ABSTRACT
Lumican is overexpressed in lung cancer cells and has been implicated in the pathogenesis of tumorigenesis and regulation of cancer cell invasion. Lumican is robustly associated with the binding of p120-catenin protein to modulate cell metastasis. However, its role in cancer cell proliferation is still unclear. This study investigated the effect of lumican on the cell division including mitosis and cytokinesis in non-small lung cancer cells. We found that the downregulation of lumican prolonged the doubling time of cells and retarded the cell growth in H460 and A549 cells. Along with tubulin, lumican localized to the mitotic spindle and centrosome during the metaphase-anaphase stage. The cell cycle was retained in the G2/M phase after the downregulation of lumican. Interestingly, lumican was found to play important roles in central spindle and midbody formation during cytokinesis. Lumican interacted with the midbody-associated proteins such as MKLP1, Aurora B, and ECT2. Notably, the downregulation of lumican decreased the level of MKLP1 accompanied by the retention of midbody-residual that resulted in multi-nucleated cells. Downregulation of lumican promoted the chromosome missegregation and the increment of the bi-/multinucleated cells. The results of this study indicated that lumican associated with tubulin is crucial for spindle fiber formation and midbody assembly in cell division. Downregulation of lumican displayed the defects in mitotic spindle assembly/dynamics and improper kinetochore-microtubules attachment that led to increase aneuploidy. This emerging property of lumican is suggested to tightly control chromosome segregation during cell division in lung cancer cells.
Abbreviations: ESCRT: endosomal sorting complex required for transport; PRC1: protein regulator of cytokinesis 1; Nci: negative control siRNA; Lumi: lumican siRNAs; MKLP1: mitotic kinesin-like protein 1; H460LD and A549LD: H460 and A549 cell lines with less expressed lumican
1 Introduction
Lung cancer cells have been shown to overexpress lumican, which is associated with the modulation of cell invasion and cell viability [Citation1]. Lumican is a small proteoglycan harboring leucine-rich tandem motifs. It has been established that binding of lumican, in an integrin-dependent manner, to polymorphonuclear leukocytes regulates their activation and migration/extravasation [Citation2]. By binding to collagen fibers, lumican regulates collagen fibrillogenesis, tumor-associated fibrosis, and collagen degradation [Citation2]. Lumican is highly expressed in several types of cancer cells and/or stromal tissue and is emerging as a key modulator of the tumor microenvironment. The capacity of lumican to regulate cell proliferation, adhesion, migration, and invasion is of particular interest in the control of cancer progression. More recently, we deciphered a role for lumican in inflammation [Citation1]. Downregulation of lumican could enhance cell invasion via p120 catenin/Rho signaling pathway [Citation1]. These data suggested an interaction between lumican and p120 catenin through inside-outside signaling circuits.
The eukaryotic cell cycle is required for proper growth and division. Defects in mitosis and cytokinesis are associated with various human diseases and might evoke chromosome imbalance that can lead to tumorigenesis [Citation3,Citation4]. During the process of cytokinesis, the midbody is the intercellular bridge that connects the two dividing cells. The midbody is a densely packed antiparallel microtubule array, with an electron-dense structure (stem body) sitting in the midbody’s center [Citation5]. The midbody is the platform that brings together the abscission machinery, including membrane trafficking components that narrow the intercellular bridge [Citation6] and the endosomal sorting complex required for transport (ESCRT) machinery, which executes the final scission event [Citation7,Citation8]. While the protein required for cytokinesis 1 (PRC1) and the chromosomal passenger complex remain associated with midbody microtubules during chromosome movement and segregation [Citation9], centralspindlin transitions in its localization from the midbody to the midbody ring [Citation9,Citation10]. In human cells, the ESCRT machinery is recruited by CEP55, which binds to centralspindlin late in cytokinesis.
It has been shown that lumican is expressed in injured epithelium and may contribute to corneal epithelial wound healing [Citation11], which might implicate its role in the regulation of cell division. This suggests that the involvement of lumican was in the regulation of mitotic process. In this study, we found the effect of lumican on the chromosome segregation during mitotic stage associated spindle fiber and midbody formation in lung cancer cells.
2 Materials and methods
2.1 Cell culture
The non-small lung cancer cell lines A549 (ATCC CCL-185) and H460 (ATCC HTB-177) were purchased from American Type Culture Collection (ATCC; Manassas, Virginia, United States). The H460LD and A549LD stable cell lines with downregulated lumican are created by using short hairpin RNA (shRNA) specific against lumican and selected by puromycin at 2.0 μg/mL of medium starting 24 h after transfection [Citation1].
2.2 Reagents and antibodies
Unless otherwise indicated, all chemical reagents were purchased from Sigma-Aldrich. Antibody to lumican (ab168348) was purchased from Abcam. Antibodies to anti-α-tubulin, anti-MKLP1, anti-ECT2, anti-Aurora A, anti-Aurora B, and anti-centrolin antibodies were purchased from Cell Signaling Technology Inc.
2.3 Cell cycle analysis
Double thymidine synchronization was performed as previously described [Citation12]. In brief, cells at 25–30% confluence were incubated in medium containing 2 mM thymidine for 18 h (first block). Cells were washed with PBS to remove thymidine and were released into fresh medium. After 9 h, the cells were incubated with medium containing 2 mM thymidine for 17 h (second block). Cells arrested at the G1/S boundary were released into fresh media and harvested for cell cycle processed by FACS analysis. Flow cytometry enables the calculation of timings for a collection of cells at distinct cell cycle phases from G1/S (following treatment with thymidine). Flow cytometric data analysis was performed using FlowJo and FCS Express software.
2.4 Confocal immunofluorescence microscopy
Lung cancer cells were grown in chamber-slide glass and incubated for 24 h. The cells were then fixed in 4% formaldehyde at room temperature. After being rinsed in PBS, the cells were incubated overnight with anti-lumican, anti-Aurora A, anti-Aurora B, anti-tubulin, anti-centrolin, anti-Ect2, or anti-MKlP1 at 4°C. Samples were stained with 4ʹ,6-diamidino-2-phenylindole (DAPI, Invitrogen) for 1 min and then visualized using a ZEISS LSM 510/780 META/Confocal microscope (Carl Zeiss MicroImaging) with the pinhole set at 1 Airy unit using 488, 546, and/or 633 nm excitation and a 20x-100x oil objective lens.
2.5 Western blot
Cells were lysed in cold lysis buffer (1% NP-40, 50 mM Tris pH 7.4, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 10% Glycerol and the Halt Protease and Phosphatase Inhibitor Cocktail) diluted at 1:100. To prepare cell lysates, cell pellets were sonicated in 0.3 mL of lysis buffer on ice, and the lysate was spun down at 13,000 g for 15 min at 4°C. The cellular extracts underwent SDS-PAGE and blotted onto polyvinylidene difluoride membranes (Immobilon(TM)-P, Millipore). Blots were probed with primary antibodies then appropriate horseradish peroxidase-conjugated secondary antibodies and visualized by chemiluminescence.
2.6 Construction of lumican variants
To determine the interaction of lumican and other proteins, the cDNA of lumican subdomains were cloned into pCDNA4 c-flag. The full-length cDNA of lumican is indicated as LUMFL. The variants of lumican were designed as LUMN (N-terminal of lumican), LUM14 (LRP1 to LRR 4), LUM57 (LRR 5 to LRR 7), LUM810 (LRR 8 to LRR 10). These fragments were amplified by PCR with specific primers (, supplement materials). The resulting PCR products were digested with BamHI and XhoI, and ligated with pCDNA4-c-flag at the compatible sites. The ligation mixtures were introduced into E. coli DH10β by transformation.
Table 1. The primers for the variant of lumican
2.7 Statistical analysis
Data are presented as the mean ± SD. Statistical significance between groups was assessed by unpaired Student’s t-test. A p value of less than 0.05 was significant.
3 Results
3.1 Downregulation of lumican delayed cell growth
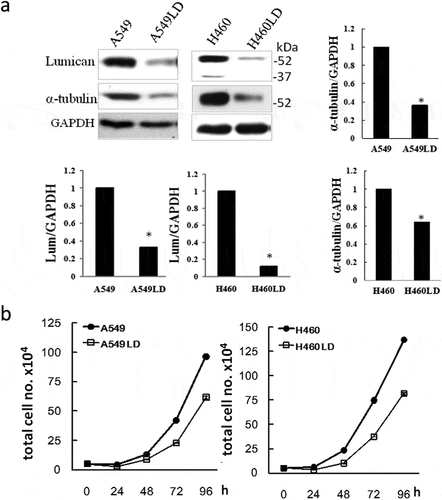
Two stable clones of less-expressed lumican (A549LD and H460LD) were established with the transfection of lumican shRNA in A549 and H460 lung cancer cells [Citation1]. The lumican protein expression level decreased in H460LD and A549LD cells compared with their respective parental H460 and A549 cells ()). Downregulation of lumican significantly decreased the expression of α-tubulin, a component of microtubules ()) (P < 0.05). A cell growth assay indicated that the time for cell doubling in A549/A549LD cells and H460/H460LD cells was 15.43 ± 0.08 h/16.89 ± 0.12 h and 14.91 ± 0.07 h/15.50 ± 0.09 h, respectively ()) (P < 0.05). These results showed that the downregulation of lumican increased the doubling time and affected the cell growth.
Figure 1. Lumican regulates the cell growth.
3.2 Lumican was associated with regulation of cell cycle
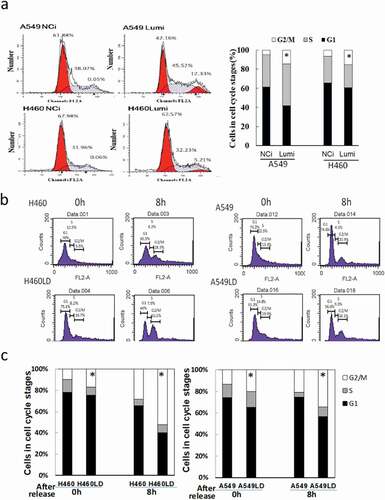
To explore the effects of lumican on cell cycle, lung cancer cells were transfected with negative control siRNA (NCi) or lumican siRNAs (Lumi). After transfection with the siRNAs for 24 h, the transfected cells subsequently were arrested at the beginning of S phase by using a double thymidine block. The cell cycle profile of the time points was monitored by flow cytometry of propidium iodide-stained cells. After release from the double thymidine block, the population of NCi-transfected cells at the G2/M phase in A549 or H460 cells was 0.05% and 0.06%, which indicated that arrest occurred at the beginning of the S phase (). In contrast, the population of siRNA-transfected A549 or H460 cells retained at the G2/M phase was approximately 12% or 5%, respectively ()). Furthermore, the profiles of cell cycle were also examined in A549/A549LD and H460/H460LD cells. The cells were starved and subsequently were arrested at the beginning of the S phase by using a double thymidine block and then released at the indicated time ()). As a similar result of lumican-specific shRNA transfection, the cells of the G2/M phase were approximately 4.77% vs. 14.69% and 6.24% vs. 15.12% after the release of thymidine block in A549 vs. A549LD and H460 vs. H460LD cells, respectively. The results suggested silencing of lumican interrupted the process of cell cycle and particularly blocked it at the G2/M phase.
Figure 2. The profile of the cell cycle.
3.3 Lumican co-localized with microtubule devoted to cell division
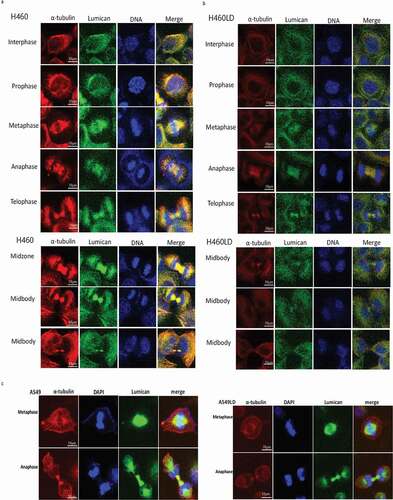
Immunofluorescence stain with anti-lumican and anti-tubulin antibodies was assayed to verify the role of lumican in the process of mitosis. Lumican was co-localized with tubulin at the interphase of the cell cycle in H460 and A549 cells ((a, c)). We found lumican coupled with tubulin in the mitotic spindle during mitosis ((a, c)). At the metaphase and anaphase, lumican was localized to the spindle fiber. Notably, lumican was localized at the midbody of cytokinesis stage and abundantly associated with the formation of spindle midzone and the midbody during cytokinesis ((a, c)). The co-localization of lumican and tubulin was markedly reduced at the stages of mitosis and cytokinesis in H460LD cells ()). The specificity of these immunostaining results was also validated in A549/A549LD cells ()).
Figure 3. Lumican is required for mitosis and cytokinesis.
Lumican plays a role in determining the architecture of microtubules [Citation2]. The interaction and regulation of lumican and tubulin has been provided by the immunoprecipitation assay and lumican gRNAs CRISPR/Cas9 plasmid transfection [Citation1], indicating the localization of lumican in the microtubule network. To elucidate the interaction between the domains of lumican and microtubule, the fragments of lumican cDNA were cloned into pCDNA4 c-flag. The fragments of lumican gene were designed for four fragments, LumN (N-terminal of Lum), Lum14 (LRP 1 to LRP 4), Lum57 (LRR 5 to LRR 7) and Lum810 (LRP 8 to LRP 10) and LumFL (full-length cDNA of lumican gene) (). The desired flag-tagged fragments of lumican were immunostained with α-tubulin (). The variants of lumican contain N-terminal, and spanning LRP1-LRP4, LRP5-LRP7 and LRP8-LRP10 fragments remained localized at the microtubules. The results indicated that the fragments of lumican were co-localized with microtubule. Together, these results indicated that lumican was localized to the mitotic apparatuses and was involved in the regulation of chromatid separation.
3.4 Lumican and aneuploidy
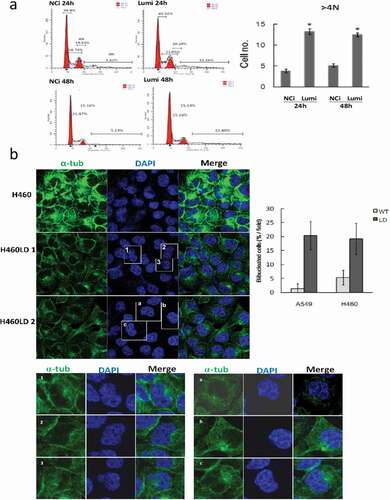
We investigated if downregulation of lumican caused the bi-/multi-nucleated cells. H460 cells were transfected with NCi or Lumi for 24 h and 48 h. The cell cycle profile of the transfected cells was determined by FACS analysis and quantified by FloJo. FACS analysis indicated that cells with more than 4N DNA (≥4N) content were 13.24% and 12.48% in Lumi-transfected cells, whereas it was 3.82% and 5.13% in NCi-transfected cells 24 h and 48 h after transfection, respectively ()). We also quantified the bi-/multinucleated cells in H460/H460LD and A549/A549LD cells by immunofluorescence staining with anti-tubulin and anti-lumican antibodies ()). We found the bi-/multinucleated cells increased about 10-fold or 4-fold in A549LD to A549 or H460LD to H460 cells, respectively (), lower panel).
Figure 4. Downregulation of lumican elicits aberrant mitosis.
3.5 Lumican regulated the midzone proteins
To investigate the cellular consequence of lumican-knockdown in cells, we analyzed the localization of selected cytokinesis regulator proteins. We assayed the proteins associated with the midbody microtubules, such as mitotic kinesin-like protein 1 (MKLP1), ECT2, Aurora A, and Aurora B by Western blotting. We found the expression levels of these midbody-regulator proteins were decreased in H460LD and A549LD cells when compared with that in H460 and A549 cells ()). The regulation of MKLP1 expression by lumican was then verified by transfection with lumican siRNAs. The results showed that α-tubulin and MKLP1 were obviously less expressed in the lumican siRNA-transfected cells than in the NCi-transfected cells (Figure 5A1).
Figure 5. Downregulation of lumican decreases the levels of midbody-associated proteins and increased the residual midbodies.
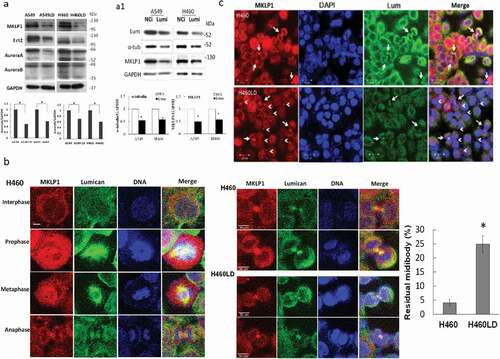
We next elucidated how lumican depleted the progress of cells through cytokinesis by immunostaining in H460/H460LD cell line. The MKLP1 is a component of centralspindlin complex that has been implicated in the assembly of midzone/midbody during mitosis and is essential for cytokinesis [Citation13]. As shown in ), lumican and MKLP1 shared the same immunofluorescence localization in the interphase and the mitotic stage including prophase, metaphase, and anaphase in H460 cells. MKLP1 associated with lumican in the formation of midbody. MKLP1 was localized on the bulge of midbody (Flembody), whereas lumican localized within or beside the two arms of the stem body in H460 cells ()). MKLP1 apparently accumulated as a smaller midbody bulge in H460LD cells compared with that in H460 cells in telophase ((b, c)). In addition, the midbody remnants were detected and retained in the H460LD cells (arrowheads, )). These results indicated that lumican cross-linked with microtubules that contacted MKLP1 to stabilize the bulge of the midbody. These data suggest that the presence of lumican was required for the midbody formation.
3.6 Downregulation of lumican decreased ECT2 and increased the multi-nucleation
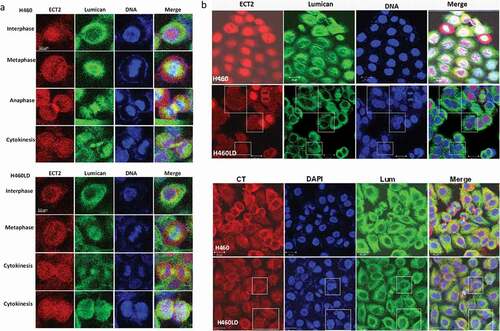
The effect of the decreased expression of ECT2 induced by the downregulation of lumican was investigated by immunostaining ()). ECT2 was expressed in the nucleus at the interphase stage. The co-localization of ECT2 and lumican was found at the mitotic spindle in the metaphase and anaphase. ECT2 associated with lumican was involved in the formation of mid-zone and midbody formation. Downregulation of lumican decreased their co-localization at the mitotic spindle and midbody in H460LD cells. The binucleated cells induced by the downregulation of lumican were also detected by the immunofluorescence staining with anti-ECT2 and anti-centrolin (CT) in H460 and H460LD cells ()).
Figure 6. Downregulation of lumican decreases ECT2 expression and disrupts the processes of cytokinesis.
4 Discussion
Chromosomal imbalances including aneuploidy might have detrimental effects on cellular fitness and might trigger cell cycle arrest [Citation14]. This study demonstrated the roles of lumican on the chromosome segregation during cell division in lung cancer. Depletion of lumican delayed the doubling time for cell cycle through retention at the G2/M phase. We found that lumican was localized to the mitotic spindle at the mitotic stage and the cytokinesis stage and targets a number of structural and regulatory proteins required for completion of cytokinesis, including Aurora A, centrolin, MKLP1, and ECT2. Importantly, we found depletion of lumican disrupted the arms formation of the midbody and induced the retention of MKLP1-associated residual midbody. The depletion of lumican decreased the spindle fiber might trigger defects in cell cycle progression, checkpoint control, and proper chromosome segregation that led to the increase of bi-/multi-nucleated cells. This aneuploidy might increase the chromosome instability and retarded cell growth. Together, our study highlights a new function of lumican in mitosis and cytokinesis in lung cancer cells.
Lumican has attracted attention as a molecule of the extracellular matrix possibly involved in signaling pathways affecting cancer cell behavior [Citation2]. Lumican colocalizes with p120-catenin in the juxta-membrane compartment and is associated with microtubule-modulated p120ctn signaling [Citation1]. This study indicated that lumican was associated with microtubule and regulated the expression of tubulin. As mitosis approaches, the microtubules assemble from their interphase arrangement and reorganize into the spindle arrangement, which grows until it fills almost the entire cell [Citation15]. After sister chromatids separate, motor proteins move them along the kinetochore microtubules toward opposite ends of the cell [Citation15]. The M checkpoint is called the spindle checkpoint and the cells check that all sister chromatids are properly attached to the spindle microtubules. The cell with decreased spindle fiber cannot actually scan the metaphase plate to confirm that all of the chromosomes are there. If a chromosome is misplaced, the cell will pause mitosis, giving the spindle time to capture the stray chromosome. Thus, depletion of lumican reduced the expression of tubulins, which may disrupt the formation of spindle fibers and lead to cells being stuck in the G2/M-phase. Lumican might play a key role in the checkpoint control and proper chromosome segregation in cell cycle progression.
Cytokinesis is the final stage of cell division, and the central spindle and midbody are thought to be essential structures for the initiation and completion of cytokinesis. The central spindle determines the position of the cleavage plane and constriction of the actomyosin ring drives furrow ingression. As the central spindle is remodeled to form a stable intercellular bridge that connects the two daughter cells with the Flemming body, the midbody provides anchorage to the cleavage furrow ingression and serves as a platform for the assembly of the abscission machinery. Imaging of lumican during cytokinesis by confocal microscope showed that they accumulate at the arms of the Flemming body. Depletion of lumican resulted in reduced levels of MKLP1, Aurora A, ECT2, and centrolin as indicated by Western blot. Imaging of MKLP1 and lumican indicated the localization of lumican in the arms of midbody, which may contribute to the binding of MKLP1 to the Flemming body [Citation13]. MKLP1 protein localized to the spindle poles and spindle in early mitosis [Citation13], distributed to the spindle midzone in anaphase, and then concentrated to the central portion of the midbody in telophase in H460 cells. The cells lacking MKLP1 progressed normally from prophase to early anaphase, but the proper formation of the midbody was severely inhibited in telophase of H460LD cells with a reduced level of MKLP1. Downregulation of lumican might abolish the MKLP1 bulge of the midbody and result in the abrogation of midbody formation and completion of cytokinesis. The midbody is a transient structure formed during cytokinesis in animal cells by the ingression of the cleavage furrow. The midbody and its adjacent regions are decisive and serve as a platform to recruit the proteins necessary for abscission [Citation16]. The central portion of the midbody, a cytoplasmic bridge between nascent daughter cells at the end of cell division, has generally been thought to be retained by one of the daughter cells, but was recently shown to be released into the extracellular space [Citation16,Citation17]. Midbody-release emerges as a characteristic feature of cells capable of differentiation [Citation17]. The fate of this midbody remnant has crucial consequences for cell differentiation and tumorigenesis in mammalian cells [Citation17]. Cells with such impaired midbody-release exhibit enhanced responsiveness to differentiation stimulus. This study indicated that the downregulation of lumican caused the retention of MKLP1-positive midbody from the bulk of midbody and displayed a midbody residual (remnant) in the intracellular compartment ()). This is likely because the depletion of lumican disrupts the spindle fiber and the intercellular bridge, resulting in the accumulation of MKLP1-associated midbody residual.
This study indicated the geometry of cleavage furrow ingression was dependent on lumican. Lumican targeted a number of structural and regulatory proteins required for completion of cytokinesis to the midbody including Aurora A and ECT2, demonstrating that lumican was indispensable for midbody structure maintenance. ECT2 is a guanine nucleotide exchange factor for the activation of Rho GTPases and is required for the assembly of contractile ring and initiation of cytokinesis. ECT2 localizes to the nucleus in interphase, condensed mitotic spindles in mitosis, and then to the midbody during cytokinesis. Depletion of ECT2 impaired cleavage furrow formation, and RhoA and Citron kinase fail to accumulate at the cleavage furrow [Citation18]. Centrolin, one of the centrosomal proteins is required for proper cytokinesis. Depletion of centrolin leads to defects in the final stages of cytokinesis, where cells remain connected by the intercellular bridges and are unable to complete abscission [Citation19]. It has been shown that the mislocalization of structurally altered ECT2 might cause the untimely activation of cytoplasmic Rho GTPase signaling pathways leading to malignant transformation [Citation20].
Conclusions
This study indicated the association of lumican with tubulin functioned in chromosome segregation during cell cycle progression. Depletion of lumican induced the chromosome missegregation and cytokinesis failure that associated with the regulation of spindle fiber and midbody formation. The changes in chromosome copy number could positively contribute to cancer evolution [Citation14]. In contrast, aneuploidy may enhance chromosomal imbalances and has detrimental effects on cellular fitness [Citation14]. Depletion of lumican elicited aneuploidy and might facilitate more severe disabling of checkpoint signaling and growth retardation. Future studies are required to investigate the therapeutic effect of targeting lumican for lung cancer treatment.
Availability of data and materials
Please contact the corresponding author for all data requests.
Authors’ contributions
SEC and CTY designed the manuscript and wrote the manuscript. PCH performed the in vitro experiments. All authors reviewed and approved the manuscript.
Consent for publication
All authors give consent for the publication of the manuscript in Molecular Cancer.
Supplemental Material
Download Zip (793.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
Additional information
Funding
References
- Yang C-T, Li J-M, Chu W-K, et al. Downregulation of lumican accelerates lung cancer cell invasion through p120 catenin. Cell Death Dis. 2018;9(4): 414.
- Nikitovic D, Papoutsidakis A, Karamanos NK, et al. Lumican affects tumor cell functions, tumor-ECM interactions, angiogenesis and inflammatory response. Matrix Biol. 2014;35:206–214.
- Fujiwara T, Bandi M, Nitta M, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437(7061):1043–1047.
- Vinciguerra P, Godinho SA, Parmar K, et al. Cytokinesis failure occurs in Fanconi anemia pathway-deficient murine and human bone marrow hematopoietic cells. J Clin Invest. 2010;120(11):3834–3842.
- Hutterer A, Glotzer M, Mishima M. Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody. Curr Biol. 2009;19(23):2043–2049.
- Schiel JA, Prekeris R. Membrane dynamics during cytokinesis. Curr Opin Cell Biol. 2013;25(1):92–98.
- Agromayor M, Martin-Serrano J. Knowing when to cut and run: mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013;23(9):433–441.
- McCullough J, Colf LA, Sundquist WI. Membrane fission reactions of the mammalian ESCRT pathway. Annu Rev Biochem. 2013;82:663–692.
- Hu CK, Coughlin M, Mitchison TJ. Midbody assembly and its regulation during cytokinesis. Mol Biol Cell. 2012;23(6):1024–1034.
- Elia N, Sougrat R, Spurlin TA, et al. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A. 2011;108(12):4846–4851.
- Saika S, Shiraishi A, Saika S, et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275(4):2607–2612.
- Rodrigue A, Coulombe Y, Jacquet K, et al. The RAD51 paralogs ensure cellular protection against mitotic defects and aneuploidy. J Cell Sci. 2013;126(1):348–359.
- Zhu C, Bossy-Wetzel E, Jiang W. Recruitment of MKLP1 to the spindle midzone/midbody by INCENP is essential for midbody formation and completion of cytokinesis in human cells. Biochem J. 2005;389(Pt 2):373–381.
- Giam M, Rancati G. Aneuploidy and chromosomal instability in cancer: a jackpot to chaos. Cell Div. 2015;10(1):3.
- Brouhard GJ, Rice LM. The contribution of αβ-tubulin curvature to microtubule dynamics. J Cell Biol. 2014;207(3):323.
- Crowell EF, Gaffuri A-L, Gayraud-Morel B, et al. Engulfment of the midbody remnant after cytokinesis in mammalian cells. J Cell Sci. 2014;127(17):3840.
- Ettinger AW, Wilsch-Bräuninger M, Marzesco A-M, et al. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat Commun. 2011;2:503.
- Chalamalasetty RB, Hümmer S, Nigg EA, et al. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J Cell Sci. 2006;119(14):3008.
- Fong K-W, Leung JW-C, Li Y, et al. MTR120/KIAA1383, a novel microtubule-associated protein, promotes microtubule stability and ensures cytokinesis. J Cell Sci. 2013;126(Pt 3):825–837.
- Saito S, Liu X-F, Kamijo K, et al. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation. J Biol Chem. 2004;279(8):7169–7179.
