ABSTRACT
In mammals, male gonocytes are derived from primordial germ cells during embryogenesis, enter a period of mitotic proliferation, and then become quiescent until birth. After birth, the gonocytes proliferate and migrate from the center of testicular cord toward the basement membrane to form the pool of spermatogonial stem cells (SSCs) and establish the SSC niche architecture. However, the molecular mechanisms underlying gonocyte proliferation, migration and differentiation are largely unknown. Cyclin A2 is a key component of the cell cycle and required for cell proliferation. Here, we show that cyclin A2 is required in mouse male gonocyte development and the establishment of spermatogenesis in the neonatal testis. Loss of cyclin A2 function in embryonic gonocytes by targeted gene disruption affected the regulation of the male gonocytes to SSC transition, resulting in the disruption of SSC pool formation, imbalance between SSC self-renewal and differentiation, and severely abnormal spermatogenesis in the adult testis.
Introduction
There are two mammalian A-type cyclins, both of which have been shown to be essential for certain aspects of cell cycle progression. Cyclin A2 (Ccna2) is a core component of the mitotic cell cycle and impacts cell proliferation by promoting both G1/S and G2/M transition [Citation1,Citation2]. Ccna2 is widely expressed and has been shown to be essential for development as the homozygous null mutation in the mouse model resulted in early embryonic lethality [Citation3]. In contrast, mouse cyclin A1 (Ccna1) is most abundantly, if not exclusively, expressed in the testis, specifically in late pachytene to diplotene spermatocytes [Citation4], and loss of cyclin A1 function results in an arrest of spermatogenesis in late meiotic prophase and complete sterility [Citation5]. Cyclin A2 is also expressed in the testis, but in a strikingly different pattern of expression, specifically in spermatogonia [Citation4,Citation6,Citation7]. However, whether Ccna2 is also essential for male germ cell differentiation during spermatogenesis remained to be determined.
To overcome the embryonic lethality so as to begin to understand the role of cyclin A2 in cell cycle regulation in specific cellular lineages in various embryonic and adult tissues, we developed a floxed Ccna2 mouse line which could then be mated with lineage-specific Cre carrying mouse strains to generate lineage-specific conditional knockouts [Citation8]. Interestingly, we showed that cyclin A2 was essential in both hematopoietic stem cells (HSCs) and embryonic stem cells (ESCs), but not in more differentiated mouse embryonic fibroblasts [Citation8]. We have recently discovered that Ccna2 is also essential in spermatogonial stem cells (SSCs) (in preparation), but whether this requirement may actually be manifested even earlier, in the precursors of these SSCs, the gonocytes, is not known.
Gonocytes are formed after the proliferation and homing of primordial germ cells (PGCs) to the embryonic gonads [Citation9]. A developmental sexual dimorphism then occurs, in which female gonocytes differentiate into oogonia and then shortly thereafter enter the process of meiosis [Citation9]. In contrast, male gonocytes (also referred to as pro-spermatogonia or pre-spermatogonia) continue to proliferate until embryonic day 15.5 (E15.5), at which point they become quiescent and remain in the G1/G0 phase of the cell cycle until birth. In the early neonatal period, from postnatal day (pnd) 0.5 to 4.5, male gonocytes undergo phases of proliferation, migration, and differentiation, which occur in a highly regulated manner [Citation10,Citation11]. Curiously, although some early gonocytes appear to undergo proliferation, their absolute number does not change, indicating that this is not a productive mitosis but rather one that leads to apoptosis [Citation12,Citation13].
Starting at pnd 3, male gonocytes extend their cytoplasmic processes to the basement membrane and move from the central region to the basement membrane of the seminiferous tubules [Citation10,Citation12]. Upon reaching the basement membrane, they develop into SSCs and give rise to the first wave of spermatogenesis [Citation10,Citation14]. Maintenance of male germ cell production throughout the lifetime of an individual involves establishing a tightly controlled balance between SSC self-renewal and differentiation [Citation15].
In the present study, we showed that cyclin A2 was expressed in mouse male gonocytes starting from pnd 1.5, and that its subcellular localization was nuclear. To investigate the role of cyclin A2 during male gonocyte proliferation, migration, and transformation from gonocytes to SSCs, we generated mice in which there was a conditional loss of cyclin A2 in embryonic gonocytes by crossing mice carrying a floxed Ccna2 allele with those with a Vasa-Cre allele. Loss of cyclin A2 function in gonocytes yielded a normal number of viable progeny but a virtually complete absence of germ cells in seminiferous tubules in the adult testes of these animals. The loss of male germ cells was seen at pnd 7.5 and indeed the number of germ cells was significantly decreased even earlier, at pnd 2.5. We conclude that cyclin A2 is essential in male mouse gonocyte differentiation and hence, essential for the establishment of SSCs and the first wave of spermatogenesis.
Materials and methods
Animals
All mice were housed in a pathogen-free facility, and manipulations were performed in strict adherence to IACUC guidelines. The generation of the conditional cyclin A2 “floxed” allele has been described previously [Citation8]. Vasa-Cre male mice [Citation16] were obtained from The Jackson Labs (FVB-Tg(Ddx4-cre)1Dcas/J; catalog # 006954).
Tissue preparation and histology
Testes of mice at selected ages were dissected and fixed in 4% paraformaldehyde overnight at 4°C. Fixed tissues were dehydrated, embedded in paraffin, sectioned at 5 μm-thick, and mounted on slides. For initial morphological examination, the sections were stained with hematoxylin and eosin according to our standard procedures [Citation17].
Antibodies used
Rabbit anti-cyclin A2 antibody (Santa Cruz 596), 1:100 dilution; rabbit anti-Foxo1 (Cell Signaling # 2880), 1:100 dilution; rabbit anti-Vasa (Abcam13840), 1:500 dilution; mouse anti-Gata4 (Santa Cruz #25,310), 1:100 dilution; Alexa 594-conjugated donkey anti-mouse IgG (H + L) (Jackson Immuno Research, #715-545-150) and Alexa 488-conjugated donkey anti-rabbit IgG (H + L) (Jackson Immuno Research, #711-545-152), 1:200 dilution.
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed using a Vectastain ABC kit for Rabbit IgG (# PK-6101, Vector Laboratories, Burlingame, CA) as described [Citation5]. In brief, histological sections were de-paraffinized in histoclear, hydrated through an alcohol series followed by a washing with distilled water. Antigen retrieval was performed by boiling the slides in a microwave for 10 min in the 0.01 M-citrate buffer, pH 6.0. Endogenous peroxidase was inactivated by incubating the slides with 3% hydrogen peroxide for 30 min and then incubated with blocking solution (2.5% goat serum) for 1 hr at room temperature. The sections were then incubated with rabbit-anti cyclin A2 antibody at 4°C overnight. The sections were washed 3 times, 5 minutes each, in TBST (TBS with 0.1% TritonX-100), and then followed the protocol for Vectastain ABC kit. For visualization, the slides were incubated with diaminobenzidine solution for 5 min, washed 3 times with distilled water and counterstained with hematoxylin. The sections were dehydrated through an alcohol series and mounted with permount mounting medium (Fisher Scientific # SP15-100). Slides were examined on a Nikon microscope under brightfield optics and the images were recorded with a digital camera.
For immunofluorescent staining, after antigen retrieval, slides were first incubated with blocking solution, then primary antibody at 4°C overnight. After rinsing in PBST, the slides were incubated with appropriate secondary antibodies. Slides were counter-stained with DAPI and mounted with Prolong Gold (Thermo Fisher Scientific, # P36930). Slides were examined using a Nikon Eclipse E800 microscope equipped with epifluorescence optics and the images were photographed with a high-definition cooled color camera head DS-Fi1 c.
Statistical analysis
The quantification is presented as mean ± standard error of mean (SEM). A statistical t-test was performed using a nonparametric Mann-Whitney signed-rank test to determine the difference between the genotypes, and the threshold of significance was set at 0.05 (GraphPad Prism 5, GraphPad Software, Inc.)
Results
Expression of cyclin A2 in postnatal gonocytes
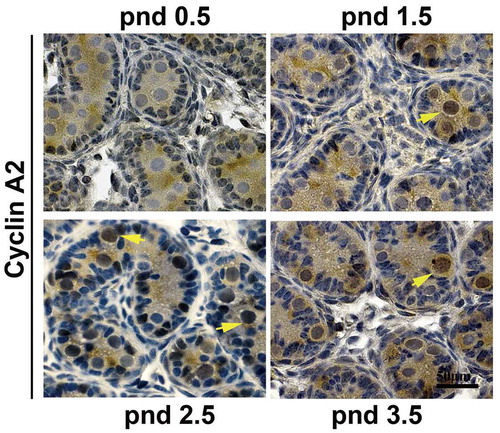
To determine whether cyclin A2 is involved in the development of gonocytes, we first needed to establish that cyclin A2 is expressed in the gonocytes. In the seminiferous tubules of pnd 0.5–3.5 mouse testes, gonocytes can be distinguished from Sertoli cells by their nuclear morphology and localization [Citation13]. We had previously shown by immunohistochemical analysis that cyclin A2 can be detected in male germ cells as early as pnd 4.5 (in preparation) and also in gonocytes in embryonic day 10.5–15.5 (E10.5-E15.5) male gonads [Citation7]. Cyclin A2-positive cells are infrequently detected at E10.5, but are readily detectable at E13.5, and then are scarce by E15.5, likely reflecting their entry into a quiescent state [Citation7], but its expression in the intervening developmental stages had not been determined. Therefore, we performed immunostaining on pnd 0.5, 1.5, 2.5 and 3.5 mouse testes with anti-cyclin A2 antibody and found that cyclin A2 was first expressed in pnd 1.5 gonocytes but not in pnd 0.5 gonocytes () and continued to be detected in both pnd 2.5 and 3.5. The staining appeared to be predominantly nuclear with only diffuse, nonspecific staining in the cytoplasm of the gonocytes which fill the lumen of the tubules.
Figure 1. Cyclin A2 protein is expressed in mouse gonocytes. Immunohistochemistry with anti-cyclin A2 antibodies at pnd 0.5, pnd 1.5, pnd 2.5, and pnd 3.5 testes showed that gonocytes express cyclin A2 from pnd 1.5 but not at pnd 0.5; the yellow arrowheads in pnd 1.5, 2.5, and 3.5 indicate cyclin A2 positive gonocytes. Bar indicates 50 μm.
Targeted deletion of cyclin A2 from embryonic gonocytes results in severely disrupted spermatogenesis in adult testes
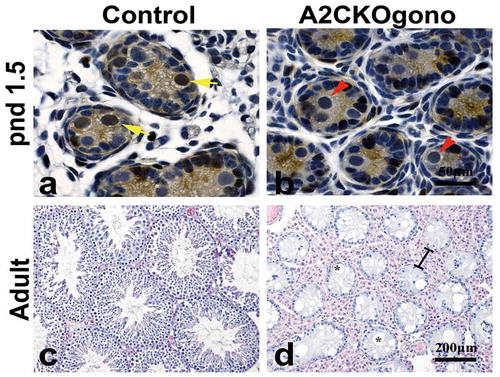
As mentioned previously, deletion of the mouse cyclin A2 gene (Ccna2) resulted in early embryonic lethality [Citation3]. To study the function of cyclin A2 in gonocytes, we generated a conditional knockout strain using a germ-line specific Cre-driver, Vasa-Cre (also known as Ddx4-Cre) [Citation16]. The endogenous Vasa gene is specifically expressed in the germ cells of both male and female mice beginning at E12.5 and continues throughout life [Citation16]. In the Vasa-Cre strain we used, Cre-mediated recombination can be detected in embryonic gonads on E15 and the deletion is 95% complete at birth [Citation16]. To excise floxed Ccna2 alleles (Ccna2fl/fl), resulting in the deletion of exon 1 to exon 8 and functionally null Ccna2 [Citation8] only in gonocytes, we crossed male Vasa-Cre carrying mice with female Ccna2fl/fl mice to generate Vasa-Cre; Ccna2fl/+ male mice. These males were then crossed with female Ccna2fl/fl mice to obtain the Vasa-Cre;Ccna2fl/fl (hereafter referred to as A2CKOgono). Immunostaining with anti-cyclin A2 antibody showed that cyclin A2 was no longer expressed in gonocytes in pnd 1.5 testes (,b)) or later (data not shown). Histological examination of adult control and A2CKOgono testes revealed that the seminiferous tubules in the testes of normal littermates contained, as expected, different layers of germ cells at different stages of spermatogenesis ()). In striking contrast, all the seminiferous tubules in A2CKOgono testes were virtually devoid of germ cells and the morphology of the seminiferous tubules was extremely abnormal, with severe disruption of the tubule architecture, vacuolation of the tubules, and hypertrophy of the interstitial tissue ()). It should be noted that the Vasa-Cre is expressed in embryonic germ cells in female gonads as well and we have previously shown expression of cyclin A2 in oogonia [Citation18]. However, female cyclin A2-deficient ovaries contained follicles with oocytes and the females were fertile.
Figure 2. Loss of cyclin A2 expression in gonocytes results in severely abnormal spermatogenesis and few if any germ cells in the adult testis. (a,b) Testicular sections from control and A2CKOgono at pnd 1.5 were stained with anti-cyclin A2 antibody using immunohistochemistry. The yellow arrows indicate cyclin A2 positive germ cells and red arrows indicate the cyclin A2 negative germ cells. Bar indicates 50 μm. (c,d) H&E staining in adult mouse testes from control (c) and A2CKOgono (d). The black lines indicate the hypertrophy of the interstitial tissue. Bar indicates 200 μm. The asterisks indicate the vacuolation of the tubules.
The germ cell depletion phenotype occurs in the initial wave of spermatogenesis
To investigate the time of onset of the phenotype of spermatogenic failure in A2CKOgono mouse testes, we examined the testicular morphology at four progessively earlier timepoints during the first wave of spermatogenesis, i.e. pnd 7.5, 4.5, 3.5 and 2.5. We used antibodies against Vasa protein as a germ cell marker [Citation19] to evaluate the germ cell deficiency in sections of mutant testes. At pnd 7.5, the seminiferous tubules of testes from control mice had readily detectable Vasa-positive germ cells (5.03 ± 1.61) that had migrated to the basement membrane (,b)). In contrast, in A2CKOgono mouse testes, there were only rare Vasa-positive germ cells in the seminiferous tubules (0.02 ± 0.01, P < 0.001). The cells remaining in the A2CKOgono seminiferous tubules had the typical appearance of Sertoli cells and stained positive for the Sertoli cell marker Gata4 [Citation20].
Figure 3. The number of germ cells decreased in A2CKOgono compared with controls as early as pnd 2.5. (a) Paraffin-embedded testis sections from A2CKOgono and control mice were co-stained with anti-Vasa antibody and anti-Gata4 antibody and germ cell numbers per tubule quantified at pnd 7.5 (126 tubules from 2 mice were counted in A2CKOgono testis, 88 tubules from 2 mice in control); pnd 4.5 (195 tubules from 2 mice in A2CKOgono, 143 tubules from 2 mice in control); pnd 3.5 (224 tubules from 3 mice in A2CKOgono, 107 tubules from 3 mice in control); and pnd 2.5 (108 tubules from 3 mice in A2CKOgono, 97 tubules from 3 mice in control). Bar indicates 50 μm. (b) Quantitative analysis depicting the number of Vasa-positive cells per tubule (y axis) in A2CKOgono and control from pnd 7.5 to pnd 2.5 (x axis). Data represent Mean ± SEM. * P < 0.05; **P < 0.001.
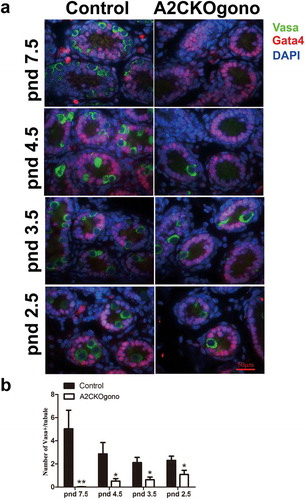
During normal development, at pnd 4.5, the gonocytes have migrated to the basement membrane of the seminiferous tubules, resume proliferation, and undergo the transition from gonocytes to SSCs to produce undifferentiated spermatogonia and some differentiating spermatogonia [Citation13]. Interestingly, we found that the number of Vasa-positive germ cells in A2CKOgono pnd 4.5 testes was significantly lower than in controls (0.52 ± 0.21 vs. 2.86 ± 0.99, P < 0.05) (,b)). Testes from mutant and control mice at pnd 3.5 and as early as pnd 2.5 were then examined to explore the role of cyclin A2 in even earlier gonocytes. We found that the number of Vasa-positive germ cells in A2CKOgono testes was significantly decreased as compared with controls as early as pnd 3.5 (0.63 ± 0.23 vs. 2.12 ± 0.45, P < 0.05) and even pnd 2.5 (1.09 ± 0.36 vs. 2.30 ± 0.37, P < 0.05) (,b)).
Together, these observations suggest that spermatogenesis in A2CKOgono testes was disrupted early in the first wave and that cyclin A2 starts to function in male germ cells before the gonocyte-to-SSC transition at pnd 4.5.
Cyclin A2 appears to not be required for the migration of gonocytes to the basement membrane
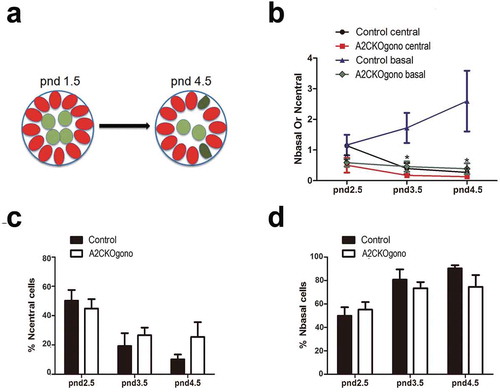
During differentiation within the testicular cords at pnd 2.5 and 3.5, gonocytes begin to migrate from the central luminal region to the basement membrane, which is essential for the formation of the SSC pool and the establishment of niches [Citation21]. As above mentioned, at pnd 4.5 the germ cells are present as undifferentiated spermatogonia and some differentiating spermatogonia [Citation13]. As it has been reported that cyclin A2 can play a role in cell migration [Citation22], we therefore next asked whether cyclin A2 is required for gonocytes to undergo this outward migration to the basement membrane (depicted in the cartoon in )). To identify germ cells within the testicular cords, immunostaining was performed on testicular sections for Vasa (germ cell marker) and Gata4 (Sertoli cell marker) (similar as in )) using the testes from pnd 2.5, 3.5, and 4.5 mice. As shown in ), in the controls, the number of Vasa positive germ cells increased with time in the basal compartment (Nbasal) and concomitantly decreased in the central compartment (Ncentral). In the A2CKOgono testes, the absolute numbers of germ cells in both the central and basal regions of the A2CKO testicular tubules were decreased compared with controls ()). However, the percentage of germ cells located in the central compartment (44.9% ± 6.4% vs. 50.2% ± 7.3%, P > 0.05, pnd 2.5; 26.6% ± 5.3% vs. 19.3% ± 8.8%, P > 0.05, pnd 3.5; 25.5% ± 1.0% vs. 10.2% ± 3.3%, P > 0.05, pnd 4.5 ()) and in the basal compartment (55.1% ± 6.4% vs. 49.8% ± 7.3%, P > 0.05, pnd 2.5; 73.4% ± 5.3% vs. 80.7% ± 8.8%, P > 0.05, pnd 3.5; 74.5% ± 1.0% vs. 90.3% ± 2.8%, P > 0.05, pnd 4.5) ()) was not significantly different compared with controls, suggesting that cyclin A2 does not play a significant role in gonocyte migration.
Figure 4. The number of gonocytes in A2CKOgono is decreased compared with control in pnd 1.5 to 4.5 but their location is not affected. (a) Schematic of location of germ cells in seminiferous tubules in pnd 1.5 and pnd 4.5 testes: red ovals indicate Sertoli cells; light green indicates the germ cells located in the central compartment; dark green indicates a germ cell located in the basal compartment, touching the basement membrane; (b) The number of germ cells located at either basal or central region of the testicular cords was quantified in A2CKOgono and controls from pnd 2.5 (n = 3), 3.5 (n = 3), and 4.5 (n = 3); (c, d) The percentage of the total germ cells located at the central (% Ncentral cells) or basal (% Nbasal cells) regions of testicular tubules in A2CKOgono and control (y axis) from pnd2.5 to pnd4.5 (x axis). Data represent Mean ± SEM. * P < 0.05, ** P < 0.001.
Cyclin A2 is required for the differentiation of gonocytes and their transition into SSCs
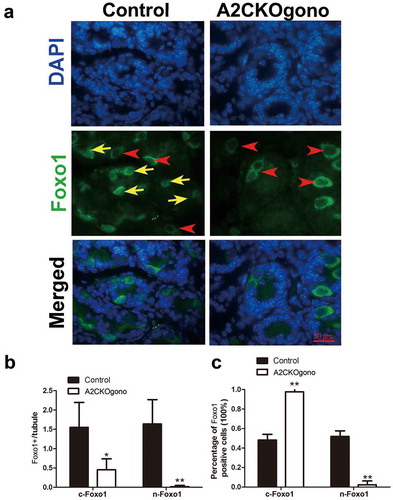
In early postnatal testis, gonocytes extend their cytoplasmic process to basement membrane and then differentiate into spermatogonial stem cells (SSCs) and establish the SSC pool around pnd 4.5 [Citation10,Citation14]. We next asked whether the gonocyte-to-SSC transition is disturbed by loss of cyclin A2 function. It has been shown that Foxo1 (forkhead box protein O1), a unique marker of gonocytes and SSCs, translocates from the cytoplasm to the nucleus around postnatal day 4 when gonocytes differentiate into SSCs [Citation23]. We thus used Foxo1 as a marker to investigate whether cyclin A2 deficiency interferes with the differentiation of gonocytes into SSCs. Immunofluorescent detection Foxo1 revealed that the ratio between nuclear of Foxo1 positive (n-Foxo1+) and cytoplasmic Foxo1 positive (c-Foxo1+) cells was dramatically decreased in A2CKOgono compared with control testes at pnd 4.5 (). This result suggests that cyclin A2 deletion affected the gonocyte-to-SSC transition.
Figure 5. Sub-cellular localization of Foxo1 in A2CKOgono and controls to indicate transition from gonocytes to SSC. (a) Paraffin-embedded testis sections from A2CKOgono and control at pnd 4.5 were stained with anti-Foxo1 antibody. Bar indicates 50 μm. Red arrowheads indicate the cytoplasmic Foxo1-positive cells; yellow arrows indicate nuclear Foxo1-positive cells. (b) Quantitative analysis of nuclear versus cytoplasmic Foxo1-positive cells per tubule and (c) the percentage of nuclear versus cytoplasmic Foxo1-positive cells in A2CKOgono (n = 158 tubules from three mice) and controls (n = 186 tubules from three mice. Data represent Mean ± SEM. *P < 0.05, **P < 0.001.
Discussion
The present study demonstrated that conditional knockout of Ccna2 in E15.5 gonocytes resulted in severely abnormal morphology of adult seminiferous tubules and few if any germ cells. To interrogate at which stage this loss of germ cells occurred, we examined the presence of germ cells during the first wave of spermatogenesis. We first showed that in the germ cells in control postnatal testes, cyclin A2 starts to express at pnd 1.5. As early as pnd 2.5, one day after cyclin A2 started to express, the number of germ cells in A2CKOgono testes had already decreased compared with control. The number of germ cells continued to decrease with time, such that in pnd 7.5 A2CKOgono testes, only rarely were germ cells observed. These results indicate that cyclin A2 is essential for postnatal male gonocyte proliferation and differentiation, the establishment of the SSC pools, and the differentiation of spermatogonia.
It has been reported that the postnatal gonocyte migration toward the basal lamina within the testicular tubules is crucial for the formation of SSC pool and the establishment of SSC niche architecture [Citation12]. It has also been shown that cyclin A2 interacts with actin and RhoA during mitosis [Citation24] and negatively regulates cell motility by promoting RhoA activation [Citation22]. Cyclin A2 has also been reported to play a role in cytoskeleton rearrangements and migration [Citation22]. These observations suggested that cyclin A2 might be involved in mouse gonocyte migration. We therefore analyzed the tubular distribution or location of germ cells in the tubules between postnatal day 2.5 and 4.5 in A2CKOgono and control testes. Our data suggested that cyclin A2 is not required for the migration of male mouse gonocytes in the early post-natal testis.
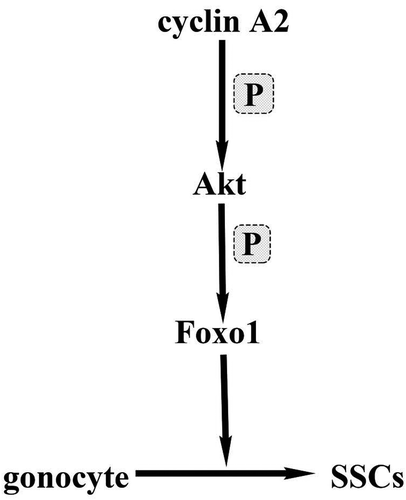
Previous studies have shown that the sub-cellular localization of Foxo1 is an important determinant of its biological activity [Citation25]. During the gonocyte to SSC transition starting from pnd 3.5, Foxo1 protein translocates to the nuclei of future SSCs [Citation23]. Our results showed that without cyclin A2, Foxo1 protein remains in the cytoplasm and germ cells are arrested in the gonocyte stage and cannot progress from gonocytes to SSCs. The question remains as to how cyclin A2 affects Foxo1 translocation from the cytoplasm to the nucleus during the gonocyte to SSC transition. Cdk2/cyclin A directly regulates Akt (also called protein kinase B, a serine/threonine kinase) activation in a phosphorylation-dependent manner during the cell cycle as acute depletion of cyclin A2 or Cdk2 resulted in decreased Akt phosphorylation and activation [Citation26,Citation27]. Akt, which plays a key role in cell proliferation, survival, and metabolism has been suggested to regulate the subcellular localization of Foxo proteins upon phosphorylation [Citation26,Citation28]. Our hypothesis is that cyclin A2 affects phosphorylation of Akt, which then impacts Foxo1 translocation from the cytoplasm to the nucleus (). We thus speculate that cyclin A2 deletion affects the function of Akt and subsequently of Foxo1, thereby disrupting SSC pool formation and spermatogenesis.
Figure 6. Cartoon depicting the possible molecular mechanism of cyclin A2 function in the gonocyte-SSC transition. In this model, cyclin A2 directly regulates Akt activation in a phosphorylation–dependent manner and activated Akt regulates the localization of Foxo1 through phosphorylation.
It is of interest that female germ cells were not overtly affected as the female mice were fertile. This is actually predicted since the Vasa-Cre strain we used expressed Cre after the oogonia had divided mitotically and already initiated meiosis in the embryonic gonad. To address the possible function of cyclin A2 in comparable stages of oogenesis, it will be necessary to use Cre strains that can excise floxed Ccna2 alleles in germ cells at earlier stages of development. The Dppa-Cre mice [Citation29] might therefore be useful in this regard.
It has, however, been reported that conditional deletion of cyclin A2 in mouse oocytes has no discernible effect on MI but did result in a disruption of MII spindles and an increase of merotelic attachments [Citation30]. On stimulation of exit from MII, a dramatic increase in lagging chromosomes and an inhibition of cytokinesis was reported. Such defects are associated with an increase in microtubule stability in MII spindles. The authors suggested that cyclin A2 mediates the fidelity of MII by maintaining microtubule dynamics during the rapid formation of the MII spindle. In our in vivo study using conditional gene ablation, we did not observe an overt effect on female reproductive ability after deletion of cyclin A2 in oocytes after meiosis has been initiated. This suggested that the effects of cyclin A2 deletion on formation of the MII spindle observed in cultured oocytes did not significantly alter female fertility in vivo.
In conclusion, using gonocyte-specific gene ablation of cyclin A2, we have shown that cyclin A2 is essential for regulating the proliferation of male gonocytes and for their transition into SSCs. In the absence of cyclin A2, the sub-cellular localization of Foxo1 is aberrant in that cytoplasmic Foxo1 does not undergo translocation to the nucleus, a hallmark of gonocyte to SSC transition. Given that this translocation of Foxo1 involves phosphorylation by Akt and the role of cyclin A2 in phosphorylation, we speculate that cyclin A2 deletion affects the function of Akt and disrupts SSC pool formation, which results in the loss of spermatogenesis.
Contributions
DJW conceived the study and DJW and FM designed the study. PS provided the floxed Ccna2 mice. All experiments were designed, performed, and analyzed by FM with help from XYW, SSWC, and ES. The initial draft of the manuscript was written by FM in consultation with DJW and was subsequently edited by FM, DJW, PS, SSWC, and ES.
Disclosure statement
The authors declare there are no conflicts of interest.
Additional information
Funding
References
- Pagano M, Pepperkok R, Verde F, et al. Cyclin A is required at two points in the human cell cycle. Embo J. 1992;11(3):961–971.
- Blanchard JM. Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem Pharmacol. 2000;60:1179–1184.
- Murphy M, Stinnakre MG, Senamaud-Beaufort C, et al. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet. 1997;15:83–86.
- Sweeney C, Murphy M, Kubelka M, et al. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64.
- Liu D, Matzuk MM, Sung WK, et al. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20:377–380.
- Ravnik SE, Wolgemuth DJ. Regulation of meiosis during mammalian spermatogenesis: the A-type cyclins and their associated cyclin-dependent kinases are differentially expressed in the germ-cell lineage. Dev Biol. 1999;207:408–418.
- Wolgemuth DJ, Manterola M, Vasileva A. Role of cyclins in controlling progression of mammalian spermatogenesis. Int J Dev Biol. 2013;57:159–168.
- Kalaszczynska I, Geng Y, Iino T, et al. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell. 2009;138:352–365.
- Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012;4:a008375.
- Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;87:1–26.
- Manku G, Culty M. Mammalian gonocyte and spermatogonia differentiation: recent advances and remaining challenges. Reproduction. 2015;149:R139–57.
- Nagano R, Tabata S, Nakanishi Y, et al. Reproliferation and relocation of mouse male germ cells (gonocytes) during prespermatogenesis. Anat Rec. 2000;258:210–220.
- Drumond AL, Meistrich ML, Chiarini-Garcia H. Spermatogonial morphology and kinetics during testis development in mice: a high-resolution light microscopy approach. Reproduction. 2011;142:145–155.
- Yoshida S, Sukeno M, Nakagawa T, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505.
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–286.
- Gallardo T, Shirley L, John GB, et al. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis. 2007;45:413–417.
- Chung SS, Wang X, Wolgemuth DJ. Prolonged oral administration of a pan-retinoic acid receptor antagonist inhibits spermatogenesis in mice with a rapid recovery and changes in the expression of influx and efflux transporters. Endocrinology. 2016;157:1601–1612.
- Persson JL, Zhang Q, Wang XY, et al. Distinct roles for the mammalian A-type cyclins during oogenesis. Reproduction. 2005;130:411–422.
- Fujiwara Y, Komiya T, Kawabata H, et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci U S A. 1994;91:12258–12262.
- Chen SR, Tang JX, Cheng JM, et al. Loss of Gata4 in Sertoli cells impairs the spermatogonial stem cell niche and causes germ cell exhaustion by attenuating chemokine signaling. Oncotarget. 2015;6:37012–37027.
- Xu J, Wan P, Wang M, et al. AIP1-mediated actin disassembly is required for postnatal germ cell migration and spermatogonial stem cell niche establishment. Cell Death Dis. 2015;6:e1818.
- Arsic N, Bendris N, Peter M, et al. A novel function for Cyclin A2: control of cell invasion via RhoA signaling. J Cell Biol. 2012;196:147–162.
- Goertz MJ, Wu Z, Gallardo TD, et al. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011;121:3456–3466.
- Loukil A, Izard F, Georgieva M, et al. Foci of cyclin A2 interact with actin and RhoA in mitosis. Sci Rep. 2016;6:27215.
- Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868.
- Liu P, Begley M, Michowski W, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545.
- Gao Y, Moten A, Lin H-K. Akt: a new activation mechanism. Cell Res. 2014;24:785.
- Zhang X, Tang N, Hadden TJ, et al. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986.
- Hirota T, Ohta H, Shigeta M, et al. Drug-inducible gene recombination by the Dppa3-MER Cre MER transgene in the developmental cycle of the germ cell lineage in mice. Biol Reprod. 2011;85:367–377.
- Zhang QH, Yuen WS, Adhikari D, et al. Cyclin A2 modulates kinetochore-microtubule attachment in meiosis II. J Cell Biol. 2017;216:3133–3143.
