ABSTRACT
Background
Circular RNAs (circRNAs) play a pivotal regulatory role in a variety of tumors.Nevertheless, the detailed function of circ_0000003 in non-small cell lung cancer (NSCLC) and its regulatory mechanism remain elusive.
Methods
RT-PCR was carried out to detect the expressions of circ_0000003, miR-338-3p and insulin receptor substrate 2 (IRS2)in NSCLC tissues. Besides, western blot was done to monitor IRS2 expression in NSCLC cells. The correlation between circ_0000003 and clinicopathologic characteristics of NSCLC patients was analyzed as well.CCK8 and BrdUassays were used to monitor cell proliferation; flow cytometry was used to detect apoptosis; and transwell assay was conducted to detect its migration and invasion.Moreover, dual luciferase reporter gene assay was done to verify the targeting relationship between circ_0000003 and miR-338-3p.Additionally, the effect of circ_0000003 on the growth of NSCLC cells in vivo was evaluated by tumorigenesis assay in nude mice.
Results
The expression of circ_0000003 was significantly high in NSCLC tissues and cell lines, and its high expression level was notably correlated with lymph node metastasis andTNM staging.In vitro experiments showed that overexpression of circ_0000003 facilitated the proliferation, migration, invasion and inhibited the apoptosis of NSCLC cells, while the knockdown of circ_0000003 had the opposite effect.In vivo experiments revealed that knockdown of circ_0000003 impeded tumor growth and metastasis. Further, the underlying mechanism showed that circ_0000003 functioned as endogenous competitive RNA and directly targeted miR-338-3p to positively regulated IRS2 expression.
Conclusion
Circ_0000003 promotes the proliferation and metastasis of NSCLC cells via modulating miR-338-3p/IRS2 axis.
KEYWORDS:
1. Introduction
Lung cancer is the leading cause of cancer-related mortality both in male and female worldwide. Non-small cell lung cancer (NSCLC) is the main histologic type of lung cancer, accounting for nearly 80% of the total cases of lung cancer [Citation1,Citation2]. Although great progress has been made in diagnosis and treatment, the long-term survival rate is only 15 to 30%, and the local control rate is only 40 to 50% [Citation3,Citation4]. Unfortunately, a majority of patients are diagnosed at an advanced stage, resulting in a high mortality rate [Citation5]. Hence, an in-depth investigation into the underlying mechanism of NSCLC cells and effective targets that can facilitate disease detection, staging and prediction of therapeutic outcome are highly desirable to improve survival rate and help to determine optimized treatment for NSCLC.
Circular RNAs (circRNAs) is a novel class of circularendogenous non-coding RNA, produced by linear premessenger RNAs through a process called “back splicing”, in which the 3 ‘end and the 5’ end are connected together to form a circular structure [Citation6,Citation7]. CircRNA, without 5-3’polarity or polyadenine tail, can exist stably in cells by resisting the activity of exonuclease [Citation8]. Interestingly,circRNA are more widely expressed than linear RNAs [Citation9]. In recent years, it has been gradually recognized that circRNAs is a new group of clinical biomarkers and potential targets for tumor therapy [Citation10]. The significance of circRNA in the onset and progression of multiple cancers, including NSCLC, has been validated by the experimental demonstrations of their oncogenic and tumor-suppressive properties [Citation11,Citation12]. For instance, circ_0043278 accelerates the proliferation of NSCLC cells, and its high expression is regarded as an indicator of poor prognosis [Citation13]. Circ_0067934 facilitates the metastasis of NSCLC cells, and its expression level was significantly correlated with the higher TNM stage andearlier metastasis of NSCLC patients [Citation14]. As a tumor suppressor, circ_FOXO3 can restrain the proliferation and metastasis of NSCLC cells [Citation15]. Unfortunately, the defined role and regulatory mechanism of circ_0000003, a novel circ-RNA, in NSCLC remain poorly understood.
MicroRNA (miRNA) is a class of small non-coding RNA, containing 18 to 25 nucleotides that specifically binds to the target gene 3-untranslated region (3’-UTR) to cause translation inhibition or mRNA degradation, thus regulating in gene expression [Citation16,Citation17]. For example, miR-621 can inhibit the malignant progress of NSCLC cells by directly targeting SIX4, while miR-522-3p plays a role in promoting cancer in NSCLC [Citation18,Citation19]. MiR-338-3p has been shown to be lowly expressed in NSCLC tissues and plays an anti-tumor role in NSCLC metastasis [Citation20]. However, the mechanism of the low expression of miR-338-3p remains unclear.It is worth noting that bioinformatics (https://circinteractome.nia.nih.gov/) indicates that there is a potential binding site between circ_0000003 and miR-338-3p, suggesting that circ_0000003 may be a sponge molecule of miR-338-3p. This regulatory relationship during the progression of NSCLCremains to be further verified.
Insulin receptor substrate 2 (IRS2) is a cellular signaling molecule that acts as a molecular adaptor mediatinginsulin, insulin-like growth factor-1 and other cytokines in cells [Citation21]. Several studies indicate that the expression of IRS2 is significantly elevated inmalignant tumors such as thyroid cancer and liver cancer, which is closely related to poor prognosis and early relapsing [Citation22,Citation23].On the basis of bioinformatics analysis, we revealed for the first time that the expression of circ_0000003 was significantly increasedin NSCLC tissues and cell lines, and its high expression level was significantly correlated with several unfavorable pathological parameters. Ultimately, we found that circ_0000003 facilitated the progress of NSCLC by sponging miR-338-3p and up-regulating IRS2 expression.
2. Materials and methods
2.1. Tissue collection
NSCLC tissues and adjacent tissues were collected under the approval of Ethics Review Committee of ZhuJiang Hospital of Southern Medical University. No patients received neoadjuvant therapy (chemotherapy or radiotherapy) before the surgery. All patients involved gave informed consent to the study and signed a written consent form. The resected tissues were stored in liquid nitrogen for further analysis.
2.2. Cell culture
Human NSCLC cell lines (A549, H1650, H1299, H358 and H226) and normal bronchial epithelial cells (BEAS-2B cells) were purchased from the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in Dulbecco’s Modified Eagle Medium(DMEM; Hyclone, Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Grand Island, NY, USA), 100U/ml penicillin and 100 μg/ml streptomycin(Invitrogen, Grand Island, NY, USA)in 5% CO2 at 37°C. 0.25% trypsin (Americesco,Frramingham,MA,USA) was used for the subculture.The culture medium was replaced at an interval of 3 to 4 days.
2.3. Cell transfection
Overexpressed circ_0000003 plasmid, empty plasmid, siRNA negative control (si-NC), siRNA-circ_0000003, miRNA negative control (miR-NC), miR-338-3p mimics and miR-338-3p inhibitors were purchased from RiboBio (Guangzhou, China).A549 and H226 cells were transfected uisng Lipofectamine 3000(Invitrogen; Thermo Fisher Scientific, Inc.). The sequences of siRNA-circ_0000003:siRNA, 5’-AAGTATCCCAGGTTGAAGTCT-3’. The transfection efficiency was validated by RT-PCR.
2.4. Real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells or tissues with TRIzolRegent (Invitrogen, Shanghai, China). 1 μg total RNA was reversely transcribed to cDNA with First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Then real-time PCR was performed using LightCycler FastStart DNA MasterPlus SYBR Green I kit (Roche Diagnostics, Burgess Hill, UK). The relative expressions of circ_0000003, miR-338-3p and IRS2 were calculated using 2−ΔΔCT method. The primers sequences were shown in .
Table 1. Sequences used for qRT-PCR.
2.5. CCK8 assay
A549 and H226 cells were harvested in logarithmic phase and trypsinized. After that, cells with the adjusted density of 1 × 104/mL were seeded in a 96-well plate, 100 μl cell suspension per well. Following that, the 96-well plate was cultured in the incubator. 10 μlCCK8 solution (Dojindo, Japan) was added to the well following the incubation for 12, 24, 48 and 72 hours, respectively. The culture was continued for another 1 hour. As the culture was terminated, the plate was placed in the microplate reader, and the absorbance (OD value) of each well was determinedat 450 nm wavelength.
2.6. BrdU assay
NSCLC cells were inoculated in 24-well plate. When the cells grew to 80% confluence, they were incubated with 10 μmol/L BrdU at 37 °C for 4 h.The culture medium was discarded and the plate was washed with PBS for 3 times.The cells were fixed at 4 °C for 10 min with 70% anhydrous ethanol. 70% anhydrous ethanol was discarded and the cells were rinsed with PBS for 3 times.The DNA of the cells was denatured by adding 2 mol/L HCl at 37 °C for 40 min.HCI was abandoned and cells were then washed with PBS3 times. Cells were then cultured in 1%BSAat room temperature for 1 hour. PBS was used to wash cells for 3 times, 5 min each time. The PBS on the cell surface was sucked and cells were incubated with anti-BrdU monoclonal antibody (Abcam, ab8152, 1: 300) (100μl per well) overnight at 4 °C. On the next day, the secondary fluorescent antibody (sheep anti-mouse antibody) labeled with FITC was added to continue the incubation at room temperature for 2 h.After stained with DAPI, the nucleus was observed under a fluorescence microscope.Ten non-overlapping visual fields were randomly selected and the number of BrdU positive cells was counted. The experiments were repeated three times.
For living mice, BrdU (10 μmol/kg body)was injected intravenously one day before the tumor-bearing mice were sacrificed.The tumor tissues were removed from mice, fixed with 4% paraformaldehyde and sliced using a freezing microtome. The obtained slices were immersed in xylene and different concentrations of ethanol (100%, 95%, 90%, 85% and 75%). Afterward, slices were washed with tap water and then PBS. The slices were incubated with 1% methanol hydrogen peroxide at room temperature for 10min. After washing with distilled water and PBS, antigen repair solution was added to the slices to continue the incubation at room temperature for 10min. After being washed with PBS, the slices were incubated with goat serum at room temperature for 20 min.Then the anti-BrdU monoclonal antibody was added and the following procedures were similar with the assay for NSCLC cells.
2.7. Apoptosis assay
The cells in each group were trypsinized and washed twice with pre-cooled PBS after the centrifugation. Afterward, the cells were resuspended in binding buffer and the concentration was adjusted to 1 × 104/ml. The cells were stained according to the proportion of 10 μl Annexin V and 5 μl PI per 1ml cell suspension and incubated in dark at room temperaturefor 15min. Then 400 μl binding buffer was added. Ultimately, The apoptosis rate of cells was detected by flow cytometry.
2.8. Western blot
The cells were lysed in RIPAbuffer (Beyotime Biotechnology, Shanghai, China) with protease inhibitor PMSF. After the high-speed centrifugation, the supernatant was collected and heated in a water bath at 100°C for 10 minutes to denaturate the protein.The content of the protein was then determined by bicinchoninic acid (BCA) analysis (Beyotime, China). Followingthat, SDS-PAGE gel electrophoresis and transmembrane were performed.After PVDFbeing blockedwith 5% slim milk, the primary antibodieswere added to shake the bed at 4°Covernight. Subsequently, the secondary antibody goat anti-rabbit IgG H&L(Abcam, ab150077, 1:1000) was added to incubate at room temperature for 1 hour. After two times of incubation, TBST was used to wash the membrane three times. Ultimately, color rendering was performed using enhanced chemiluminescence kit (Pierce, Waltham, MA,USA). The primary antibodies included: IRS2 antibody (Abcam, ab134101,1:1000), Bax (Abcam, ab32503,1:1000), Bcl-2 (Abcam, ab185002,1:1000) and internal reference β-actin antibody (Abcam,ab20272,1:100).
2.9. Transwell assay
In the migration assay, A549 and H226 cells in logphase were collected and trypsined. 200μl single cell suspension resuspended in the serum-free medium was inoculated in a 24-well plate withtranswell chamber (8 μ m pore diameter, BD Biosciences,CA,USA). 1ml complete medium was then added to each wellof the plate. Following that, the plate was cultured for 24 hours. Afterward, the cells on the upper surface of the transwell chamber were gently wiped off with a cotton swab. The cells invading the lower chamber were fixed with 95% ethanol for 20 minutes, and then stained with 0.1% crystal purple for 10 minutes. The cells were counted under a microscope. In the invasion experiment, except that a layer of matrix glue (BD Biosciences,CA,USA) was coated on the bottom of the transwell chamber, the other steps were the same as the migration assay.
2.10. Dual luciferase report assay
The sequences of circ_0000003 were subcloned into the luciferase reporter psiCHECK2 (Promega, Madison, WI) to construct psiCHECK2-circ_0000003-WT and psiCHECK2-circ_0000003-MUT.The cells were then inoculated on a 24-well plate with 5000 cells per well. Afterward, cells were co-transfection with plasmid and miR-338-3p mimics or negative controls.Luciferase activity was determined by dual luciferase system (Promega,Madison,WI,USA). The experimentwas repeated three times.
2.11. In vivo experiment
In vivo experiment was approved by the Animal Model Research Center of ZhuJiang Hospital of Southern Medical University.4-week-old male BALB/c athymic nude mice were used in this experiment.H226 cells (2 × 107/ml) transfected with si-NC or si-circ_0000003 werewashed with PBS for three times and re-suspended in PBS.100 μl cell suspension was inoculated to the right and left sides of each mouse.The longest and shortest diameters of the tumorwere measured every 3 days with a caliper until the tumor was removed 13 days later.The tumor volume was calculated by using the formula: volume = (length × width2 × 0.5). On the 14th day after injection, the growth of subcutaneous tumor lesion was observed.
2.12. Statistic analysis
The results were presented as mean ±standard deviation (SD). Student’s t test and one-way ANOVA were carried out to analyze the difference of measurement data. Chi-square test was performed to the correlation between the expressionof circ_0000003 and clinicopathological indexes. GraphPad Prism 7 was adopted for statistical analysis. P < 0.05 indicated statistical significance.
3. Results
3.1. The expressions of circ_0000003, miR-338-3p and IRS2 in NSCLC cells
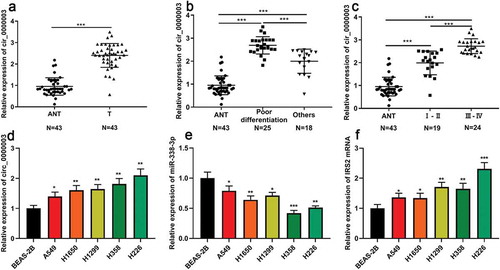
RT-PCR showed that the expressions of circ_0000003 in NSCLC tissues was significantly higher than that in adjacent tissues ()). Further analysis revealed that the expressionof circ_0000003 was significantly increased in poorly differentiated cases compared to other cases ()). In addition, it was found that the high expressionof circ_0000003 was also significantly correlated with higher TNM staging ()). Subsequently, we examined the expressions of circ_0000003, miR-338-3p and IRS2 mRNA in five human NSCLC cells (A549 cells, H1650 cells, H1299 cells, H358 cells and H226 cells). The results suggested that compared with normal bronchial epithelial cells (BEAS-2B cells), the expressions of circ_0000003 and IRS2 mRNA were significantly increased in the above five NSCLC cells, while the expressionof miR-338-3p wasnotably decreased ()).
3.2. The expression of circ_0000003 was associated with multiple pathological indicators in NSCLC patients
Then, we further analyzed the relationship between the expressionof circ_0000003 and the clinicopathological indexes of NSCLC. It was found that the high expression of circ_0000003 in tumor tissues was significantly correlated withlocal lymph node metastasis and higher T stage in NSCLC patients, but had no link to gender, age, smoking, histology and tumor size ().This suggested that high expression of circ_0000003 may be involved in the progression of NSCLC .
Table 2. Correlations between circ_0000003 expression and clinical characteristics in NSCLC patients.
3.3. Circ_0000003 regulated the proliferation, apoptosis, migration and invasion of NSCLC cells
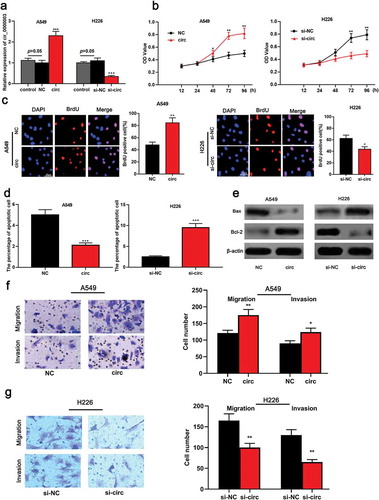
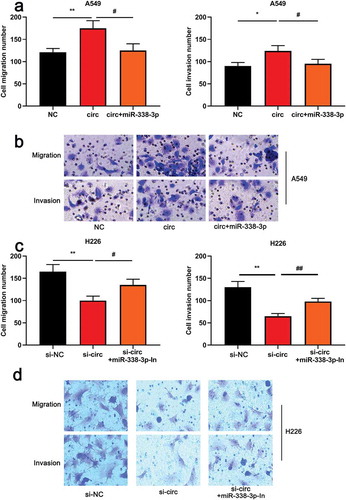
To further investigate the roles of circ_0000003 and miR-338-3p in NSCLC cells, cell modelswith over-expressed and low-expressed circ_0000003 were successfully established ()). On this basis, CCK8, BrdUand flow cytometry were carried out. The results showed that overexpressed circ_0000003 significantly promoted the proliferation of NSCLC cells compared to the control group ()) and inhibited cell apoptosis ()). On the other hand, the knockdown of circ_0000003 significantly arrested the proliferation of H226 cells and increased the proportion of apoptosis()). Subsequently, the expressions of the apoptotic marker molecule were detected by western blot. It was found that up-regulated circ_0000003 markedly increased Bcl-2 expression and decreased Bax expression ()). Compared with the control group, knock-down circ_0000003 inhibited the expression of Bcl-2 but caused an increase in Bax expression. The above data indicated that circ_0000003 promotedthe proliferation of NSCLC cells and repressed apoptosis. The malignancy of the tumor is not only related to the proliferative capacity of the cell, but also closely related to the metastatic potential. Subsequently, the metastatic ability of NSCLC cells was explored by transwell migration and invasion assays. The results informed us that the number of A549 cells with overexpressed circ_0000003 to migrate and invade was significantly higher than that of the control group ()). On the other hand, knock-down circ_0000003 in H266 cells blocked the migration and invasion compared to the control group()). Our data suggested that circ_0000003 regulatedthe malignant phenotypes of NSCLC cells.
3.4. Circ_0000003 sponged miR-338-3p and negatively regulated its expression
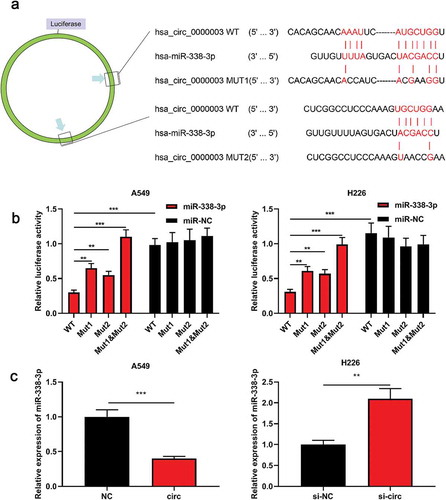
Bioinformatics databasepredicted that miR-338-3p was one of the targetmiRNAs of circ_0000003 ()). To further verify whether miR-338-3p can bindto circ_0000003, a luciferase reporter vector containing wild-type or mutant circ_0000003 was constructed. The results showed that miR-338-3p significantly reduced the luciferase activity of psi-circ_0000003-WT, but had no significant effect on psi-circ_0000003-MUT1&2 ()). We also demonstrated that upregulation of circ_0000003 significantly reduced miR-338-3p expression in A549 cells, while knocking down circ_0000003 exerted the opposite effect in H226 cells ()). The above data indicated that circ_0000003 regulated the expression of miR-338-3p.
3.5. Circ_0000003 was involved in the regulation of proliferation and apoptosis of NSCLC cells via miR-338-3p
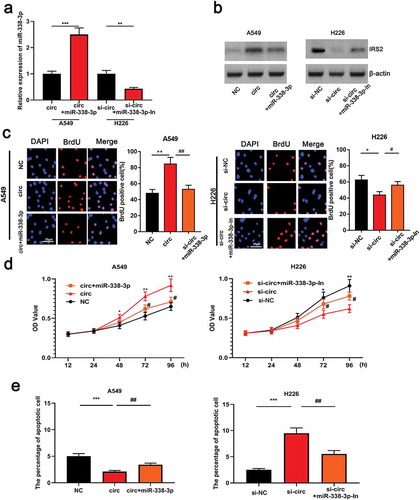
In order to further explore the potential mechanism ofcirc_0000003 in NSCLC, miR-338-3p mimics were transfected into A549 cells, and miR-338-3p inhibitor was transfected into H226 cells, and transfection efficiencywas confirmed by qRT-PCR ()). Previous studies have demonstrated that miR-338-3p can target the regulation of IRS2 expression [Citation20]. This study revealed that the upregulation of circ_0000003 can induce an increase in the expression of IRS2 in NSCLC cells, and this promotion can be partially reversed by miR-338-3p mimics()). On the other hand, knocking down the expression of circ_0000003 resulted in a decrease in the expression of IRS2 in H226 cells. Subsequent transfection ofmiR-338-3p inhibitor into H226 cells resulted in a significant increase in the expressionof IRS2 ()). Furthermore, the proliferation of A549 cellstransfected with miR-338-3p mimic was obviously repressed compared tooverexpressed circ_0000003 group ()). Flow cytometry results suggested thatmiR-338-3p mimics significantly facilitated the apoptosis of NSCLC compared tooverexpressed circ_0000003 group, whereas miR-338-3p inhibitor significantly blockedthe apoptosis ()). The above data indicated thatcirc_0000003/miR-338-3p axis regulated the proliferation and apoptosis of NSCLC cells.
3.6. Circ_0000003 regulates the migration of NSCLC cells via miR-338-3p
Based on the data above, it was clear that circ_0000003 was involved in the regulation of proliferation and apoptosis of NSCLC via miR-338-3p. Here, we are curious whether circ_0000003/miR-338-3p axis can facilitate the migration of NSCLC. Consistent with expectations, transwell assay revealed that the transfection ofmiR-338-3p mimics notably impeded the migration and invasionof A549 cells compared to the overexpressed circ_0000003 group ()). On the other hand, the transfection of miR-338-3p inhibitor into H226 cells enhanced the migration and invasion ()). Our data suggested that circ_0000003 promoted the migration and invasion of NSCLC cells via miR-338-3p .
3.7. The knockdown of circ_0000003 restrained the tumor growth in vivo
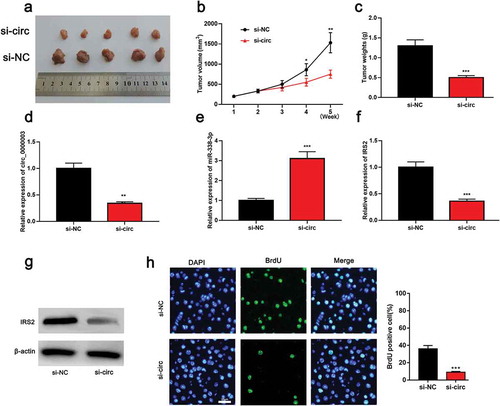
After the determination of the biological effects of circ_0000003 in vitro, we embarked on exploring the role of circ_0000003 in vivo. Tumor size and body weight were observed after the inoculation of H226 cells transfected with si-NC or si-circ_0000003 into the back of each mouse ()). After knocking down circ-0000003, the tumor was remarkably smaller than that in the control group ()). Besides, the mean weight of tumors in the si-circ_0000003 group was significantly lower than in the si-NC group ()). qRT-PCR results revealed that the expressions of circ_0000003 and IRS2 were significantly down-regulated in tumor tissues, while the expression of miR-383-3p was notably increased ()). Western blot suggested that IRS2 expression was decreased at the protein level after circ_0000003knockdown ()). Following that, BrdU assay was used to detect the proliferation of tumor cells in the back of mice. It was turned out that the proliferationof NSCLC cells with knock-down circ_0000003 was significantly weakenedthan that of the control group ()). These results validated the carcinogenic effects of circ_0000003 in NSCLC.
4. Discussion
CircRNAs are widely expressed in multiple tumor cells, including NSCLC cells [Citation24]. Their abnormal expressioncan be indicators for disease prognosis [Citation25]. Further discussion of the relationship between circRNA and NSCLC helps to elucidate the mechanisms involved in the progression of NSCLC [Citation26]. In this study, we first found that circ_0000003was up-regulated in NSCLC tissues. In addition, it was first discovered by gain- and loss-experiment that circ_0000003 facilitated the proliferation and metastasis of NSCLC cells. Thus, circ_0000003 plays a cancer-promoting effect in the progression of NSCLC.
CircRNA has some notable features. First and foremost, circRNA has a closed-loop structure that exhibits resistance to exonuclease-mediated degradation to render highly conserved andstablecircRNA [Citation27,Citation28]. Secondly, circRNA is mainly composed of exons and can act as a sponge of endogenous competitive RNA to target the regulation of miRNA expression [Citation29–Citation31]. In recent years, circRNA is a key regulator for tumors, includingNSCLC [Citation32]. Circ_PTK2 represses the epithelial-mesenchymal transition and the metastasis of NSCLC cells, while circPIP5K1A serves as a tumor promoter [Citation7,Citation9,Citation33]. In this study, we found that the expressionof circ_0000003 in tumor tissues was significantly increased, and its high expression level was correlated with the local lymph node metastasis and TNM stage in NSCLCpatients. These results indicated that circ_0000003 was expected to be an indicator of poor prognosis of NSCLC. In addition, we successfully constructed a NSCLC cell model with over-expressed circ_0000003. By cell experiment, it was found that the proliferation, migration and invasion ability of NSCLC cells were significantly enhanced, while the proportion of apoptotic cells werenotably reduced. On the other hand, knock-down circ_0000003 impeded the proliferationof NSCLC cells and induced apoptosis. Further, we confirmed by in vivo experiments that knock-down circ_0000003 restrained tumor growth. The above data suggested that circ_0000003 enhanced the proliferation and metastasis of NSCLC and suppressed the apoptosis.
Extensivestudies verify miRNAs are implicated in the tumorigenesis and progression of diverse tumors [Citation34]. To explore the link between miRNAs and the biological behavior of malignant tumor cells can provide a novel indicators for early diagnosis, monitoring of progression, and prognosis [Citation35]. Previous studies have indicated that multiple members from miR-338 family can block the progression of malignancies, such as ovarian cancer and colorectal cancer [Citation36,Citation37]. From a mechanistic point of view, miR-338-3p can directly bind to the 3’UTR of MACC 1 mRNA, thusimpeding the proliferation and migration of ovarian cancer cells [Citation36]. MiR-338-3p arrests the progression of NSCLC via the targeting inhibition on the expressionsof neuropilin 1 and sphingosine kinase 2 [Citation38,Citation39]. In the present study, the expression of miR-338-3p was decreased in NSCLC tissues and cell lines.Previous studies also indicated that the decreased expression of miR-338-3p was significantly associated with poor prognosis of NSCLC patients [Citation20]. These studies have fully confirmed that miR-338-3p can be used as an indicator of poor prognosis in NSCLC.
In recent years, it has been observed that circRNA can sponge miRNAs, thereby regulating gene expression [Citation7,Citation8]. For example, circ_0016760 acts as a molecular sponge to promote the proliferation and invasion of NSCLC cells via miR-1287 [Citation11]. Therefore, we are curious about the mechanism that causes the low expression of miR-338-3p. Through bioinformatics analysis,a binding site was found between circ_0000003 and miR-338-3p. Moreover, dual luciferase reporter gene assay confirmed that circ_0000003 can modulate miR-338-3p. In addition, the knock-down of circ_0000003 triggered an increase in miR-338-3p expression. Subsequently, following the transfection of miR-338-3p inhibitors into NSCLC cells with lowly-expressed circ_0000003, it was found that the inhibitory effect induced by the knockdown of circ_0000003 on cell proliferation was partially reversed by miR-338-3p inhibitors. Consistent with expectations, miR-338-3p inhibitors attenuated the increase in the proportion of apoptotic cells induced by knockdown circ_0000003. On the other hand, the upregulation of circ_0000003 facilitated the proliferation and metastasis of NSCLC cells, and restrained the apoptosis. Further, this effect can be partially offset by miR-338-3p. Therefore, we concluded that circ-0000003 was involved in the regulation of proliferation, apoptosis, migration and invasion of NSCLC cells viasponging miR-338-3p.
IRS is known as cytoplasmic protein that integrates and coordinates signals from the extracellular to the intracellular environment through transmembrane receptors, which plays a crucial role in regulating cell growth, metabolism, survival and proliferation. The PI3K/AKT/MTOR and MAPK signaling pathways are the most typical downstream signaling pathways for IRS signal activation [Citation40]. As an important member of IRS family, IRS2 is involved in the promotion of malignant progression in multiple tumors, such and NSCLC [Citation20,Citation22,Citation41].In addition, IRS2 is validated to be regulated by miRNAs. For example, miR-766 restrains the progression of papillary thyroid carcinoma by directly targeting IRS2 and modulating the PI3K/Akt pathway [Citation22]. Up-regulated miR-146a inhibits the malignant biological behavior of esophageal squamous cell carcinoma via IRS2 [Citation42]. Moreover, IRS2 is directly negatively regulated by miR-338-3p, miR-448, and miR-146a in NSCLC [Citation20,Citation43,Citation44]. Our study confirmed that miR-338-3p can be sponged by circ_0000003. The expression of IRS2 was down-regulated as well after knocking down circ_0000003 in NSCLC cells. In contrast, up-regulated circ_0000003 induced an increase in the expression of IRS2 in NSCLC cells. Collectively, the above data indicated that the knockdown of circ_0000003 impeded the proliferation and metastasis of NSCLC cells by modulatingmiR-338-3p/IRS2 axis.
In summary, our findings highlight a unique circRNA-regulated mechanism in NSCLC, that is circ_0000003 facilitates the malignant behaviors of NSCLC cells via miR-338-3p/IRS2 axis. The thorough exploration of the NSCLC mechanism helps us to improve survival rate and determine optimized treatment for NSCLC.However, this study is limited to single-center samples, and a larger sample is needed to confirm this conclusion in the future.
Authors’ contribution
Conceived and designed the experiments : SBL, YSY;
Performed the experiments : SBL, XGL, HL, YNL, Z YS, YSY;
Statastic analysis : SBL;
Wrote the paper : SBL, YSY.
All authors read and approved the final manuscript.
Ethics statement
Our study was approved by the ethics review board Zhujiang Hospital of Southern Medical University,
Disclosure statement
No potential conflict of interest was reported by the authors.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):7–34.
- Wei S, Zheng Y, Jiang Y, et al. The circRNA circPTPRA suppresses epithelial-mesenchymal transitioning and metastasis of NSCLC cells by sponging miR-96-5p. EBioMedicine. 2019 Jun;44:182–193.
- Dubaere E, Goffaux M, Wanet M, et al. Long term outcome after 48 Gy stereotactic ablative body radiotherapy for peripheral stage inon-small celllungcancer. BMC Cancer. 2019 Jun 28;19(1):639.
- Antonia SJ, Balmanoukian ABrahmer J, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol. 2019 Oct;14(10):1794–1806.
- Saigi MAlburquerque-Bejar JJ. Determinants of immunological evasion and immunocheckpoint inhibition response in non-small cell lung cancer: the genetic front. Oncogene. 2019 Aug;38(31):5921–5932.
- de Fraipont F, Gazzeri S, Cho WC, et al. Circular RNAs and RNA splice variants as biomarkers for prognosis and therapeutic response in the liquid biopsies of lung cancerPatients. Front Genet. 2019 May;7(10):390.
- Chi Y, Luo QSong Y, et al. Circular RNA circPIP5K1A promotes non-small cell lung cancer proliferation and metastasis through miR-600/HIF-1α regulation. J Cell Biochem. 2019 Nov;120(11):19019–19030.
- Wu J, Qi X, Liu L, et al. Emerging epigenetic regulation of circular RNAs in human cancer. Mol Ther Nucleic Acids. 2019 Jun;7(16):589–596.
- Maass PG, Glažar P, Memczak S, et al. A map of human circular RNAs in clinically relevant tissues. J Mol Med (Berl). 2017 Nov;95(11):1179–1189.
- Verduci L, Strano S, Yarden Y, et al. ThecircRNA-microRNA code: emerging implications forcancerdiagnosisand treatment. Mol Oncol. 2019 Apr;13(4):669–680.
- Li Y, Hu J, Li L, et al. Upregulated circular RNA circ_0016760 indicates unfavorable prognosis inNSCLCand promotes cell progression through miR-1287/GAGE1 axis. Biochem Biophys Res Commun. 2018 Sep 10;503(3):2089–2094.
- Chang H, Qu J, Wang J, et al. Circular RNA circ_0026134 regulates non-small cell lung cancer cell proliferation and invasion via sponging miR-1256 and miR-1287. Biomed Pharmacother. 2019 Apr;112:108743.
- Cui J, Li W, Liu G, et al. A novel circular RNA, hsa_circ_0043278, acts as a potential biomarker and promotes non-small cell lung cancer cell proliferation and migration by regulating miR-520f. Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):810–821.
- Wang J, Li H. CircRNAcirc_0067934silencing inhibits the proliferation, migration and invasion ofNSCLCcells and correlates with unfavorable prognosis inNSCLC. Eur Rev Med Pharmacol Sci. 2018 May;22(10):3053–3060.
- Zhang Y, Zhao H, Zhang L. Identification of the tumor-suppressive function of circular RNA FOXO3 in non-small cell lung cancer through sponging miR-155. Mol Med Rep. 2018 Jun;17(6):7692–7700.
- Lee SS, Cheah YK. The interplay betweenmicrornasand cellular components of Tumour Microenvironment (TME) on Non-Small-CellLungCancer (NSCLC) progression. J Immunol Res. 2019 Feb;13:3046379.
- Cui M, Yao X, Lin Y, et al. Interactive functions ofmicroRNAsin the miR-23a-27a-24-2 cluster and the potential for targeted therapy incancer. J Cell Physiol. 2019 Jun 13; DOI:10.1002/jcp.28958
- Zhang M, Shi H, Zhang C, et al. MiRNA-621 inhibits the malignant progression of non-small cell lung cancer via targeting SIX4. Eur Rev Med Pharmacol Sci. 2019 Jun;23(11):4807–4814.
- Shi J, Ma H, Wang H, et al. Overexpression of LINC00261 inhibits non-small cell lung cancer cells progression by interacting with miR-522-3p and suppressing Wnt signaling. J Cell Biochem. 2019 Jun 12; DOI:10.1002/jcb.29149
- Zhang P, Guoguang S, Xingyu L, et al. MiR-338-3pinhibits the growth and invasion of non-small cell lung cancer cells by targetingIRS2. Am J Cancer Res. 2017;7(1):53–63.
- Wang H, Zhao F, Cai S, et al. MiR-193a regulates chemoresistance of human osteosarcoma cells via repression ofIRS2. J Bone Oncol. 2019 May 11;17:100241.
- Zhao J, Li Z, Chen Y, et al. MicroRNA-766 inhibits papillary thyroidcancerprogression by directly targeting insulin receptor substrate 2 and regulating the PI3K/Akt pathway. Int J Oncol. 2019 Jan;54(1):315–325.
- Jeong SH, Kim HB, Kim MC, et al. Hippo-mediated suppression ofIRS2/AKT signaling prevents hepatic steatosis and livercancer. J Clin Invest. 2018 Mar 1;128(3):1010–1025.
- Meng S, Zhou H, Feng Z, et al. CircRNA: functions and properties of a novel potential biomarker forcancer. Mol Cancer. 2017 May 23;16(1):94.
- Chinniah C, Aguarin LCheng P, et al. Early detection of recurrence in patients with locally advanced non-small-cell lung cancer via circulating tumor cell analysis. Clin Lung Cancer. 2019 Sep;20(5):384–390.e2.
- Kowanetz M, Zou W, Gettinger SN, et al. Differential regulation of PD-L1 expression by immune and tumor cells inNSCLCand the response totreatmentwith atezolizumab (anti-PD-L1). Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):E10119–E10126.
- Sun H, Xi P, Sun Z, et al. Circ-SFMBT2promotestheproliferationofgastriccancercellsthroughspongingmiR-182-5ptoenhanceCREB1expression. Cancer Manag Res. 2018 Nov;16(10):5725–5734.
- Chen S, Li T, Zhao Q, et al. Using circular RNA hsa_circ_0000190 as a new biomarker in thediagnosisof gastric cancer. Clin Chim Acta. 2017 Mar;466:167–171.
- Hentze MW, Preiss T. Circular RNAs: splicing’s enigma variations. Embo J. 2013 Apr 3;32(7):923–925.
- Zhang HD, Jiang LH, Sun DW, et al. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018 Jan;25(1):1–7.
- Zhou X, Natino D, Qin Z, et al. Identification and functional characterization of circRNA-0008717 as an oncogene in osteosarcoma through sponging miR-203. Oncotarget. 2017 Dec 20;9(32):22288–22300.
- Kristensen LS, Hansen TB, Venø MT, et al. Circular RNAs incancer: opportunities and challenges in the field. Oncogene. 2018 Feb 1;37(5):555–565.
- Longqiang W, Tong X, Zhengyu Z, et al. Circular RNA hsa_circ_0008305 (circPTK2) inhibits TGF-β-induced epithelial-mesenchymal transition and metastasis by controlling TIF1γ innon-small celllungcancer. Mol Cancer. 2018 Sep 27;17:140. Published online.
- Weidle UH, Birzele F, Nopora A. MicroRNAs as potential targets for therapeutic intervention with metastasis of non-small cell lung cancer. Cancer Genomics Proteomics. 2019 Mar - Apr;16(2):99–119.
- Szejniuk WM, Robles AI, McCulloch T, et al. Epigenetic predictive biomarkers for response or outcome to platinum-based chemotherapy in non-small cell lung cancer, current state-of-art. Pharmacogenomics J. 2019 Feb;19(1):5–14.
- Zhang R, Shi H, Ren F, et al. Down-regulation ofmiR-338-3p and up-regulation of macc1 indicated poor prognosis of epithelial ovarianCancerPatients. JCancer. 2019 Feb 23;10(6):1385–1392.
- Li M, Bian Z, Jin G, et al. LncRNA-SNHG15 enhances cell proliferation in colorectalcancerby inhibitingmiR-338-3p. CancerMed. 2019 May;8(5):2404–2413.
- Ding Z, Zhu J, Zeng Y, et al. The regulation of neuropilin 1 expression bymiR-338-3p promotesnon-small celllungcancer via changes in EGFR signaling. Mol Carcinog. 2019 Jun;58(6):1019–1032.
- Zhang G, Zheng H, Zhang G, et al. MicroRNA-338-3p suppresses cell proliferation and induces apoptosis of non-small-cell lung cancer by targeting sphingosine kinase 2. Cancer Cell Int. 2017 Apr 17;17:46.
- Machado-Neto JA, Fenerich BA, Rodrigues Alves APN, et al. Insulin Substrate Receptor (IRS) proteins in normal and malignant hematopoiesis. Clinics (Sao Paulo). 2018 Oct 11;73(suppl 1):e566s.
- Zhu S, Ward BM, Yu J, et al. IRS2 mutations linked to invasion in pleomorphic invasive lobular carcinoma. JCI Insight. 2018 Apr 19;3(8): pii: 97398.
- Liu H, Ren G, Zhu L, et al. The upregulation of miRNA-146a inhibited biological behaviors of ESCC through inhibition of IRS2. Tumour Biol. 2016 Apr;37(4):4641–4647.
- Park DH, Jeon HS, Lee SY, et al. MicroRNA-146a inhibits epithelial mesenchymal transition in non-small cell lung cancer by targeting insulin receptor substrate 2. Int J Oncol. 2015 Oct;47(4):1545–1553.
- Gao J, Feng X, Wang F, et al. microRNA-448 inhibits the progression of non-small-cell lung cancer through regulating IRS2. 2019 Aug;120(8):13453–13463.