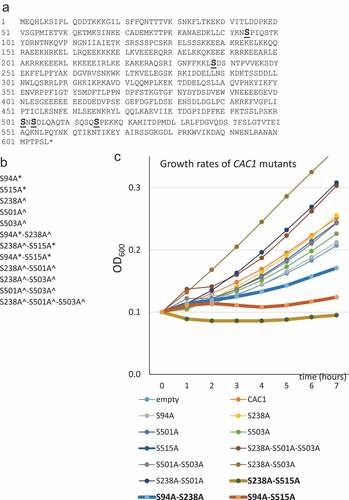
In agreement with earlier studies [Citation14,Citation23,Citation24] cac1∆ cells expressing no Cac1p, wild type Cac1p or any single S→A substitutions in the DDK kinase target sites showed little difference in their growth rates ()). The same applies to mutants with double/triple substitutions in the putative DDK target sites. The single S→A substitutions in the CDK target sites (S94, S515) moderately reduced growth. Remarkably, mutations at both CDK target sites (S94A-S515A) or at one CDK target site in combinations with a S238A substitution (S94A-S238A and S238A-S515A) caused more than three-fold decrease in the doubling times of the exponentially growing cultures. We asked if the reduced growth rates could be caused by reduced stability of the proteins or maintenance of the expressing plasmids. In ) (see also Suppl. Figure 1) we show that most mutant Cac1p proteins were expressed at comparable levels as judged by anti-FLAG Western blotting. However, the S94A-S238A and S238A-S515A double mutants were less abundant (); Suppl. Figure 1). We considered the possibility that the stability of the expressing plasmids could contribute to the reduced abundance of these proteins. Plasmid maintenance was tested by a routine mini-chromosome maintenance assay as in [Citation21,Citation25]. Briefly, cac1∆ and isogenic BY74742 cells harboring the expression plasmids (pRS315; LEU2 ARS4 CEN4) were selected on SC-leu plates, grown in liquid nonselective medium for 20 generations and then spread on SC and SC-leu plates. The proportion of leu+ cells was used to calculate the loss of plasmid per generation as in [Citation21]. In the wild type BY4742 strain all plasmids were lost at 1–3% per generation ()). Similar rates were observed for all single mutants in the cac1∆ cells. In the double/triple mutants plasmid loss was increased to 4–5% loss per generation ()). For comparison, the loss rate in the control mcm5-1 (Mini-Chromosome Maintenance 5–1) strain [Citation26] was 27% per generation ()). These results showed that plasmid maintenance was modestly affected in the double deletion mutants, but plasmid instability did not correlate to the reduced level of mutant proteins or reduced growth rates in the strains. For example, the S501A-S503A mutant showed 4% loss per generation as compared to about 5% in the S94A-S515A, S94A-S238A and S238A-S515A mutants, but grew three times faster than them. On the other hand, the mcm5-1 mutant grows at higher rates as compared to these three CAC1 mutants (not shown), but has about five times higher rates of plasmid loss.
Figure 2. Protein and plasmid stability in CAC1 mutants and association with PCNA
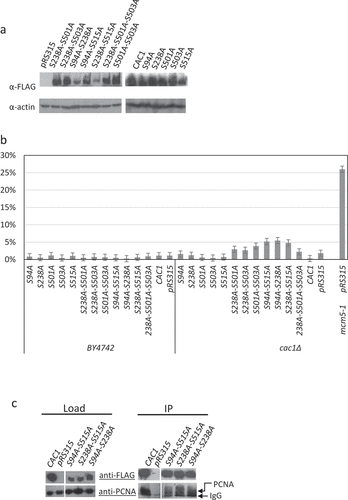
Previous studies have demonstrated that Cac1p directly binds to the sliding DNA replication clamp, PCNA, and that mutations in individual phosphorylation sites of Cac1p do not alter this association [Citation14,Citation27]. Nevertheless, the strong phenotypes of the S94A-S238A, S94A-S515A and S238A-S515A mutants prompted us to test their association with PCNA as in [Citation14]. These co-immunoprecipitation assays indicated that the mutants bind to PCNA almost as efficiently as wild type Cac1p ()). The results strongly suggest that growth retardation rates observed in the mutants are not related to a reduced association of CAF-I with PCNA, and as such likely do not reflect reduced availability of CAF-I at the replication fork.
Effects of CAC1 mutations on cell cycle progression, DNA damage and mutation rates
We noticed multiple elongated/enlarged cells in the S94A-S238A and the S238A-S515A, but not in S94A-S515A mutants ()) or any other of the mutants (not shown). Similar morphology has been previously related to the de-repression of the mating type loci [Citation28] or to a prolonged S-phase [Citation29]. For this reason we tested the cell cycle progression ()) and the silencing of the mating type loci () in these mutants.
Figure 3. Deregulation of cell cycle in CAC1 mutants
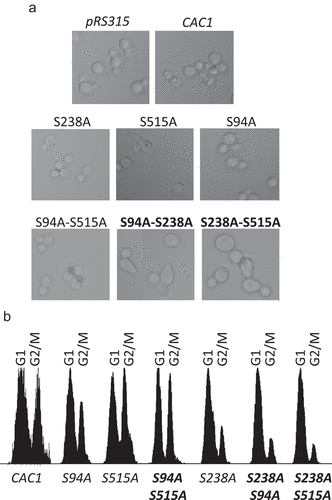
The analysis of cell cycle demonstrated that the cells harboring the S515A and the double S94A-S515A mutations had an increased proportion of cells in G2/M ()). On the other hand, the cells harboring the S94A-S238A and S238A-S515A mutations produced a significantly lower proportion of cells in G2/M ()). These observations correlated with the altered morphology of the two mutants ()). The single S238A mutant had similar, but less pronounced reduction of cells in G2/M ()). Since S238 is a putative DDK target and S94 and S515 are known CDK targets, our results point to the possibility that phosphorylation by both DDK and CDK regulate the activity of CAF-I in S phase. Phosphorylation by CDK alone does not produce the same effect, suggesting that CDK phosphorylation regulates a different aspect of CAF-I activity during the cell cycle.
It is known that the deletion of CAC1 increases the sensitivity to DNA damage and produces higher rates of spontaneous mutations [Citation11,Citation13]. We tested if our mutants suppressed or exacerbated these phenotypes. All strains were grown to mid-exponential phase and aliquots were spotted on plates containing 0.005%, 0.01% and 0.02% Methyl Methane Sulfonate (MMS). As shown earlier, cells without CAC1 were sensitive to 0.01% MMS ()). Similar sensitivity was seen in the slowly growing S94A-S515A, S94A-S238A and S238A-S515A while none of the other mutants was sensitive to this DNA damaging agent ()). All strains tested, including those harboring the plasmid expressing wildtype CAC1, were highly sensitive to MMS at 0.02%. We also used the canavanine resistance fluctuation assay [Citation11] to measure the frequency of spontaneous mutations in the mutant strains. Canavanine is a translation inhibitor and is internalized by the CAN1 transporter. Consequently, mutations in CAN1 produce canavanine resistance. In wild type cells the rates of forward CAN1 mutations is about 3 × 10−7 [Citation30,Citation31]. In cac1∆ cells the rates moderately increase to 1–3 × 10−6 [Citation11,Citation32]. While reproducing these earlier observations, we saw no significant differences between any of the mutants and the cells expressing wild type CAC1 ()).
Figure 4. Mutation rates and sensitivity to DNA damage
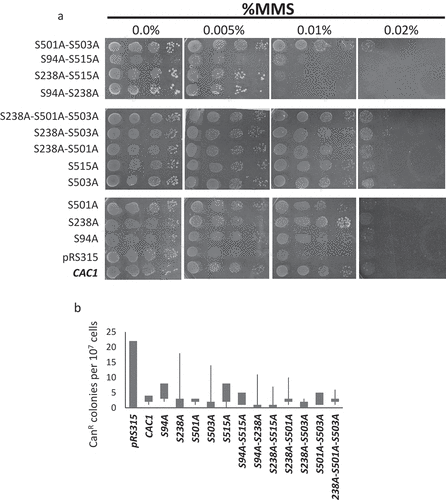
We concluded that the loss of phosphorylation of Cac1p at S94, S238 and S515 does not increase the rates of spontaneous mutations, but reduces its activity in DNA repair.
Flocculation-like phenotypes and expression of FLO genes
When grown in liquid cultures, the S94A-S238A and S238A-S515A mutants formed visible clusters and were sedimenting faster than the other strains ()). None of the single S→A mutants or other double/triple mutants, including S94A-S515A, displayed a similar phenotype. These traits were reproducible upon multiple transformations of two different cac1∆ strains (cac1::KanMX, BY4742 background; ZGY450 cac1::LEU2, W303 background). The phenotype is reminiscent of flocculation, which is frequently observed in industrial strains but rarely seen in laboratory strains [Citation33–Citation35].
Figure 5. Flocculation in S94A-S238A and S238A-S515A mutants
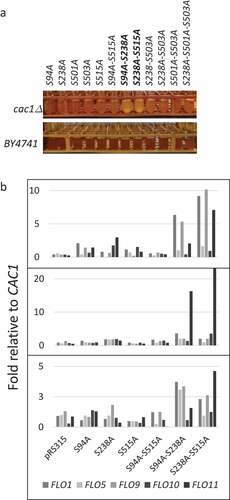
Flocculation in budding yeasts is regulated by the FLO genes, which encode carbohydrate-binding proteins. The Flo proteins mediate cell-to-cell contacts and are expressed upon exposure to ethanol and other stressors [Citation33]. The FLO genes contain multiple internal repeats. It is known that in industrial yeasts they undergo intergenic recombination, which contribute to length variation and diversified flocculation phenotypes [Citation33,Citation36,Citation37]. For this reason, we looked at possible length variations and measured the levels of expression of all FLO genes in them.
The analysis of length variation was performed exactly as in [Citation37] and showed that all FLO genes maintain the length seen in BY4742 (Suppl. Figure 2). We therefore focused on the measurement of the abundance of FLO gene RNAs in the single S94A, S238A and S515A and the double S94A-S238A, S238A-S515A and S94A-S515A mutants. A representative experiment is shown in ). We noticed up to 2 fold increase in the expression of FLO genes in the S238A mutant and much higher (2–10 fold) increase in the S94A-S238A and S238A-S515A mutants ()). While this trend was preserved in three independent measurements of all FLO genes, the magnitude of increase of individual FLO genes varied considerably. We have observed similar variations in FLO expression in other laboratory strains that were forced to flocculate by various mutations (see appended manuscript). We have also seen variations in the extent of flocculation between biological replicates of these strains. While we can not rule out experimental variations, we suspect that oscillating expression of FLO genes and variable cell adhesion properties could be an intrinsic feature that provides for multiple aggregation options for the strain.
We concluded that de-repression of the FLO genes, and not their intragenic recombination, was causing flocculation in the S94A-S238A and S238A-S515A mutants. In a related study (manuscript appended) we have reached a similar conclusion about the cause of flocculation in strains that harbor a deletion of CAC1 in conjunction with deletions of any of ASF1, HIR1, RRM3 and HDA1 genes.
Analysis of gene silencing at the mating type and subtelomeric loci
Previous studies have shown that the deletion of CAC1 does not affect the mating efficiency of the cells[Citation9]. We performed similar assays and showed that none of our single or double CAC1 mutants produced detectable loss of mating efficiency (Suppl. Figure 3). The silencing of the mating loci was also tested by a recombinant HMR locus, which harbors an expression cassette for GFP (Green Fluorescence Protein)[Citation38]. Earlier reports have shown that the deletion of CAC1 does not allow the expression of GFP from this position, however a double cac1∆asf1∆ deletion does [Citation11,Citation38]. In Suppl. Figure 3 we show that cac1∆asf1∆ cells readily display GFP signals, but we observed no detectable GFP in any of our CAC1 mutants.
Next, we used the highly sensitive CRASH assay [Citation10,Citation39] for transient de-repression of HML. Cells harboring the HML alpha1→CRE and LoxPRFPLoxP/GFP CRASH cassettes normally express RFP; however transient de-repression of HML results in expression of CRE and subsequent loss of RFP and irreversible transition to GFP expression [Citation39]. Consequently, even temporary transient loss of silencing at HML results in the formation of a green segment in an otherwise red colony and can be readily observed by low resolution fluorescence microscopy. We expressed the CAC1 mutants in a cac1Δ strain harboring these constructs. ) shows colony images from each of the tested mutants. ) shows the average number of segments observed in 4–5 colonies from each strain, except for the cac1Δ strain with empty pRS315 vector where the numerous GFP segments made it impossible to count. We found an average of 6.5 segments in cac1Δ cells complemented with wild type CAC1, which is in keeping with what is observed in wild type cells. There was a small increase in the number of segments in the S94A-S515A, S94A and S515A mutants. The S238A, S94A-S238A and S238A-S515A mutants showed approximately 4 times higher (22–26 segments) numbers of segments as compared to wild type CAC1. However, none of these mutants showed levels of HML de-repression to the extent observed in cac1Δ colonies. We concluded that all cac1 mutants complemented, albeit to somewhat different extent, the transient loss of silencing at HML observed upon deletion of CAC1. It seems that the phosphorylation of Cac1p at S94, S515 and S238 alone or in combination does not substantially contribute to the silencing at HML.
Figure 6. Analysis of silencing at Sir2p-regulated loci
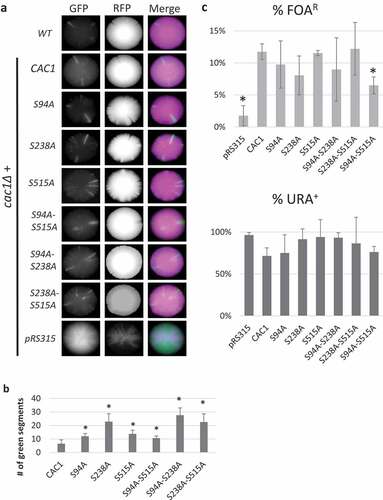
We followed up by analyses of sub-telomeric silencing as in [Citation14]. We expressed the CAC1 mutants in a cac1Δ strain with a URA3 reporter adjacent to the VII-L telomere. Briefly, the strains were selected on SC-ura-leu plates, then three colonies were separately grown for 20 generations in liquid SC-leu to maintain the expression plasmids but allow unrestricted variegation of the URA3 reporter. Cultures were then serially diluted and grown on SC-ura-leu, Sc-leu and SC-leu+5FOA plates to calculate the percentage of cells with silenced/active URA3. We have previously shown that none of the single S→A mutations on CAC1 cause significant detriments in sub-telomeric silencing [Citation14]. Similarly, we found no significant changes of silencing in the S94A-S238A and S238A-S515A relative to a strain expressing wild type CAC1 (). A reduction in URA3 silencing was observed in the S94A-S515A mutant, although the effect was not to the extent observed in cac1Δ cells with the empty expression vector (). Unfortunately, the cac1∆ strains expressing CAC1 mutants showed significant deterioration, slow growth and loss of viability upon selection on SC+FOA plates and subsequent growth in nonselective medium thus precluding the possibility to test frequency of epigenetic conversions as previously shown in [Citation11].
We concluded that S94, S238 and S515 phosphorylation does not significantly contribute to the silencing of the mating and subtelomeric loci. These findings are in contrast with our observations that the S94A-S238A and S238A-S515A mutants substantially de-repress the FLO genes (). This suggests that the phosphorylation patterns of Cac1p can be used to regulate gene silencing at various loci.
Discussion
The destruction of CAF-I in S. cerevisiae is known to cause substantial loss of gene silencing and variegation at the telomeres, transient de-repression of the mating type loci and increased sensitivity to DNA damage [Citation10,Citation11,Citation13]. These are surprisingly mild phenotypes for a factor postulated to be central to the replication-coupled assembly of nucleosomes [Citation1,Citation2]. In this study we show that the presence of dysfunctional CAF-I leads to prominent and more severe phenotypes. In particular, we demonstrate that S→A mutations at the two CDK phosphorylation sites on Cac1p (S94A-S515A) cause severe reduction of growth rate and an increased sensitivity to DNA damage. The consequences of mutating either one of these sites in a combination with a S238A mutation are even more intriguing and interesting. The S94A-S238A and S238A-S515A mutants have severe growth defects and sensitivity to DNA damage, but additionally display altered morphology, slow progression through S-phase and de-repression of the FLO genes. Together, these observations suggest that the function of CAF-I extends beyond reassembly of nucleosomes at the replication fork and could involve the coordination of chromatin re-assembly with DNA replication and the progression through the cell cycle. Previous studies have shown that cac1Δasf1Δ mutants progress slowly through G2/M following the activation of DNA damage checkpoints [Citation6]. Here we show that defective CAF-I can alter cell cycle even in the presence of ASF1 and that this role of CAF-I is mediated by the phosphorylation of Cac1p by CDK and potentially by DDK. The mechanism of this enigmatic link between chromatin assembly and cell cycle progression should certainly be addressed in the future.
While it is well established that both yeast and mammalian CAF-I are phosphorylated in vivo [Citation14,Citation16], it is not clear how these phosphorylation events regulate its activity. Even more, it is not clear precisely how CAF-I functions at the replication fork. Earlier studies have indicated that the majority of “old” H3/H4 tetramers are transmitted to the new DNA strands without being split [Citation40,Citation41]. Other evidence indicates that Asf1p, which disassembles H3/H4 tetramers ahead of the fork, interacts with H3/H4 in a manner that disrupts the tetramer [Citation42] and that two CAF-I complexes interact with each tetramer [Citation5,Citation7]. These findings indicate that the tetramers must be split prior to their deposition and that CAF-I may form tetramers of H3/H4 at the time of assembly onto DNA. However, recent evidence points out that un-split H3/H4 tetramers can be ferried by the MCM helicase thus providing alternatives in the transmission of the old H3/H4 histones [Citation43]. Some researchers go as far as suggesting that CAF-I assembles only “new” histones while the “old” ones are transmitted by different means [Citation44]. A recent review has put forward the idea that different mechanisms of transmission of H3/H4 could operate when the replication fork is advancing or pausing [Citation1]. It is possible that the phosphorylation of CAF-I could contribute to these alternative mechanisms. For example, it has been shown that the replicative clamp PCNA and CAF-I are continuously recycled during elongation [Citation10] and failure to disassemble/recycle PCNA and CAF-I leads to transient loss of silencing at the mating type loci and to higher retention of CAF-I away from the replication forks [Citation10]. It is possible that the phosphorylation of CAF-I is involved in these processes. Our understanding of the regulation and activities of CAF-I and other chaperones behind the replication fork remains limited, thus multiple scenarios are possible.
The role of DDK at the advancing replication fork is another open and very interesting question. Upon arrest of the replication fork DDK phosphorylates the MCM helicase and the Fork Protection Complex FPC (Mrc1/Tof1p/Csm3p) [Citation45]. Another study has demonstrated that during replication stress DDK phosphorylates Histone H3 at position T45 [Citation46]. These DDK activities are different from its essential role in the initiation of DNA replication [Citation47] and meiosis [Citation48].We have previously shown that S238, S501 and S503 are phosphorylated by DDK in vitro [Citation14]. A recent study has shown that Cdc7p and Cac1p interact in S, but not G1 phase of the cell cycle[Citation17]. Even more, a cac1-S238A mutant failed to reproduce the effects of wild type CAC1 on the silencing of a defective HMR mating type locus [Citation17]. All these observations point to yet another role of DDK during the elongation stage of DNA replication, with potential implications on fork stalling and resumption and during replication stress.
A very intriguing aspect of our studies is the gain of flocculation-like phenotypes in the S94A-S238A and S238A-S515 strains via the loss of repression of FLO genes. Flocculation is reminiscent of biofilm formation by other microorganisms. In industrial strains flocculation is readily observed upon starvation, exposure to ethanol and by other stressors [Citation33,Citation49]. On the other hand, S288C and other laboratory strains of S. cerevisiae do not flocculate, most likely because of the prolonged passive selection in favor of planktonic growth [Citation33,Citation34]. Our findings strongly suggest that CAF-I contributes to the repression of FLO genes and potentially other Tup1/Cyc8 regulated genes (). Even more, the S94A-S238A and S238A-S515A strains did not show substantial de-repression of the mating type and subtelomeric loci (). It seems that CAF-I operates by different mechanisms at the FLO loci as compared to the SIR-dependent mating type and subtelomeric loci. This is in opposition to most current models, which favor the idea of a universal mechanism for the reassembly of chromatin at all loci. Our findings indicate that the rebuilding of silent chromatin in the wake of replication forks could be more complex than previously thought and may involve multiple cyclin-dependent kinases. This complexity can not be revealed by the destruction of CAF-I alone, highlighting the need for further studies of CAF-I phosphorylation.
In summary, our study strongly suggests that CDK and DDK control the activity of CAF-I. Deregulation of this phosphorylation leads to dominant phenotypes including cell cycle defects and de-repression of FLO genes. Further investigation of these phenotypes would provide details on the function of CAF-I and on the coordination between DNA replication, chromatin re-assembly and progression through the cell cycle.
Supplemental Material
Download MS Power Point (4.3 MB)Acknowledgments
We want to thank Dr. Z. Zhang and Dr. J. Rine for providing strains for this study, and to Dr. G. van der Merwe, Dr. R. Shapiro and Dr. B. Duncker for valuable discussion and advice.
This study was supported by a NSERC grant (RGPIN-2015-06727) to KY and financial support to HR, AC and KS by the University of Guelph.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Almouzni G, Cedar H. Maintenance of epigenetic information. Cold Spring Harb Perspect Biol. 2016;8(5).
- Rowlands H, Dhavarasa P, Cheng A, et al. Forks on the run: can the stalling of DNA replication promote epigenetic changes? Front Genet. 2017;8:86.
- Rolef Ben-Shahar T, Castillo AG, Osborne MJ, et al. Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function. Mol Cell Biol. 2009;29:6353–6365.
- Alabert C, Groth A. Chromatin replication and epigenome maintenance. Nat Rev Mol Cell Biol. 2012;13:153–167.
- Liu WH, Roemer SC, Zhou Y, et al. The Cac1 subunit of histone chaperone CAF-1 organizes CAF-1-H3/H4 architecture and tetramerizes histones. Elife. 2016;5:e18023.
- Kim J-A, Haber JE. Chromatin assembly factors Asf1 and CAF-1 have overlapping roles in deactivating the DNA damage checkpoint when DNA repair is complete. Proc Natl Acad Sci U S A. 2009;106:1151–1156.
- Mattiroli F, Gu Y, Yadav T, et al. DNA-mediated association of two histone-bound complexes of yeast chromatin assembly factor-1 (CAF-1) drives tetrasome assembly in the wake of DNA replication. Elife. 2017;6:e22799.
- Houlard M, Berlivet S, Probst AV, et al. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2006;2:e181.
- Sharp JA, Franco AA, Osley MA, et al. Chromatin assembly factor I and Hir proteins contribute to building functional kinetochores in S. cerevisiae. Genes Dev. 2002;16:85–100.
- Janke R, King GA, Kupiec M, et al. Pivotal roles of PCNA loading and unloading in heterochromatin function. Proc Natl Acad Sci U S A. 2018;115:E2030–E9.
- Jeffery DC, Wyse BA, Rehman MA, et al. Analysis of epigenetic stability and conversions in Saccharomyces cerevisiae reveals a novel role of CAF-I in position-effect variegation. Nucleic Acids Res. 2013;41:8475–8488.
- Wyse B, Oshidari R, Rowlands H, et al. RRM3 regulates epigenetic conversions in Saccharomyces cerevisiae in conjunction with Chromatin Assembly Factor I. Nucleus. 2016;7:405–414.
- Kaufman PD, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357.
- Jeffery DC, Kakusho N, You Z, et al. CDC28 phosphorylates Cac1p and regulates the association of chromatin assembly factor I with chromatin. Cell Cycle. 2015;14:74–85.
- Gerard A, Koundrioukoff S, Ramillon V, et al. The replication kinase Cdc7-Dbf4 promotes the interaction of the p150 subunit of chromatin assembly factor 1 with proliferating cell nuclear antigen. EMBO Rep. 2006;7:817–823.
- Sadowski I, Breitkreutz BJ, Stark C, et al. The PhosphoGRID Saccharomyces cerevisiae protein phosphorylation site database: version 2.0 update. Database. 2013;2013:bat026.
- Young TJ, Cui Y, Irudayaraj J, et al. Modulation of gene silencing by Cdc7p via H4 K16 acetylation and phosphorylation of chromatin assembly factor CAF-1 in saccharomyces cerevisiae. Genetics. 2019;211:1219–1237.
- Nougarede R, Della Seta F, Zarzov P, et al. Hierarchy of S-phase-promoting factors: yeast Dbf4-Cdc7 kinase requires prior S-phase cyclin-dependent kinase activation. Mol Cell Biol. 2000;20:3795–3806.
- Masai H, Matsumoto S, You Z, et al. Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem. 2010;79:89–130.
- Huang S, Zhou H, Tarara J, et al. A novel role for histone chaperones CAF-1 and Rtt106p in heterochromatin silencing. Embo J. 2007;26:2274–2283.
- Kramer DJ, Gauthier L, Yankulov K. Higher-accuracy method for measuring minichromosome stability in Saccharomyces cerevisiae. Biotechniques. 2002;32:1036–1040.
- Smukalla S, Caldara M, Pochet N, et al. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell. 2008;135:726–737.
- Krawitz DC, Kama T, Kaufman PD. Chromatin assembly factor I mutants defective for PCNA binding require Asf1/Hir proteins for silencing. Mol Cell Biol. 2002;22:614–625.
- Sharp JA, Fouts ET, Krawitz DC, et al. Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr Biol. 2001;11:463–473.
- Tye BK. Minichromosome maintenance as a genetic assay for defects in DNA replication. Methods. 1999;18:329–334.
- Dziak R, Leishman D, Radovic M, et al. Evidence for a role of MCM (mini-chromosome maintenance)5 in transcriptional repression of sub-telomeric and Ty-proximal genes in Saccharomyces cerevisiae. J Biol Chem. 2003;278:27372–27381.
- Zhang Z, Shibahara K, Stillman B. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature. 2000;408:221–225.
- Pillus L, Rine J. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell. 1989;59:637–647.
- Li X, Cai M. Inactivation of the cyclin-dependent kinase Cdc28 abrogates cell cycle arrest induced by DNA damage and disassembly of mitotic spindles in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:2723–2734.
- Huang ME, Rio AG, Nicolas A, et al. A genomewide screen in Saccharomyces cerevisiae for genes that suppress the accumulation of mutations. Proc Natl Acad Sci U S A. 2003;100:11529–11534.
- Davidson MB, Katou Y, Keszthelyi A, et al. Endogenous DNA replication stress results in expansion of dNTP pools and a mutator phenotype. Embo J. 2012;31:895–907.
- Myung K, Pennaneach V, Kats ES, et al. Saccharomyces cerevisiae chromatin-assembly factors that act during DNA replication function in the maintenance of genome stability. Proc Natl Acad Sci U S A. 2003;100:6640–6645.
- Verstrepen KJ, Klis FM. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol. 2006;60:5–15.
- Matheson K, Parsons L, Gammie A. Whole-genome sequence and variant analysis of W303, a widely-used strain of saccharomyces cerevisiae. G3 (Bethesda). 2017;7:2219–2226.
- Van Mulders SE, Christianen E, Saerens SM, et al. Phenotypic diversity of Flo protein family-mediated adhesion in Saccharomyces cerevisiae. FEMS Yeast Res. 2009;9:178–190.
- Fidalgo M, Barrales RR, Jimenez J. Coding repeat instability in the FLO11 gene of Saccharomyces yeasts. Yeast. 2008;25:879–889.
- Gemayel R, Vinces MD, Legendre M, et al. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu Rev Genet. 2010;44:445–477.
- Huang S, Zhou H, Katzmann D, et al. Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc Natl Acad Sci U S A. 2005;102:13410–13415.
- Dodson AE, Rine J. Heritable capture of heterochromatin dynamics in Saccharomyces cerevisiae. Elife. 2015;4:e05007.
- Katan-Khaykovich Y, Struhl K. Splitting of H3-H4 tetramers at transcriptionally active genes undergoing dynamic histone exchange. Proc Natl Acad Sci U S A. 2011;108:1296–1301.
- Xu M, Long C, Chen X, et al. Partitioning of histone H3-H4 tetramers during DNA replication-dependent chromatin assembly. Science. 2010;328:94–98.
- Natsume R, Eitoku M, Akai Y, et al. Structure and function of the histone chaperone CIA/ASF1 complexed with histones H3 and H4. Nature. 2007;446:338–341.
- Clement C, Almouzni G. MCM2 binding to histones H3-H4 and ASF1 supports a tetramer-to-dimer model for histone inheritance at the replication fork. Nat Struct Mol Biol. 2015;22:587–589.
- Sarkies P, Sale JE. Propagation of histone marks and epigenetic memory during normal and interrupted DNA replication. Cell Mol Life Sci. 2012;69:697–716.
- Bastia D, Srivastava P, Zaman S, et al. Phosphorylation of CMG helicase and Tof1 is required for programmed fork arrest. Proc Natl Acad Sci U S A. 2016;113:E3639–48.
- Baker SP, Phillips J, Anderson S, et al. Histone H3 Thr 45 phosphorylation is a replication-associated post-translational modification in S. cerevisiae. Nat Cell Biol. 2010;12:294–298.
- Sheu YJ, Stillman B. The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature. 2010;463:113–117.
- Matsumoto S, Masai H. Regulation of chromosome dynamics by Hsk1/Cdc7 kinase. Biochem Soc Trans. 2013;41:1712–1719.
- Reynolds TB, Fink GR. Bakers’ yeast, a model for fungal biofilm formation. Science. 2001;291:878–881.
