ABSTRACT
YKL-40 is a chitinase-like protein which was significantly elevated in asthma patients and related closely to asthma severity and airway remodeling. Airway remodeling in asthma involves complicated physical and pathological processes, including increased airway smooth muscle mass due to proliferation, migration of airway smooth muscle cells, epithelial-mesenchymal transition (EMT) and sub-epithelial fibrosis. However, the precise effect and underlying mechanism of YKL-40 in this pathological alteration remained unelucidated. In this study, we demonstrated that YKL-40 could promote asthma airway remodeling by increasing airway smooth muscle mass, inducing EMT and sub-epithelial fibrosis. Furthermore, we identified that FAK and MAPK signaling pathways are activated in the process. Inhibiting FAK or MAPK pathway could significantly ameliorate airway remodeling induced by excessive secretion of YKL-40 in vitro. and in vivo. In conclusion, this study shed light upon the effects of YKL-40 in asthma airway remodeling and provided potential novel targets in asthma patients with high YKL-40 level.
Introduction
Asthma is a common chronic inflammatory and heterogeneous pulmonary disease [Citation1–Citation3], characterized by reversible airway obstruction, chronic inflammation, hyper-responsiveness, and airway remodeling [Citation4–Citation6]. It affects 334 million people worldwide, with the highest prevalence in developed countries [Citation1,Citation7,Citation8]. Based on the “inflammation theory”, steroids could greatly relieve the symptoms in the early phase [Citation5,Citation9]. However, in most cases, the effect of steroid treatment is unsatisfactory as expected due to asthma-related airway remodeling [Citation10]. Several publications have confirmed the involvement of multiple pathological changes in the asthma airway remodeling, such as epithelial alterations, increased airway smooth muscle mass and sub-epithelial fibrosis, [Citation11–Citation15], but the intrinsic factor residing in the microenvironment of airway remodeling has not yet been determined. Moreover, the underlying molecular mechanism linking airway remodeling and paralleled chronic inflammation remains unclear.
Chitinase-3-like protein 1 (CHI3L1), also known as YKL-40, is a 40-kDa secreted chitinase-like glycoprotein and has been implicated in many inflammatory diseases and tissue remodeling, including asthma [Citation16–Citation20]. A variety of cells are reported to express YKL-40, including macrophages, neutrophils, epithelial cells, smooth muscle cells, and chondrocytes [Citation21–Citation24], most of which compose the airway microstructure. Importantly, the levels of YKL-40 in both airway and serum have been clarified to be significantly elevated in asthma patients and correlated with the severity of the disease [Citation17,Citation19,Citation25,Citation26]. The thickness of sub-epithelial basement membrane, the main feature of airway remodeling [Citation27], was found to be positively correlated with YKL-40 level [Citation17,Citation26] in asthma patients. These data indicated that YKL-40 might play a role in the pathological development and progress of airway remodeling in asthma patients, however, its specific effects on asthma and underlying mechanisms needed to be illustrated.
Here, we aimed to investigate the biological contribution of YKL-40 to airway remodeling in the context of asthma. In this study, we evaluated how YKL-40 promoted asthma via altering the biological function of smooth muscle cells and airway epithelial cells as well as the aberrant formation of sub-epithelial fibrosis in ovalbumin (OVA)-induced murine model of asthma. Furthermore, the molecular mechanism underlying airway remodeling due to excess secretion of YKL-40 was invested by antibody microarray and small molecule inhibition. FAK and MAPK pathway were identified as activated. By using FAK or MAPK pathway specific inhibitor, airway remodeling mediated by YKL-40 could be alleviated in vitro. and in vivo. Thus, we provided evidence that YKL-40 regulated airway remodeling in asthma model via activating FAK and MAPK pathway, which might shed new light on the development of new treatment for asthma patients.
Materials and methods
Animals and in vivo. treatment
The animal experiments in this study were approved by the Institutional Animal Care and Use Committee of Second Military Medical University (Shanghai, China). Six-week-old sex-matched C57/B16 mice were used to establish the OVA-induced murine model of asthma following the standard procedure [Citation28,Citation29]. Firstly, on days 0, 7, and 14, each mouse was pre-sensitized by intraperitoneal injection of 50 μg OVA (Sigma-Aldrich) emulsified in 1 mg of potassium aluminum sulfate. Subsequently, on consecutive Day 21, 22 and 23, groups were stimulated by injecting 100 μg OVA dissolved in 40 μl sterile saline through the trachea.
Cells and reagents
Airway epithelial cell Beas2B, bronchial smooth muscle cell (BSMC) and tracheal smooth muscle cell (TSMC) were purchased from LONZA Inc. (Basel, Swiss), cultured in BEBM and SMCM culture media (LONZA Inc.) respectively. Cells were cultured at 37 °C in a 5% CO2 incubator. Human recombinant YKL-40 was purchased from Quidel Corporation (California, USA). CDK inhibitor palbociclib, FAK inhibitor PF562271 and MAPK inhibitor SCH772984 were purchased from Selleck (Houston, USA).
Cell proliferation detection by CCK8
The appropriate concentration of the primary BSMCs was cultured in a 96-well cell culture plate for 36 hours. Cell proliferation was measured at 0 h, 12 h, 24 h, and 36 h using Cell Counting Kit-8 (CCK8; Dojindo, Rockville, MD, USA). At the indicated time points, cells were incubated with CCK8 solution at 37°C for 1 hour, and the absorbance at 450 nm was detected.
Wound healing assay and cell migration assay
For wound healing assay, an equal number of BSMCs were seeded into six-well plates under the same culture condition. Monolayers of cells were wounded by scraping with a plastic pipette tip. After treated with 200 ng/ml YKL-40 or vehicle, the migrated BSMCs into the gap were observed and photographed under a microscope at the indicated time points. For migration assay, 5 × 104[Citation4] BSMCs were cultured in 24-well transwell plates with inserts (Corning). The culture medium in the inserts was free of FBS, whereas 10% FBS was added into the medium in the lower chamber. To observe the role of YKL-40 on cell migration, indicated concentration of recombinant YKL-40 or vehicle were added into the lower chamber. After a certain time of incubation, the migratory cells were fixed, stained and captured under the microscope according to the manufacturer’s protocols. Representative fields were showed and the number of migrated cells was counted.
qRT-PCR and Western blot
qRT-PCR: Total RNA was extracted by using Trizol reagent (Invitrogen). Then, Superscript III RT (Invitrogen) and random primer N6 were used to perform reverse transcription. Furthermore, the amounts of specific transcripts were measured according to the standard procedure of qRT-PCR with an ABI PRISM 7300 sequence detector (Applied Biosystems).
Western blot: Protein extracting was conducted following the standard protocols. Proteins were analyzed by immunoblot with primary antibodies (Abcam) and IRDye 800CW-conjugated second antibody (LI-COR Biosciences).
Immunofluorescence and immunohistochemistry
Immunofluorescence was performed on frozen sections of lungs of mice by overnight primary antibody incubation with E-cadherin, N-cadherin, DSP or vimentin (Abcam) followed by incubation with the appropriate fluorophore-labeled secondary antibody. For immunohistochemistry, lung sections of mice were used to detect YKL-40 expression. Briefly, the sections were incubated with YKL-40 primary antibody (Abcam) at 4°C overnight and, with horseradish peroxidase-conjugated secondary antibody at 37°C for 30 min on the second day. Finally, the sections were incubated with diaminobenzidine and counter-stained with hematoxylin for detection.
Phospho-kinase screening
Phospho-kinase screening was performed using a phospho-kinase array (R&D Systems, ARY003B) according to the manufacturer’s instructions.
Statistical analysis
All results presented as the mean ± SD are from at least three independent experiments. Statistical comparisons of different groups were evaluated by using the Student’s t-test or Mann-Whitney tests and Pearson’s χ2 tests. SPSS software (IBM, version 21.0) was used to complete the statistical analysis. p values of less than 0.05 were considered to be statistically significant.
Results
YKL-40 promotes the proliferation of BSMCs via modulating cyclin D1/CDK activation
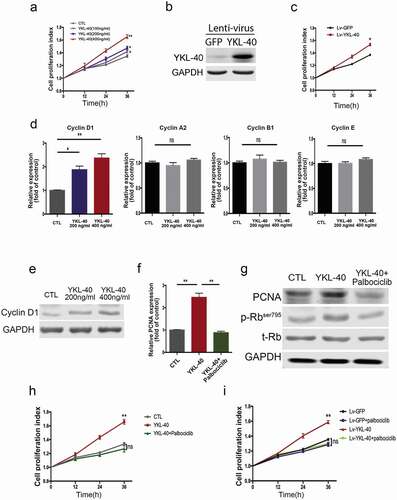
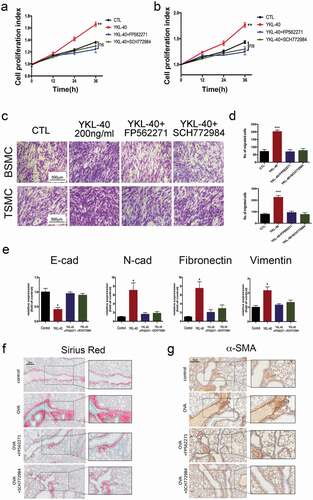
Increased airway smooth muscle mass, a major hallmark of airway remodeling, greatly impairs the intrinsic plasticity of bronchial smooth muscle cells and deteriorates the normal airway structure in the pathogenesis of asthma. Proliferation and migration of airway smooth muscle cells have been demonstrated to contribute to airways remodeling triggered by chronic inflammation. Hence, we determined the effect of YKL-40 on the proliferation of bronchial smooth muscle cell (BSMC) in vitro. As shown in ), human recombinant YKL-40 significantly promotes the proliferation of BSMCs in a dose-dependent manner compared with the vehicle control. This effect was confirmed in TSMCs ()). To further validate the long-term proliferation promotion effect induced by YKL-40 expression, stable BSMC cell lines were established by Lenti-virus ()). Similarly, stable expression of YKL-40 could facilitate the proliferation of BSMC cells ()). Furthermore, the expression profile of cell cycling in the BSMC exposed to YKL-40 was analyzed by qRT-PCR. Interestingly, YKL-40 only induced the expression of cyclin D1, while showing little effect on other cyclins ()), which is further demonstrated in protein level presenting a dose-dependent increase of cyclin D1 via YKL-40 administering in BMSC ()). As one of the essential signaling pathways involved in cell cycle progression, CDK4 associates with cyclin D and regulates the cell cycle through hyperphosphorylation and deactivation of the tumor suppressor retinoblastoma protein (Rb) [Citation30]. To further confirm the role of YKL-40 in the cell cycle, we treated BSMC with potent CDK4/6 inhibitor palbociclib. As indicated by PCNA expression, the elevated proliferation of BSMC cells induced by YKL-40 treatment was significantly inhibited ()). Decreased expression of proliferating cell nuclear antigen (PCNA) was accompanied by dephosphorylation of Rb in cells treated by both YKL-40 and palbociclib ()). Consistently, palbociclib significantly inhibited recombinant YKL-40 administration induced BSMC proliferation ()), as well as in the context of stable YKL-40 expression ()). These results support the notion that YKL-40 promotes the proliferation of BSMC via cyclinD1/CDK4/6 dependent signaling pathway.
YKL-40 enhances the migration ability of BSMC
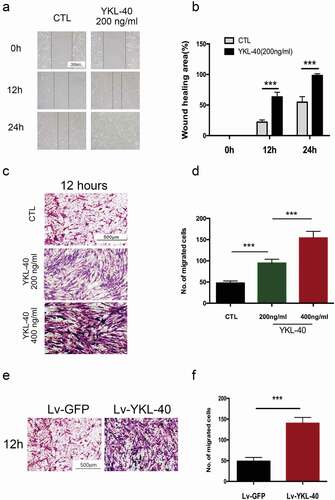
Besides proliferation, smooth muscle cells migration cooperatively contributes to increased airway smooth muscle mass and remodeling process. Wound healing assay was used to determine the migration ability of BSMC in vitro. Our data indicated a significant increase in the migration ability of BSMCs incubated with YKL-40 than the vehicle group at different time points(). It was further supported by Transwell assay that when treated by YKL-40, BMSCs showed enhanced migration ability in a dose-dependent manner ()). Consistently, YKL-40 treatment promoted migration ability in TSMCs ()). Then, we also observed the increased migrating ability in YKL-40-overexpressing BSMCs compared with control cells ()). These data showed that YKL-40 might improve the migration of airway smooth muscle cells, thereby contributing to airway smooth muscle mass formation.
YKL-40 enhances the epithelial-mesenchymal transition of airway epithelial cells
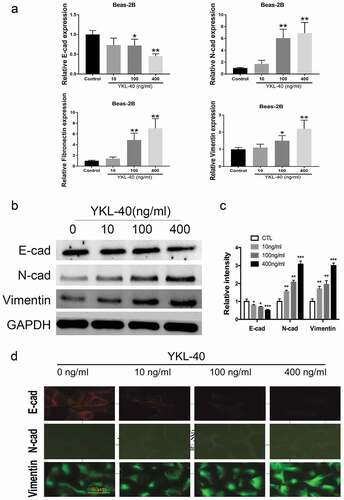
Epithelial-mesenchymal transition (EMT) happens when epithelial cells lose cell polarity and gain migratory properties, which occur in wound healing and organ fibrosis. Accumulating data support that EMT contributes to the formation of sub-epithelial fibrosisin asthma airway remodeling. As indicated by qRT-PCR in ), when Beas2B was treated with recombinant YKL-40, epithelium marker E-cadherin was significantly down-regulated, while mesenchymal markers N-cadherin, fibronectin and vimentin were up-regulated compared with the vehiclecontrol group, which demonstrated that YKL-40 promoted EMT of Beas2B cells in a dose-dependent way.Western blot consistently showed that YKL-40 decreased the expression of E-cadherin and increased N-cadherin and Vimentin, respectively ()). Furthermore, these functions of YKL-40 were reassured by immunofluorescence in Beas2B with weaker fluorescence of E-cadherin and stronger fluorescence of N-cadherin and Vimentin under higher concentration of YKL-40 ()). Taken together, YKL-40 could enhance the EMT of bronchial epithelial cells, which might accelerate airway remodeling.
YKL-40 accelerates the formation of sub-epithelial fibrosis in a murine asthma model
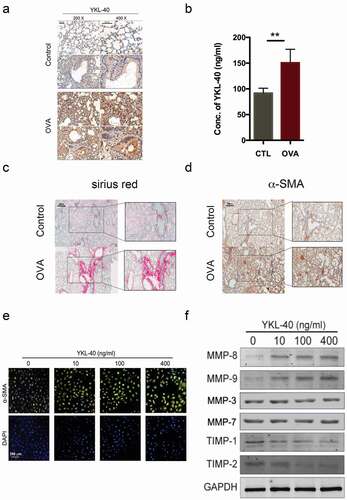
Sub-epithelial fibrosis is a major complication of chronic asthma and reflects the severity of airway remodeling. To examine the function of YKL-40 in vivo, OVA-induced murine asthma model was established according to the standard procedure as indicated previously [Citation28,Citation29]. We observed significantly elevated expression of YKL-40 in lung tissues of OVA-induced asthma mice by immunohistochemistry staining ()). Meanwhile, the serum concentration of YKL-40 significantly increased about two-fold of the control as determined by ELISA ()). Consistently, the extent of sub-epithelial fibrosis marker positively correlated to the expression of YKL-40 in lung tissue and serum, which was confirmed by Sirius red staining ()). The expression of fibrosis marker α-SMA was also increased in lung tissue of OVA-induced asthma model, paralleled with elevated YKL-40 ()). Loss of extracellular matrix (ECM) homeostasisis involved in sub-epithelial fibrosis featured by up-regulated matrix metalloproteinases (MMPs) and down-regulated tissue inhibitor of metalloproteinases (TIMPs). Thus, to further understand how YKL-40 acts on the regulation of ECM, Beas2B and BSMC was treated with recombinant YKL-40 in vitro. As shown in ), recombinant YKL-40 could induce the expression of α-SMA in airway epithelial cell Beas2B. Meanwhile, YKL-40 dose-dependently enhanced the expression of MMP-8, MMP-9 but not MMP-3, MMP-7 and down-regulated TIMP-1, TIMP-2 in BSMC, which contributes to the sub-epithelial fibrosis ()). This evidence supported the speculation that YKL-40 facilitates sub-epithelial fibrosis by regulating the formation of ECM.
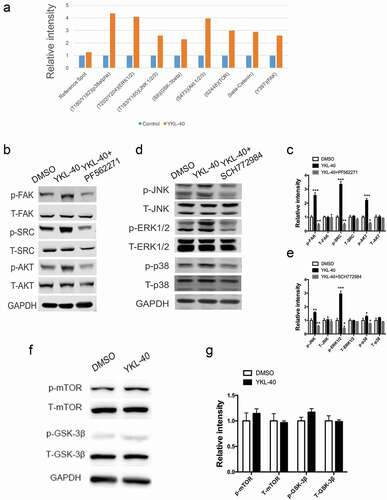
YKL-40 activates FAK/Src/Akt and MAPK pathways in BSMC
To identify the underlying mechanism through which YKL-40 regulated cell cycle progression and airway remodeling, we performed a phosphorylation-screening in BSMCs treated with recombinant YKL-40 and their counterparts. In this screen, we found molecules involved in FAK and MAPK pathway phosphorylation were strongly induced by YKL-40. In the meantime, p-mTOR, p-GSK-3β, and β-catenin were also elevated compared to the control ()). To validate the above findings, we examined the activation of these pathways. As shown by ,d), FAK signaling pathway associated downstream molecules p-SRC and p-AKT were upregulated, along with MAPK signaling pathway proteins p-ERK, p-JNK and p-P38 in YKL-40 treated cells. As well, when FAK inhibitor PF562271 or MAPK inhibitor SCH772984 was administered together with YKL-40 treatment, the activated pathway induced by YKL-40 was blocked ()). However, we didn’t observe notable discrepancy in p-mTOR, p-GSK-3β and β-catenin (data not shown) expression upon YKL-40 or vehicle treatment (,g)), which further demonstrated FAK and MAPK pathways were activated by YKL-40 in BSMCs specifically.
FAK or MAPK inhibitor ameliorates airway remodeling mediated by YKL-40
Since FAK and MAPK pathways were activated when BSMCs were treated with YKL-40. Therefore, we next examined whether FAK and MAPK inhibitors could interrupt the effects of YKL-40 in airway remodeling. Notably, either FAK inhibitor PF562271 or MAPK inhibitor SCH772984 could significantly block the proliferation of BSMC and TSMC induced by YKL-40 administration ()). Meanwhile, migration enhancement caused by YKL-40 was also suppressed as indicated by a decreased number of migrated cells ()). Furthermore, we tested the effect of FAK or MAPK inhibitor on the EMT of Beas-2B. Either FAK or MAPK inhibitor could abolish the increased expression of E-cadherin and downregulation of N-cadherin, Fibronectin and vimentin ()). In the OVA-induced mice model, we have also observed alleviated lung fibrosis by FAK or MAPK inhibitor treatment ()). α-SMA immunostaining also demonstrated that PF562271 or SCH772984 treatment could block YKL-40 induced sub-epithelial fibrosis ()). Taken together, either FAK or MAPK pathway inhibitors could ameliorate YKL-40 caused cell proliferation, migration and subsequent airway remodeling in asthma.
Discussion
Asthma, a common chronic respiratory disease, is characterized by airway inflammation and remodeling [Citation1]. Previous studies indicated that the involvement of airway epithelium, airway smooth muscles, and extracellular matrix components in asthma-related airway remodeling contributed to the development and progression of the disease, leading to several serious respiratory complications. Elevated concentration of serum YKL-40 was not only detected in asthma patients but also correlated with disease severity [Citation17,Citation31]. Notably, a correlation study suggested that YKL-40 might be involved in the development of lung fibrosis and airway remodeling in the process of asthma [Citation22]. YKL-40 is also considered a potential biomarker of inflammation and airway remodeling in severe pediatric asthma [Citation32]. The increased circulating YKL-40 observed in the airways with severe asthma may be owing to the more severe airway remodeling in these patients, which formed a vicious loop in the progressionof asthma [Citation31]. However, current knowledge regarding the underlying mechanism involved in the role of YKL-40 in airway remodeling of asthma is limited.
Our previous study found that YKL-40 increased IL-8 production in bronchial epithelial cells via MAPK (JNK and ERK) and NF-kB pathways, and induced IL-8 resulted in enhanced proliferation and migration of BSMCs [Citation33]. In this study, we showed that YKL-40 could facilitate the proliferation and migration of BSMCs via activation of FAK-Src-Akt and MAPK pathways, thus contribute to the accumulation of airway smooth muscle mass. On the other hand, YKL-40 was previously found related to cancer cell survival, transformation, migration and invasion by regulating PI3K/AKT/mTOR and Ras/Raf/MAPK signaling pathways, during which EMT is one of the pivotal biological process [Citation33–Citation35]. The inhibition of FAK or MAPK pathway could also inhibit enhanced EMT of Beas-2B in vitro and sub-epithelial fibrosis in OVA-induced asthma modelin vivo. Our data provided evidence to support that YKL-40 enhanced EMT of airway epithelial cells via MAPK pathway confirmed by downregulated epithelium marker and upregulated mesenchymal markers.
It is commonly accepted that inflammation plays a crucial role in airway remodeling of asthma. Inflammation-induced over-healing of tissue is another form of fibrosis. It has been shown that YKL-40 played an important role in inflammation driven by T cells and expressed in an exaggerated manner in tissues from patients with asthma [Citation36]. Cytokines induced by inflammation, such as interleukin (IL)-13 could stimulate YKL-40 production in macrophages and epithelial cells. In turn, YKL-40 enhances this response by preventing macrophage apoptosis and inducing alternative (M2) macrophage differentiation [Citation22]. Moreover, the produced Th2 cytokines would induce chemokine and transforming growth factor (TGF)-β1, which enhanced inflammation, tissue fibrosis, and airway remodeling. In this way, YKL-40 may also define the airway remodeling of asthma by interacting with inflammatory cytokines. However, the modeling system employed in our study limited the investigation of the role YKL-40 in cross-talk between epithelial or smooth muscle cells and immune cells. Whether YKL-40 exerts its function via regulating inflammation response in our model needs to be further explored by genetically modified mice.
Early interventions targeting YKL-40 and downstream signaling to prevent remodeling might harbor the hope of prevention of the progression of asthma. The effects of anti-YKL-40 neutralizing antibody in the prevention and treatment of asthma as a new strategy are under investigation. Here, we performed preliminary exploration on classical asthma mice model. Both administration of PF-562271 (FAK/Pyk2 Inhibitor) and SCH772984 (inhibitor of ERK1/2) showed significant amelioration of airway remodeling in OVA-induced asthma mice model. Therefore, our study shed new lights on the development of potential treatment of asthma with activated YKL-40 signaling.
Acknowledgments
Hao Tang is responsible for the conception and designing of this article. Yu Sun and Zhaoquan Shi participated in cell experiments, animal experiment and organizing results. Bing Liu, Xian’gui Li, Ge Li, and Feng Yang implemented animal experiments and statistical results. All authors contributed to approving the final version and are accountable for the accuracy and integrity of the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet. 2018;391:783–800.
- Pakkasela J, Ilmarinen P, Honkamaki J, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020;20:9.
- Khurana S, Jarjour NN. Systematic approach to asthma of varying severity. Clin Chest Med. 2019;40:59–70.
- Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. quiz 63-4.
- British Thoracic S, Scottish Intercollegiate Guidelines N. British guideline on the management of asthma. Thorax. 2014;69(Suppl 1):1–192.
- Siddique AE, Rahman M, Hossain MI, et al. Association between chronic arsenic exposure and the characteristic features of asthma. Chemosphere. 2019;246:125790.
- Mattiuzzi C, Lippi G. Worldwide asthma epidemiology: insights from the global health data exchange database. Int Forum Allergy Rhinol. 2019;10(1):75-80.
- Wark PAB. Contemporary concise review 2019: asthma. Respirology. 2020. DOI:10.1111/resp.13794
- Mishra V, Banga J, Silveyra P. Oxidative stress and cellular pathways of asthma and inflammation: therapeutic strategies and pharmacological targets. Pharmacol Ther. 2018;181:169–182.
- Cardet JC, Busse PJ, Carroll JK, et al. Adherence to adding inhaled corticosteroids to rescue therapy in a pragmatic trial with adults with asthma- a pilot study. Ann Allergy Asthma Immunol. 2020. DOI:10.1016/j.anai.2019.12.027.
- Hassan M, Jo T, Risse P-A, et al. Airway smooth muscle remodeling is a dynamic process in severe long-standing asthma. J Allergy Clin Immunol. 2010;125:1037–45 e3.
- Takeda N, Maghni K, Daigle S, et al. Long-term pathologic consequences of acute irritant-induced asthma. J Allergy Clin Immunol. 2009;124:975–81 e1.
- Holgate ST, Davies DE, Lackie PM, et al. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204.
- Holgate ST, Davies DE, Puddicombe S, et al. Mechanisms of airway epithelial damage: epithelial-mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl. 2003;44:24s–9s.
- Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013;305:L912–33.
- Ostergaard C, Johansen JS, Benfield T, et al. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin Diagn Lab Immunol. 2002;9:598–604.
- Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027.
- Ober C, Tan Z, Sun Y, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691.
- Gomez JL, Yan X, Holm CT, et al. Characterisation of asthma subgroups associated with circulating YKL-40 levels. Eur Respir J. 2017;50: 1700800.
- Shao J, Yang X, Ren D, et al. A genetic variation in CHI3L1 is associated with bronchial asthma. Arch Physiol Biochem. 2019;1–6. DOI:10.1080/13813455.2019.1634737
- Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J. 2009;50:22–30.
- Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501.
- Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2010;2:20–27.
- Shurin MR, Yanamala N, Kisin ER, et al. Graphene oxide attenuates Th2-type immune responses, but augments airway remodeling and hyperresponsiveness in a murine model of asthma. ACS Nano. 2014;8:5585–5599.
- James AJ, Reinius LE, Verhoek M, et al. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193:131–142.
- Usemann J, Frey U, Mack I, et al. CHI3L1 polymorphisms, cord blood YKL-40 levels and later asthma development. BMC Pulm Med. 2016;16:81.
- Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367:551–569.
- Debeuf N, Haspeslagh E, van Helden M, et al. Mouse models of asthma. Curr Protoc Mouse Biol. 2016;6:169–184.
- Hwang YH, Hong SG, Mun SK, et al. The protective effects of astaxanthin on the OVA-induced asthma mice model. Molecules. 2017;22. DOI:10.3390/molecules22112019
- Blain SW. Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle. 2008;7:892–898.
- Konradsen JR, James A, Nordlund B, et al. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol. 2013;132:328–35 e5.
- Guerra S, Melen E, Sunyer J, et al. Genetic and epigenetic regulation of YKL-40 in childhood. J Allergy Clin Immunol. 2018;141:1105–1114.
- Tang H, Sun Y, Shi Z, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190:438–446.
- El-Galaly TC, Bilgrau AE, Gaarsdal E, et al. Circulating tumor necrosis factor-alpha and YKL-40 level is associated with remission status following salvage therapy in relapsed non-Hodgkin lymphoma. Leuk Lymphoma. 2015;56:2476–2478.
- Hao H, Wang L, Chen H, et al. YKL-40 promotes the migration and invasion of prostate cancer cells by regulating epithelial mesenchymal transition. Am J Transl Res. 2017;9:3749–3757.
- Zhu Z, Zheng T, Homer RJ, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682.