ABSTRACT
Ring1 and Yin Yang 1-Binding Protein (RYBP) is a member of non-canonical polycomb repressive complex 1 to mediate monoubiquitination of histone H2A at lysine 119. It plays an important role in development, but its role in reproduction remains illusive. In this study, we used Rybp conditional knockout mouse model to genetically ablate Rybp in male germ cells. We found that Rybp deficiency during spermatogenesis led to smaller testes, loss of germline cells, disturbed meiosis, increased apoptosis of spermatocytes, decreased sperm motility, and reduced global H3K9me3, without impacting retrotransposon expression. Meanwhile, we depleted Rybp during oogenesis, but oocyte maturation and preimplantation development were normal. Our findings demonstrate that RYBP plays important roles in spermatogenesis through regulating meiosis and sperm motility.
KEYWORDS:
Introduction
Spermatogenesis is a complicated and precise process with a series of global epigenetic reprogramming events [Citation1,Citation2], and histone modifications as well as their writers and readers play important roles in this procedure. Monoubiquitination of Histone H2A lysine 119 (uH2AK119) is mainly catalyzed by Polycomb repressive complex 1 (PRC1) and is linked to gene silencing [Citation3]. uH2AK119 has been shown to play important roles in different stages during spermatogenesis. uH2AK119 is enriched in sex body in pachytene spermatocytes, mediating transcriptional repression of genes linked to unsynapsed axes of the sex chromosomes [Citation4,Citation5]. Loss of uH2AK119 in spermatocytes results in failed gene silencing followed by developmental arrest. Intensive uH2AK119 signal was also identified in postmeiotic elongating spermatids to facilitate nucleosome destabilization during histone-to-protamine transition [Citation6,Citation7]. Loss of uH2AK119 in spermatids results in retained histones in spermatozoa followed by disrupted sperm motility and fertilization.
PRC1 shows high heterogeneity in mammals [Citation8]. Canonical PRC1 consists of four core PcG components, a CBX family factor (CBX2, CBX4, CBX6, CBX7, CBX8), a PCGF family factor (PCGF1–PCGF6), a RING1 family factor (RING1a and RING1b) and an HPH family factor (HPH1–HPH3), while non-canonical PRC1 contains RYBP [Citation9] or its homolog YAF2 [Citation10]. RYBP plays indispensable roles in organic development, apoptosis and diseases [Citation11]. In mice, Rybp homozygous null embryos showed embryonic lethality at postimplantation stage, while a subset of heterozygous embryos did not survive beyond birth [Citation12]. PRC1 complex containing RYBP [Citation13] behaves differently from canonical PRC1. Typically, chromatin regions with H3K27me3 modification are catalyzed by the PRC2 complex, followed by PRC1 recognition for uH2AK119, resulting in chromatin compaction and gene repression. In contrast, RYBP-PRC1 complex mediates uH2AK119 in a PRC2-independent manner [Citation14]. Depletion of Rybp gene barely reduces the genome-wide uH2AK119 level, while RYBP protein may affect the enzymatic activity but not localization of PRC1 on chromatin [Citation10,Citation15]. Intriguingly, a significant number of RYBP binding sites do not colocalize with uH2AK119 sites, indicating that RYBP has functions independent of PRC1 [Citation15]. In support of this, further studies found that RYBP recruits OCT4 to Kdm2b promoter and regulates reactivation of endogenous pluripotent gene expression during reprogramming [Citation16]. Additionally, RYBP inhibits DNA double-strand break (DSB) repair in a PRC1-independent manner [Citation17]. Notably, RYBP represses germline-specific genes and endogenous retroviruses in mouse embryonic stem cells (ESCs), indicating that Rybp gene may play important roles in gametogenesis and early embryogenesis [Citation15,Citation18].
In this study, we aim to unveil the regulatory roles of Rybp in mouse reproduction using genetic modified mouse models. We found that Rybp conditional knockout (cKO) mice had smaller testes, apoptosis of leptotene to zygotene spermatocytes, less germ cells per tubule, and reduced sperm motility, indicating that RYBP plays important roles during mammalian spermatogenesis.
Materials and methods
Ethics statement
All the animal procedures were approved by the Institutional Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology. Mice were housed in the specific pathogen-free facility of Huazhong University of Science and Technology. All experiments with mice were conducted ethically according to the Guide for the Care and Use of Laboratory Animal guidelines.
Mice
Floxed Rybp mice were generated through mouse ESC targeting to insert LoxP sites in intron 2 and intron 5 at Rybp gene locus, followed by blastocyst injection for chimera formation and germline transmission. Stra8-Cre and ZP3-Cre transgenic mice were obtained from Jackson Laboratory. All mice were maintained on C57BL/6 J background.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from testes using TRIzol reagent (Invitrogen) following the manufacturer’s procedure. The purity and concentration of RNA samples were determined with Nanodrop ND-2000 spectrophotometer. Reverse transcriptional reaction was performed using Hifair 1st Strand cDNA Synthesis Kit according to the manufacturer’s procedure. qRT-PCR was performed with SYBR green master mix (Yeason) on LightCycler@96 Real-Time PCR system (Roche) according to manufacturers’ instructions. The relative gene expression was quantified using the comparative cycle threshold method, with Gapdh gene expression for normalization. For primer sequences, see Table S1.
Histological analysis
Mouse testes were collected and fixed in Bouin’s solution overnight at room temperature and then washed with 75% alcohol. Samples were then embedded in paraffin, 5 μm sections were cut and stained with periodic acid-Schiff (PAS).
Meiotic chromosome spreads for immunofluorescence
Meiotic chromosome spreads were prepared as described before [Citation19]. Generally, testes were collected, de-capsulated into 100 mM sucrose and chopped/pipetted to release germ cells. Cells were added to slides coated with 1% paraformaldehyde and dried in a humidified chamber. Slides were then washed with 0.4% Photo-Flo 200 Solution, dried and stored at −20°C. For immunofluorescence, meiotic spreads were blocked (30 min, room temperature) and incubated with primary antibody overnight, followed by incubation (2 h, room temperature) with Alexa Fluor secondary antibody.
Preparation of testicular frozen sections
Testes were fixed in 4% paraformaldehyde for 16 h at 4°C and washed with PBS. Samples were then sequentially soaked in 15% and 30% sucrose in PBS at 4°C for 16 h and embedded in Tissue-Tek O.C.T. 5μm thick cryosections were cut using CryoStar NX50 Cryostat (Thermo Scientific) and stored at −20°C.
TUNEL staining
TUNEL staining was performed using TUNEL Apoptosis Detection Kit (Yeason) according to the manufacturer’s procedure. Briefly, cryosections were washed by PBS and digested with Proteinase K, then blocked with equilibration buffer and incubated with the TUNEL reaction Mixture for 1 h at 37°C. DNA was stained with DAPI.
Immunofluorescence
Frozen testicular sections were washed with PBS and permeabilized (PBS, 0.5% TritonX-100, 20 min, room temperature). Slides were blocked (30 min, room temperature) with SuperBlock Blocking Buffer (Thermo Fisher Scientific) containing 0.05% Tween-20. Slides were then incubated with primary antibody (diluted 1:50–100, SuperBlock Blocking Buffer, 0.05% Tween-20, overnight, room temperature) followed by incubation (2 hr, room temperature) with an Alexa Fluor secondary antibody. DNA was stained with DAPI.
Superovulation and embryo collection
Adult female mice (age 6–8 wk) were injected with 10 IU of PMSG followed by 10 IU of hCG 48 h later to promote ovulation. Mice were then caged with males overnight. Successful mating was confirmed by the presence of vaginal plugs. Zygotes were collected from oviducts and cultured in Quinn´s Advantage Cleavage Medium (SAGE, ART-1026) at 37°C and in 5% CO2 for preimplantation development.
Antibodies
All antibodies are commercially available. Anti-RYBP antibody: Abcam, ab185971. Anti-SYCP3 antibody: Abcam, ab15093. Anti-γH2A.X antibody: Millipore, 05–636. Anti-uH2AK119 antibody: Cell Signaling Technology, 8240 S. Anti-H3K4me3 antibody: Abcam, ab8580. Anti-H3K9me3 antibody: ab8898. Anti-H3K27me3 antibody: Abcam, ab6002. Anti-H3K56ac antibody: Abcam, ab71956. Anti-H4K16ac antibody: Cell Signaling Technology, 13534.
RNA-sequencing analysis
Raw reads were processed with cutadapt v1.16 to remove adapters and perform quality trimming with default parameters except for quality-cutoff 20, minimum-length 20. Trimmed reads were mapped to the mouse genome, using STAR with default settings. Reads were counted in exons of the mouse genome, using the STAR-quantMode GeneCounts setting. Cufflinks was used to calculate FPKM value. Reported RNA-seq results for processing include GSE43717 and GSE22182.
Statistical analysis
The two-tailed student’s t-test was used to calculate p values. Statistically significant values for p < 0.05, p < 0.01, p < 0.001 and p < 0.0001 are indicated by single, double, triple and quadruple asterisk, respectively.
Results and discussion
Rybp is widely expressed during gametogenesis and early embryogenesis
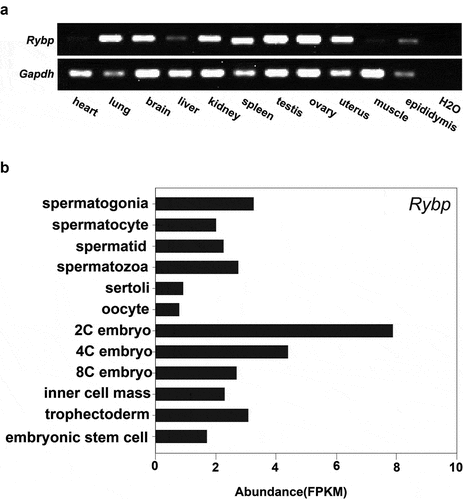
We first detected the expression level of Rybp in different tissues by RT-PCR and found that Rybp is ubiquitously expressed and is highly enriched in testis and ovary (). To elucidate a possible role of Rybp in mouse reproduction, we examined its expression in testicular cells [Citation20], oocytes and preimplantation embryos [Citation21] in mice by public RNA-seq data analysis (). The result shows that in mouse testis, Rybp mainly exists in male germ cells and is expressed from spermatogonia to spermatozoa. However, Rybp is less expressed in mouse oocytes and is enriched throughout preimplantation stages.
Rybp deficiency disturbs spermatogenesis
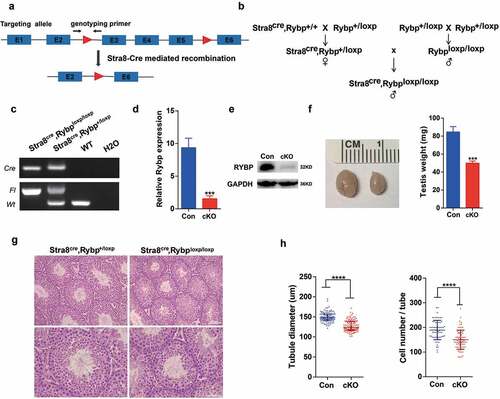
It was previously reported that Rybp homozygous null embryos exhibited lethality at early postimplantation stage [Citation12]. Therefore, we generated the cKO mouse model of Rybp through inserting LoxP sequence in intron 2 and intron 5 of Rybp locus.
To study functions of Rybp in spermatogenesis, we generated male germline-specific knockout mouse model (Rybpfl/fl; Stra8-Cre) by crossing Rybpfl/+ mice () with Stra8-Cre transgenic mice in which Cre recombinase is expressed from differentiated spermatogonia [Citation22] to delete exon 3–5 of Rybp gene (). Successful breeding of conditional knockout Rybp mouse model was confirmed by genotyping analysis (). Both mRNA () and protein () levels of Rybp in cKO testes were significantly decreased compared with that of control, indicating that Rybp was depleted in testes with high efficiency. We found that testes from adult Rybp cKO mice were significantly smaller than control, suggesting that spermatogenesis was disturbed in the absence of Rybp (). Histological analysis showed that structure of seminiferous tubules from cKO mice looked normal and all stages of seminiferous tubules were present (). However, seminiferous tubules of testes from cKO mice exhibited reduced diameter and contained lower cell numbers per tubule ().
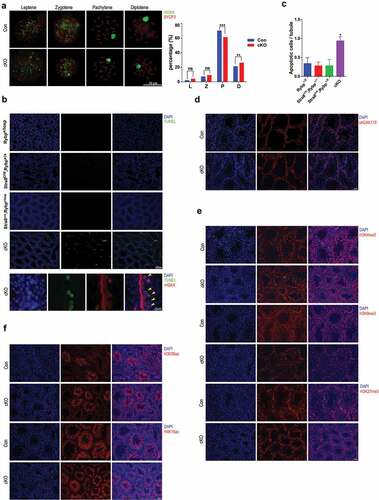
Then, we asked whether meiosis was affected when Rybp was deficient. We therefore performed immunostaining of meiotic chromosome spread of spermatocytes from control and cKO testes. Interestingly, we noticed that although different stages of meiosis could be observed without obvious morphological alterations, Rybp cKO testes had a smaller portion of pachytene spermatocytes and a larger portion of diplotene spermatocytes (). TUNEL assay (, upper panel) indicated that apoptotic cells in Rybp cKO testes were significantly increased (). Further, co-immunostaining with anti-γH2AX antibody identified co-localization of TUNEL-positive cells with leptotene to zygotene spermatocytes [Citation23], indicating apoptosis of a part of leptotene to zygotene spermatocytes (, lower panel). Moreover, we noticed that knockout of Rybp barely affects the global uH2AK119 level (). In agreement with our study, a previous report showed that global uH2AK119 was just slightly downregulated upon knockout of Rybp in mouse ESCs [Citation10]. This may be explained by that Rybp-containing non-canonical PRC1 complex only mediates a limited fraction of uH2AK119-modified chromatin, in contrast to canonical PRC1. Moreover, RYBP and its homolog YAF2, and probably other factors, may play redundant roles on uH2AK119 modification.
We also performed immunostaining of H3K4me3, H3K9me3 and H3K27me3 on testicular frozen sections (), and found that H3K9me3 level was significantly downregulated when Rybp was absent. Specifically, H3K9me3 signal was evenly distributed in control germ cells, but exhibited punctate patterns in cKO germ cells. Additionally, H3K56ac and H4K16ac were examined and no significant changes were identified between control and cKO testicular sections (). These results indicate that RYBP-mediated uH2AK119 has specific crosstalk with global H3K9me3.
RYBP was previously reported to repress endogenous retroviruses and cleavage-stage genes in mouse ESCs [Citation18]. Therefore, we performed qRT-PCR to examine the expression of retrotransposons (), cleavage-stage genes () and pachytene piRNA precursors () in control and cKO testes. However, we were unable to detect significant expression changes of these genes.
Figure 4. Rybp deficiency during spermatogenesis does not influence expression of retrotransposons or piRNA precursors.
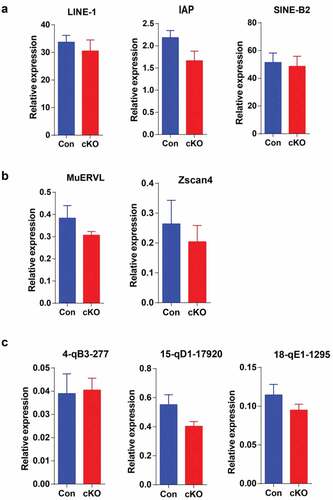
Taken together, our results show that Rybp regulates meiosis and histone modification. The phenotype that spermatogenesis was not completely blocked in absence of Rybp may be explained by functional redundancy of Rybp homolog Yaf2 [Citation24], and other PRC1 members like L3mbtl2 [Citation25], which is also enriched in testes.
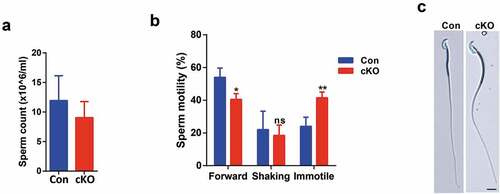
Deletion of Rybp reduces sperm motility
Next, we asked whether sperm count or sperm motility was influenced in the absence of Rybp. Despite of having smaller testes, Rybp cKO mice contained only slightly lower sperm count in cauda epididymis (). Notably, Rybp cKO mice showed a significant increase of immotile sperm (). Further examination showed that sperm morphology of Rybp cKO mice was normal (). A 3-to-4-month fertility test of six male Rybp cKO mice showed that they were absolutely infertile. Thus, we wondered whether sperm from Rybp cKO testes could be fertilized and further develop until blastocyst stage. However, we found that these sperms were able to fertilize oocytes in vivo and most of fertilized eggs (P-cKO) developed into blastocyst stage in vitro, indicating that developmental arrest happened post implantation (Figure S1 C). A previous study has shown that a significant number of heterozygous null embryos of Rybp cannot survive beyond birth [Citation12]. We proposed that sperm from Rybp cKO mice may contain abnormal epigenetic modifications which were transferred to fertilized embryos and worsened developmental potency of Rybp null heterozygotic embryos so that the embryos derived from Rybp cKO sperm failed to survive beyond birth.
Figure 5. Deletion of Rybp reduced sperm motility.
Sperm count of adult control and cKO mice. Data are presented as means ±SD, n = 3. Student’s t-test was used.
Sperm motility of adult control and cKO mice. Forward, forward motility. Shaking, shaking in place. Data are presented as means ± SE, n = 2. *P < 0.05. **P < 0.01. Student’s t-test was used.
Sperm morphology of adult control and cKO mice. Scale bar, 10 μm.
Rybp is dispensable for oocyte maturation and preimplantation development
PRC1 plays pivotal roles in embryogenesis, including Xist RNA mediated X chromosome inactivation [Citation26,Citation27], uterine decidualization [Citation28] and early lineage specification [Citation29]. To study functions of Rybp in oocyte maturation and preimplantation development, we generated female germline-specific knockout mice by crossing Rybpfl/+ mice with ZP3-Cre transgenic mice in which Cre recombinase is expressed from primary oocytes [Citation30]. Ovary morphology looked normal after Rybp deletion (Figure S1A), and Rybp depleted oocytes (M-cKO) could be fertilized by WT sperm in vivo and develop to blastocyst stage in vitro (Figure S1B). Furthermore, zygotes produced from paternal (Rybpfl/fl; Stra8-Cre) and maternal (Rybpfl/fl; ZP3-Cre) cKO (D-cKO) mice were also able to develop to blastocyst stage (Figure S1 C). Therefore, maternal and embryonic depletion of Rybp have no impact on preimplantation development. The reason why only male reproductive system produces phenotype may be explained by that spermatogenesis is a highly complex spatial-temporal process with orchestration of gene expression in a very short time window.
Taken together, we used mouse genetics to identify that Rybp gene plays important roles in spermatogenesis, but not oocyte maturation or preimplantation development. Generally, our results show that the ablation of Rybp in male germ cells impacts H3K9me3 level and leads to abnormal spermatogenesis through disturbing meiosis and sperm motility. We also noticed that embryos generated from cKO male and control female have disrupted post-implantation development, possibly because of abnormal epigenetic modifications of sperm chromatin such as H3K9me3.
Author contributions
Li-quan Zhou and Ying Yin conceived and designed the project. Qing Tian, Shi-meng Guo and Shi-ming Xie performed the experiment and analyzed the data. Qing Tian and Shi-meng Guo wrote the initial manuscript. Li-quan Zhou revised the manuscript. All authors have read and approved the final manuscript.
Supplemental Material
Download MS Word (158.1 KB)Acknowledgments
This work was supported by National Key R&D Program of China [2018YFC1004502, 2018YFC1004001], the National Natural Science Foundation of China [NSFC 31771661], the Foundation of NHC Key Laboratory of Male Reproduction and Genetics [KF201804], and the Fundamental Research Funds for the Central Universities [2019kfyXKJC074].
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Atlasi Y, Stunnenberg HG. The interplay of epigenetic marks during stem cell differentiation and development. Nat Rev Genet. 2017;18:643–658.
- Larose H, Shami AN, Abbott H, et al. Gametogenesis: a journey from inception to conception. Curr Top Dev Biol. 2019;132:257–310.
- Wang H, Wang L, Erdjument-Bromage H, et al. Role of histone H2A ubiquitination in polycomb silencing. Nature. 2004;431:873–878.
- An JY, Kim EA, Jiang Y, et al. UBR2 mediates transcriptional silencing during spermatogenesis via histone ubiquitination. Proc Natl Acad Sci U S A. 2010;107:1912–1917.
- Hasegawa K, Sin HS, Maezawa S, et al. SCML2 establishes the male germline epigenome through regulation of histone H2A ubiquitination. Dev Cell. 2015;32:574–588.
- Lu LY, Wu J, Ye L, et al. RNF8-dependent histone modifications regulate nucleosome removal during spermatogenesis. Dev Cell. 2010;18:371–384.
- Wang X, Kang JY, Wei L, et al. PHF7 is a novel histone H2A E3 ligase prior to histone-to-protamine exchange during spermiogenesis. Development. 2019;146:dev175547.
- Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol. 2013;20:1147–1155.
- Simoes da Silva CJ, Simon R, Busturia A. Epigenetic and non-epigenetic functions of the RYBP protein in development and disease. Mech Ageing Dev. 2018;174:111–120.
- Rose NR, King HW, Blackledge NP, et al. RYBP stimulates PRC1 to shape chromatin-based communication between Polycomb repressive complexes. eLife. 2016;5:e18591.
- Zhan S, Wang T, Ge W, et al. Multiple roles of Ring 1 and YY1 binding protein in physiology and disease. J Cell Mol Med. 2018;22:2046–2054.
- Pirity MK, Locker J, Schreiber-Agus N. Rybp/DEDAF is required for early postimplantation and for central nervous system development. Mol Cell Biol. 2005;25:7193–7202.
- Garcia E, Marcos-Gutierrez C, Del Mar Lorente M, et al. RYBP, a new repressor protein that interacts with components of the mammalian polycomb complex, and with the transcription factor YY1. Embo J. 1999;18:3404–3418.
- Tavares L, Dimitrova E, Oxley D, et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell. 2012;148:664–678.
- Morey L, Aloia L, Cozzuto L, et al. RYBP and Cbx7 define specific biological functions of polycomb complexes in mouse embryonic stem cells. Cell Rep. 2013;3:60–69.
- Li H, Lai P, Jia J, et al. RNA helicase DDX5 inhibits reprogramming to pluripotency by miRNA-based repression of RYBP and its PRC1-dependent and -independent functions. Cell Stem Cell. 2017;20:462–77 e6.
- Ali MAM, Strickfaden H, Lee BL, et al. RYBP is a K63-ubiquitin-chain-binding protein that inhibits homologous recombination repair. Cell Rep. 2018;22:383–395.
- Hisada K, Sanchez C, Endo TA, et al. RYBP represses endogenous retroviruses and preimplantation- and germ line-specific genes in mouse embryonic stem cells. Mol Cell Biol. 2012;32:1139–1149.
- Zhou L, Canagarajah B, Zhao Y, et al. BTBD18 regulates a subset of piRNA-generating loci through transcription elongation in mice. Dev Cell. 2017;40:453–66 e5.
- Soumillon M, Necsulea A, Weier M, et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep. 2013;3:2179–2190.
- Tang F, Barbacioru C, Nordman E, et al. Deterministic and stochastic allele specific gene expression in single mouse blastomeres. PloS One. 2011;6:e21208.
- Sadate-Ngatchou PI, Payne CJ, Dearth AT, et al. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis. 2008;46:738–742.
- Blanco-Rodriguez J. gammaH2AX marks the main events of the spermatogenic process. Microsc Res Tech. 2009;72:823–832.
- Kalenik JL, Chen D, Bradley ME, et al. Yeast two-hybrid cloning of a novel zinc finger protein that interacts with the multifunctional transcription factor YY1. Nucleic Acids Res. 1997;25:843–849.
- Trojer P, Cao AR, Gao Z, et al. L3MBTL2 protein acts in concert with PcG protein-mediated monoubiquitination of H2A to establish a repressive chromatin structure. Mol Cell. 2011;42:438–450.
- Schoeftner S, Sengupta AK, Kubicek S, et al. Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing. Embo J. 2006;25:3110–3122.
- Almeida M, Pintacuda G, Masui O, et al. PCGF3/5-PRC1 initiates polycomb recruitment in X chromosome inactivation. Science. 2017;356:1081–1084.
- Bian F, Gao F, Kartashov AV, et al. Polycomb repressive complex 1 controls uterine decidualization. Sci Rep. 2016;6:26061.
- Voncken JW, Roelen BA, Roefs M, et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci U S A. 2003;100:2468–2473.
- Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151.