 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Osteosarcoma (OS) accounts for 9 percent of cancer-related deaths in young people. The PI3K/Akt signaling, a well-known carcinogenic signaling pathway in human cancer, cooperates with other signaling pathways such as Wnt signaling to promote cancer progression. Wnt7b, as a transforming member of the Wnt family, could activate mTORC1 through PI3K-AKT signaling and is upregulated in OS. In the present study, we found that miR-342-5p inhibits Wnt7b expression via direct binding to Wnt7b 3′-UTR. miR-342-5p overexpression remarkably suppressed the viability and invasion while enhanced the apoptosis of OS cells; meanwhile, Wnt7b, β-catenin, c-myc, and cyclin D1 proteins were reduced while E-cadherin protein showed to be increased. Consistent with its expression pattern, Wnt7b exerted oncogenic effects on OS cells. Wnt7b could significantly attenuate the impacts of miR-342-5p. In conclusion, we demonstrated a miR-342-5p/Wnt7b axis that regulates the capacity of OS cells to proliferate and to invade through Wnt/β-catenin signaling. The miR-342-5p/Wnt7b axis might be novel targets for OS targeted therapy, which needs further in vivo and clinical investigations.
Introduction
As a relatively rare cancer, osteosarcoma (OS) accounts for about 9 percent of cancer-associated deaths in young people, with an overall incidence of 5 cases in every one million people per year [Citation1]. Patients with OS who have undergone modern treatment approaches, including chemotherapy and surgery, only maintain a 5-year survival rate of 60 to 70 percent [Citation1,Citation2]. Thus, developing new treatment methods for patients with OS is in an urgent need [Citation3,Citation4].
Recent advances in the identification of tumor-associated signaling pathways and mediators for the pathogenesis and prognosis of OS have promoted the development of new targeted therapeutic methods. The PI3K/Akt signaling pathway is a well-known carcinogenic signaling pathway in human cancer [Citation5], which is closely related to the initiation and progression of OS via affecting cell proliferation, cell invasion, the progression of the cell cycle, apoptosis, neovascularization, metastasis and chemoresistance [Citation6,Citation7]. Attractively, PI3K/Akt signaling cooperates with other signaling pathways to promote these aggressive behaviors. Overexpression of Wnt5a, a member of non-transforming Wnt family, could stimulate MG63 cell to migrate by promoting PI3K and Akt phosphorylation [Citation8]. Another transforming member of Wnt family, Wnt7b, could activate mTORC1 through PI3K-AKT signaling, therefore promoting bone formation [Citation9]. Unlike that the role of Wnt5a in OS has been widely revealed, the specific role of Wnt7b and the underlying mechanism remain unclear. Wnt7b is highly-expressed in many cancers [Citation10,Citation11]; more importantly, Wnt7b expression was also increased within OS tissue samples (Figure S1A) and cells (Figure S1B-C) based on online microarray profiles (GSE12865, GSE11414, GSE49003), especially in metastatic OS cell lines (Figure S1C). The overall survival in OS patients with lower Wnt7b expression showed to be remarkably higher compared to those with higher Wnt7b expression (Figure S1D). These data inspired us to determine how Wnt7b was involved in within the development of OS.
In addition to protein-coding genes, the essential roles of non-protein coding RNAs in cancer initiation and development have been recognized. miRNAs are small non-coding RNAs, and a single miRNA can modulate several gene targets simultaneously. miRNA expression profiling revealed specific miRNA expression patterns in OS; these miRNAs and their targets may serve as potential biomarkers or therapeutic targets for OS [Citation12–Citation17]. Reportedly, several miRNAs, including miR-132, miR-451, miR-133a, miR-218, and miR-223, showed to be reduced in OS and could suppress OS cell growth, invasion, and migration via targeting different downstream targets such as cyclin E1, cyclin D1, Hsp90B1, Bcl-xL, and PGE2 [Citation18–Citation21]. Interestingly, Wnt7b has been regarded as downstream targets of miRNAs in other cancers, including in glioblastoma [Citation22] and oral squamous cell carcinoma [Citation23]; since Wnt7b expression is dramatically upregulated within OS, investigating miRNAs that might target to inhibit Wnt7b in OS can be a new potent strategy for the targeted treatment of OS.
Herein, the expression and effects of Wnt7b showed to be firstly validated within OS tissues and/or cell lines. Next, miRNAs that were negatively correlated with Wnt7b and might target Wnt7b were investigated; among the candidates, miR-342-5p was selected because of its anti-tumor role in other cancers [Citation24,Citation25]. The predicted binding between miR-342-5p and Wnt7b was validated. The impacts of miR-342-5p upon the viability, invasion, migration, sensitivity to Doxorubicin, and apoptosis of OS cells, and Wnt7b expression were examined. Finally, the dynamic effects of miR-342-5p and Wnt7b upon OS cells were evaluated to investigate whether miR-342-5p plays a role through Wnt7b. In summary, we demonstrate a novel miRNA-mRNA axis representing potent targets for the OS targeted therapy.
Results
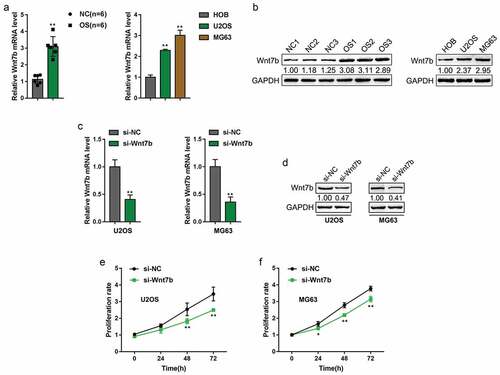
Wnt7b expression is upregulated in osteosarcoma and promotes OS cell proliferation
The PI3K/Akt signaling plays an essential role in OS [Citation26]. Wnt7b, a well-known transforming Wnt gene that is highly expressed in many cancers [Citation10,Citation11], can significantly promote the proliferation and activity of osteoblasts through PI3K/Akt/mTORC1 [Citation9]. More importantly, Wnt7b expression has been reported to be upregulated in OS tissues (Figure S1A) and OS cell lines (Figure S1B-C) based on online microarray profiles (GSE12865, GSE11414, GSE49003), especially in metastatic OS cell lines. The overall survival in OS patients with lower Wnt7b expression was significantly higher than those with higher Wnt7b expression according to the TCGA database (Figure S1D). Based on these previous findings, we speculated that Wnt7b might play a critical role in OS development.
By performing PCR-based analyzes, we found that Wnt7b expression was the most significantly upregulated Wnt gene family member in OS tissues compared to that in normal tissues (, Figure S2A), and was remarkably upregulated in OS U2OS and MG63 cell lines compared to that in normal HOB cell line (and Figure S2B). Moreover, Wnt7b protein levels were also higher in OS tissues and cell lines, compared to those in normal tissues and HOB cell (). To further investigate the function of Wnt7b in OS cells, we knockdown Wnt7b in U2OS and MG63 cells () and measured the cell proliferation. As shown, knockdown of Wnt7b significantly inhibited OS cell proliferation rate. These data suggest that Wnt7b expression is upregulated in OS tissues and cell lines, and promotes OS cell proliferation.
Figure 1. Wnt7b expression is upregulated in osteosarcoma and promotes OS cell proliferation
miR-342-5p suppresses the expression of wnt7b via targeting its 3′-UTR
Based on previous studies [Citation12,Citation27], we speculate miRNAs play a crucial role in OS development and targeted therapy, possibly in a Wnt7b-related way. Online microarray profile (GSE70414 and GSE70367) reported that 30 miRNAs were significantly negatively correlated with Wnt7b in OS cell lines (). According to online tool TargetScan, a total of 3 miRNAs might target Wnt7b (); among these miRNAs, of them, miR-342 has been regarded as an anti-tumor miRNA [Citation24,Citation25]; according to GSE70415, miR-342 was significantly negatively correlated with Wnt7b (). Thus, we hypothesize that miR-342 might target Wnt7b to inhibit Wnt7b expression.
Figure 2. miR-342-5p binds to the 3′-UTR of Wnt7b to inhibit its expression
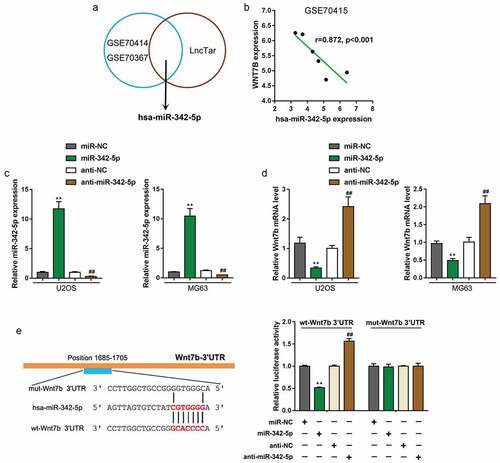
We transfected U2OS and MG63 cells with miR-342-5p or anti-miR-342-5p to conduct miR-342-5p overexpression/inhibition, and performed real-time PCR to verify the transfection efficiency (). Next, Wnt7b expression in miR-342-5p-overexpressed or miR-342-5p-inhibited U2OS and MG63 cells were determined; as shown in , Wnt7b expression showed to be remarkably downregulated via miR-342-5p overexpression while upregulated via miR-342-5p inhibition (). To further validate predicted binding between miR-342-5p and Wnt7b, we performed luciferase reporter assays by constructing Wnt7b 3′-UTR luciferase reporter vectors which contain the wild or mutated miR-342-5p binding site. These luciferase reporter vectors were then co-transfected with miR-342-5p or anti-miR-342-5p in 293T cells; the luciferase activity was then examined. Wild-type Wnt7b 3′-UTR luciferase activity was remarkably decreased upon miR-342-5p overexpression but increased upon miR-342-5p inhibition; the mutation in the predicted miR-342-5p binding site eliminated the changes in the luciferase activity (). In summary, miR-342-5p binds to Wnt7b 3′-UTR to inhibit Wnt7b expression.
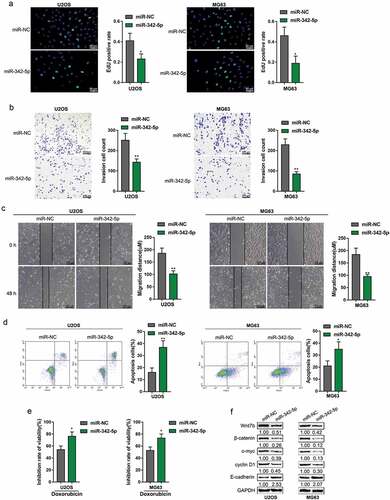
Impacts of miR-342-5p upon OS cell lines and Wnt7b
After confirming miR-342-5p binding to Wnt7b 3′-UTR, we validated the impacts of miR-342-5p upon OS cells. We transfected both OS cells U2OS and MG63 with miR-342-5p and examined DNA synthesis ability, cell invasion, cell migration, cell apoptosis, doxorubicin sensitivity, as well as Wnt7b, β-catenin, c-myc, cyclin D1, and E-cadherin protein levels. As shown in , the overexpression of miR-342-5p dramatically inhibited the DNA synthesis, cell invasion, and migration, while enhanced the apoptosis and the sensitivity to doxorubicin of both OS cell lines. Consistently, miR-342-5p overexpression reduced Wnt7b, β-catenin, c-myc, and cyclin D1 protein levels whereas increased E-cadherin (). In summary, miR-342-5p acts as an anti-tumor miRNA within OS by suppressing the capacity of proliferation, invasion, and migration.
Figure 3. Effects of miR-342-5p on OS cells and Wnt7b
Dynamic effects of miR-342-5p and wnt7b upon OS cell lines
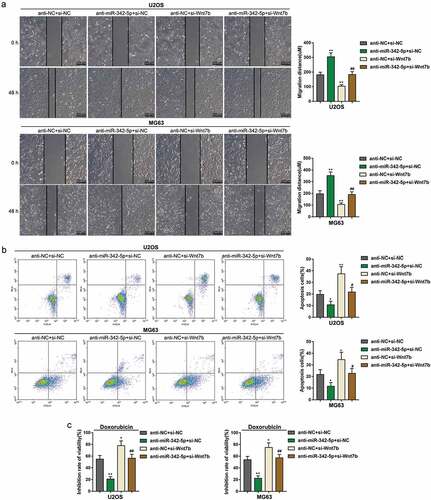
After demonstrating the anti-tumor effects of miR-342-5p, finally, we continued to evaluate the dynamic effects of miR-342-5p and Wnt7b upon OS cell lines. We co-transfected U2OS and MG63 with anti-miR-342-5p and si-Wnt7b. ) showed that Wnt7b expression could be dramatically promoted via miR-342-5p inhibition but suppressed via Wnt7b silence; the effects of miR-342-5p inhibition was partially reversed by Wnt7b silence. Consistently, the protein levels of Wnt7b, β-catenin, c-myc, and cyclin D1 showed to be reduced via Wnt7b silence whereas increased by miR-342-5p inhibition; inversely, E-cadherin protein was increased by Wnt7b silence whereas reduced by miR-342-5p inhibition; the effects of miR-342-5p inhibition were partially attenuated via Wnt7b silence ()). Regarding the cellular effects, miR-342-5p inhibition remarkably enhanced the DNA synthesis ability ()), invasion ()) and migration ()), while suppressed the apoptosis ()) and doxorubicin sensitivity ()) of OS cells; Wnt7b silence exerted opposing effects upon the DNA synthesis, invasion, migration, apoptosis and doxorubicin sensitivity of OS cells. Meanwhile, the effects of miR-342-5p inhibition on OS cells were significantly reversed by Wnt7b silence (–). In summary, miR-342-5p serves as an anti-tumor miRNA within OS by targeting Wnt7b.
Figure 4. Dynamic effects of miR-342-5p and Wnt7b on OS cells
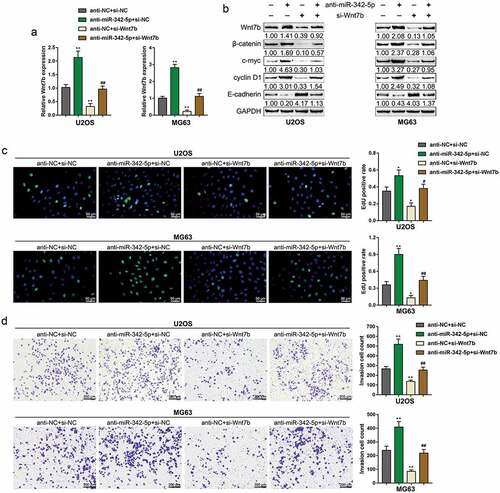
Figure 5. Dynamic effects of miR-342-5p and Wnt7b on OS cell migration, apoptosis, and sensitivity to Doxorubicin
Materials and methods
Clinical tissue sampling
A total of 6 paired normal and OS tissue samples were obtained from patients who underwent the resection of spinal primary OS in the Second Xiangya hospital with the approval of the Research Ethics Committee of the Second Xiangya Hospital. All the patients were informed that the data from the cases would be submitted for publication and their written informed consent was obtained. Subjects receiving chemotherapy or radiotherapy before surgery were excluded. Tissues were immediately stored at −80°C for further studies.
Cell lines and cell transfection
Human osteoblasts (HOB; C-12,720) were obtained from PromoCell (Heidelberg, Germany) and cultured in Osteoblast Growth Medium (C-27,001, PromoCell). U2OS (ATCC® HTB-96™) cell line was obtained from ATCC (Manassas, VA, USA) and cultured in Modified McCoy’s 5a medium (Catalog No. 30–2007, ATCC). MG63 (ATCC® CRL-1427™) cell line was obtained from ATCC and cultured in Eagle’s Minimum Essential Medium (Catalog No. 30–2003). All cells were cultured in a 5% CO2 atmosphere at 37℃.
The miR-342-5p, anti-miR-342-5p, and control (miR-NC and anti-NC, negative control; GenePharma, Shanghai, China), as well as si-Wnt7b and control (si-NC, negative control), were transfected into the target cells using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) following the protocols.
PCR-based analyses
After extracting total RNA using TRIzol reagent (Invitrogen) following the protocols, we examined the miRNA and mRNA expression using PCR-based assays. The relative expression of miRNA and mRNA was calculated normalizing to the internal control (U6 or GAPDH) via equation 2−ΔΔCt.
Immunoblotting analysis
Immunoblotting was conducted for protein expression determination. Proteins were isolated with RIPA lysis buffer containing 1 mg of protease inhibitors after 2 days of transfection. The protein content was determined using a Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were then incubated with following primary antibodies against Wnt7b (ab94915, Abcam, Cambridge, UK), β-catenin (ab32572, Abcam), c-myc (ab32072), cyclin D1 (ab134175, Abcam), E-cadherin (ab1416, Abcam), and the internal control GAPDH (ab181602). Subsequently, secondary antibodies were marked by horseradish peroxidase for 2 h at 37°C. The signal was visualized by the Enhanced Chemiluminescence (ECL) Western Blotting Substrate (Thermo Fisher Scientific). The load protein was normalized to GAPDH.
Cell proliferation
MTT assay was used to evaluate the cell proliferation rate. Cells were seeded in 96-well plates at 5 × 105 cells/mL, 24 h later, cells were transfected with si-Wnt7b or si-NC, 0, 24, 48 and 72 h later, 20 μl MTT (at a concentration of 5 mg/ml; Sigma-Aldrich) was added, and the cells were incubated for an additional 4 h in a humidified incubator. 200 μl DMSO was added after the supernatant discarded to dissolve the formazan. OD490 nm value was measured. The proliferation of the si-NC transfected cells at 0 h (control group) was defined as 1, and the proliferation rate of cells from all other groups was calculated separately from that of the control group.
DNA synthesis determined by EdU assay
The DNA synthesis ability was determined by EdU (5-Ethynyl-2ʹ-deoxyuridine) assays using an EdU assay kit (RiboBio, Guangzhou, China) following a method described previously [Citation28]. DAPI was used for the nucleus staining. The representative images were taken under a fluorescence microscope (Olympus, Tokyo, Japan) and shown.
Cell invasion assay
Cells were suspended in serum-free medium in Transwell chambers with Matrigel pre-coating; medium with 10% FBS was added to the bottom chamber. Forty-eight hours later, noninvasive cells in the upper chambers were discarded; 5% paraformaldehyde was used to fix the invasive cells for 20 min and then 0.1% crystal violet was used to stain these cells for 20 min. Invasive cells were counted at 4 randomly-selected fields in each group.
Wound healing assay
Cells were seeded in 6-well plates at 5 × 105 cells/mL, until the cell monolayer emerged, and then the cell wound healing assay was performed. The scratch area was measured under the microscope (Olympus, Japan) at 0 h and 48h. The relative distance of cell migration to scratch area was also measured under the microscope and analyzed by ImageJ software (NIH, USA).
Cell apoptosis determined by flow cytometer assay
OS cell apoptosis was determined by performing Flow cytometer analysis using an Annexin V-FITC apoptosis detection kit (Keygen, China). Forty-eight hours after transfection and/or treatment, trypsin (0.25%) without EDTA was used to harvest target cells. Cells were then washed with ice-cold PBS, re-suspended, and then incubated with Annexin V-FITC specific antibodies (5 μl) and PI (5 μl) followed by an incubation in the dark for 20 minutes. Excitation wavelength and emission wavelength used here were 488 nm and 530 nm.
Doxorubicin sensitivity measurement
Cells were seeded in 96-well plate at 5 × 105 cells/mL, 24h later, cells were treated with 0.1 μM doxorubicin for 48h. The cell proliferation was determined by MTT assays as mentioned above. The inhibition rate of viability =
Luciferase reporter assay
The wild-type Wnt7b 3′-UTR luciferase reporter vector was generated by cloning Wnt7b 3′-UTR to the downstream of psiCheck2 (Promega) to generate; a mutant type Wnt7b 3′-UTR luciferase reporter vector was generated by mutating the predicted miR-342-5p binding site within the Wnt7b 3′-UTR region. These two types of reporter vectors were co-transfected with miR-342-5p mimics or miR-342-5p inhibitor into 293T cells. 48 h later, the luciferase activity was detected using the Dual-luciferase Reporter Assay System (Promega).
Statistical analysis
All data from at least three independent experiments are processed using GraphPad software (San Diego, CA, USA) and then presented as the mean ± standard deviation (SD). A Student’s t-test was used for the comparison of the differences between groups where applicable. A one-way ANOVA was used for the comparison of the differences among more than two groups. A P value of < 0.05 is considered as statistically significant.
Discussion
Herein, we revealed that Wnt7b expression is dramatically upregulated within OS tissue samples and cells. Knockdown of Wnt7b inhibited OS cell proliferation. miR-342-5p inhibits Wnt7b expression via direct binding to Wnt7b 3′-UTR. miR-342-5p overexpression remarkably suppressed the viability and invasion while enhanced the apoptosis of OS cells; meanwhile, Wnt7b, β-catenin, c-myc, and cyclin D1 proteins were reduced while E-cadherin protein showed to be increased. Consistent with its expression pattern, Wnt7b exerted oncogenic effects on OS cells. Wnt7b could significantly attenuate the impacts of miR-342-5p.
As we have mentioned, Wnt7b could activate mTORC1 through PI3K-AKT signaling [Citation9]. Aberrant Wnt7b gene expression contributes to the pathogenesis of many cancers. Arensman et al. [Citation29] revealed that WNT7B expression was increased within pancreatic adenocarcinoma cells with high activity levels of cell-autonomous Wnt/β-catenin; WNT7B could not only mediate cell-autonomous activation of Wnt/β-catenin signaling but also promote an anchorage-independent growth phenotype. Wnt7b, which is highly expressed in castration-resistant prostate cancer cells, may activate protein kinase C isozymes to promote cancer cell androgen-independent growth [Citation30]. Moreover, we observed a remarkably upregulated Wnt7b expression and protein levels within OS tissue samples and cells. Consistent with its expression pattern, Wnt7b silence within OS cells remarkably inhibited the viability and invasion, whereas enhanced the apoptosis of OS cells, indicating that knocking down Wnt7b could inhibit the OS cell growth.
During the past decades, miRNAs have received more and more attention due to their extensive biological performance through binding to multiple downstream target genes. In carcinogenesis, miRNA plays a critical role serving as either oncogene or tumor suppressor under different conditions. Reportedly, miR-342 might be a pro-apoptotic tumor suppressor miRNA; reconstituting miR-342 within the colorectal cancer cell line HT-29 could induce the apoptosis [Citation24]. By targeting NAA10, miR-342-5p inhibits the risk of colon cancer [Citation31]. Besides, miR-342-5p has been regarded as not only an upstream regulator of the HER2 signaling pathway but also an inhibitor of the growth of HER2-positive breast carcinoma cell lines [Citation32]. In the present study, both silica and experimental analyzes revealed that miR-342-5p suppresses the expression of Wnt7b via direct binding to its 3′-UTR, indicating that miR-342-5p could serve as a tumor suppressor within OS cells.
Regarding the cellular functions of miR-342-5p within the development of OS, miR-342-5p overexpression remarkably suppressed the viability, migration, and invasion, whereas enhanced the sensitivity to Doxorubicin and apoptosis of OS cells. In the meantime, Wnt7b, β-catenin, c-myc, and cyclin D1 protein levels were reduced while E-cadherin protein showed to be increased. As we have mentioned, Wnt7b-mediated cell-autonomous Wnt/β-catenin activation could control key developmental gene expressions and thus has emerged as an underlying cancer control strategy; it has been found that Wnt antagonists could inhibit the potential for tumorigenesis and metastasis within OS [Citation33]. β-catenin protein expression level was related to OS cell invasion, and the chemical inhibition of Wnt/β-catenin promoted OS cell apoptosis mediated by MTX [Citation34]. Herein, miR-342-5p overexpression significantly reduced Wnt7b and β-catenin proteins, suggesting that miR-342-5p might play a tumor-suppressive role through inhibiting the capacity of OS cells to proliferate and to invade via targeting Wnt7b. As a further confirmation, Wnt7b silence exerted similar effects on OS cells as those of miR-342-5p overexpression; more importantly, the effects of miR-342-5p inhibition on OS cell viability, migration, invasion, sensitivity to Doxorubicin, and apoptosis were significantly reversed by Wnt7b silence, suggesting that miR-342-5p acts as a tumor suppressor within OS through targeting Wnt7b.
Regarding the limitation of the present study, the specific effects of miR-342-5p/Wnt7b axis on cancer stem cells remain unclear. Cancer stem cells are a small fraction of cancer cells that constitute a reservoir of self-sustaining cells with the exclusive ability to self-renew and initiate/maintain the tumor [Citation35]. They are tumor-initiating cells that proliferate through their unique self-renewal ability. Besides, OS cancer stem cells are proposed to be responsible for the chemoresistance [Citation36–Citation38]. miRNAs have a significant role to play in the regulation of the signaling pathways involved in oncogenesis. They are implicated in the maintenance of cancer stem cells via their ability to affect multiple pathways including cell proliferation, cell death [Citation39,Citation40], cell-cell communication and cell adhesion [Citation41]. Thus, the effects of miR-342-5p/Wnt7b axis on OS cancer stem cells should be investigated in our future study.
In summary, we demonstrate a miR-342-5p/Wnt7b axis that regulates the capacity of OS cells to proliferate and to invade by Wnt/β-catenin signaling. The miR-342-5p/Wnt7b axis might be novel targets for OS targeted therapy, which needs further in vivo and clinical investigations.
Supplemental Material
Download Zip (615.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13.
- Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790.
- Broadhead ML, Clark JC, Myers DE, et al. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011;2011:959248.
- He JP, Hao Y, Wang XL, et al. Review of the molecular pathogenesis of osteosarcoma. Asian Pac J Cancer Prev. 2014;15:5967–5976.
- Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64.
- Zhang J, Yu XH, Yan YG, et al. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. 2015;444:182–192.
- Hu K, Dai HB, Qiu ZL. mTOR signaling in osteosarcoma: oncogenesis and therapeutic aspects (Review). Oncol Rep. 2016;36:1219–1225.
- Zhang A, He S, Sun X, et al. Wnt5a promotes migration of human osteosarcoma cells by triggering a phosphatidylinositol-3 kinase/Akt signals. Cancer Cell Int. 2014;14:15.
- Chen J, Tu X, Esen E, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10:e1004145.
- Bui TD, O’Brien T, Crew J, et al. High expression of Wnt7b in human superficial bladder cancer vs invasive bladder cancer. Br J Cancer. 1998;77:319–324.
- Kirikoshi H, Sekihara H, Katoh M. Molecular cloning and characterization of human WNT7B. Int J Oncol. 2001;19:779–783.
- Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013;25:398–406.
- He H, Ni J, Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett. 2014;7:1352–1362.
- Zheng Z, Ding M, Ni J, et al. MiR-142 acts as a tumor suppressor in osteosarcoma cell lines by targeting Rac1. Oncol Rep. 2015;33:1291–1299.
- Li H, Zhang K, Liu LH, et al. MicroRNA screening identifies circulating microRNAs as potential biomarkers for osteosarcoma. Oncol Lett. 2015;10:1662–1668.
- Diao CY, Guo HB, Ouyang YR, et al. Screening for metastatic osteosarcoma biomarkers with a DNA microarray. Asian Pac J Cancer Prev. 2014;15:1817–1822.
- Wang Z, He R, Xia H, et al. MicroRNA-101 has a suppressive role in osteosarcoma cells through the targeting of c-FOS. Exp Ther Med. 2016;11:1293–1299.
- Ji F, Zhang H, Wang Y, et al. MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone. 2013;56:220–226.
- Jin J, Cai L, Liu ZM, et al. miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev. 2013;14:3681–3684.
- Wang J, Xu G, Shen F, et al. miR-132 targeting cyclin E1 suppresses cell proliferation in osteosarcoma cells. Tumour Biol. 2014;35:4859–4865.
- Xu H, Mei Q, Shi L, et al. Tumor-suppressing effects of miR451 in human osteosarcoma. Cell Biochem Biophys. 2014;69:163–168.
- Zhang C, Yang X, Fu C, et al. Combination with TMZ and miR-505 inhibits the development of glioblastoma by regulating the WNT7B/Wnt/beta-catenin signaling pathway. Gene. 2018;672:172–179.
- Shiah SG, Hsiao JR, Chang WM, et al. Downregulated miR329 and miR410 promote the proliferation and invasion of oral squamous cell carcinoma by targeting Wnt-7b. Cancer Res. 2014;74:7560–7572.
- Grady WM, Parkin RK, Mitchell PS, et al. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene. 2008;27:3880–3888.
- Cittelly DM, Das PM, Spoelstra NS, et al. Downregulation of miR-342 is associated with tamoxifen resistant breast tumors. Mol Cancer. 2010;9:317.
- Perry JA, Kiezun A, Tonzi P, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci U S A. 2014;111:E5564–73.
- Zhou W, Hao M, Du X, et al. Advances in targeted therapy for osteosarcoma. Discov Med. 2014;17:301–307.
- Liu ZB, Wang JA, Lv RQ. Downregulation of long non-coding RNA DBH-AS1 inhibits osteosarcoma progression by PI3K-AKT signaling pathways and indicates good prognosis. Eur Rev Med Pharmacol Sci. 2019;23:1418–1427.
- Arensman MD, Kovochich AN, Kulikauskas RM, et al. WNT7B mediates autocrine Wnt/beta-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma. Oncogene. 2014;33:899–908.
- Zheng D, Decker KF, Zhou T, et al. Role of WNT7B-induced noncanonical pathway in advanced prostate cancer. Mol Cancer Res. 2013;11:482–493.
- Yang H, Li Q, Niu J, et al. microRNA-342-5p and miR-608 inhibit colon cancer tumorigenesis by targeting NAA10. Oncotarget. 2016;7:2709–2720.
- Lindholm EM, Leivonen SK, Undlien E, et al. miR-342-5p as a potential regulator of HER2 breast cancer cell growth. Microrna. 2019;8:155–165.
- Lin CH, Ji T, Chen CF, et al. Wnt signaling in osteosarcoma. Adv Exp Med Biol. 2014;804:33–45.
- Ma Y, Ren Y, Han EQ, et al. Inhibition of the Wnt-beta-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun. 2013;431:274–279.
- Papagiannakopoulos T, Kosik KS. MicroRNAs: regulators of oncogenesis and stemness. BMC Med. 2008;6:15.
- Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–436.
- Fujii H, Honoki K, Tsujiuchi T, et al. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. 2009;34:1381–1386.
- Gillette J, Nielsen-Preiss S. Cancer stem cells: seeds of growth in osteosarcoma. Cancer Biol Ther. 2009;8:553–554.
- Yu F, Deng H, Yao H, et al. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29:4194–4204.
- Radisky DC. miR-200c at the nexus of epithelial-mesenchymal transition, resistance to apoptosis, and the breast cancer stem cell phenotype. Breast Cancer Res. 2011;13:110.
- Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215.
