ABSTRACT
Chronic stress which is common in the current society can be harmful to female reproduction and is associated with oocyte defects. However, the underlying mechanisms remain largely unknown. Herein, by using a mouse model of chronic restraint stress, we demonstrated that chronic stress could induce meiotic spindle abnormalities, chromatin misalignment, mitochondrial dysfunction and elevated ROS levels in oocytes in vivo, all of which were normalized by the administration of melatonin. Consistently, melatonin treatment during in vitro maturation also attenuated the meiotic defects induced by H2O2 by regulating autophagy and SIRT1, which could be abolished by SIRT1 inhibitor, Ex527 and autophagy inhibitor Bafilomycin A1 (Baf A1). These data indicate that melatonin can mitigate chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1 and autophagy, providing new understanding for stress-related meiotic errors in MII oocytes and suggesting melatonin and SIRT1 could be new targets for optimizing culture system of oocytes as well as fertility management.
Introduction
Today, 10–15% of couples are suffering from infertility problems[Citation1], possibly due to a variety of causes such as social and behavioral factors as well as environmental exposures [Citation2]. As one of the social factors, stress is present at every level of society in the form of physical, social and psychological [Citation3], posing threats to reproduction in women [Citation4]. For instance, stressful life events are associated with poor outcomes of in vitro fertilization (IVF) [Citation1,Citation5], which can reduce the number of oocytes retrieved and fertilization and pregnancy rate [Citation6]. Evidence indicates that the detrimental effects of stress on fertility could be attributed to the compromised oocytes quality, meiotic spindle abnormalities and defective chromosomal segregation [Citation7–Citation11]. Despite intensive investigation, the underlying molecular basis by which stress induces spindle abnormalities and chromatin misalignment remains elusive.
Reactive oxygen species (ROS) levels were dramatically elevated in oocytes from stressed mice [Citation8]. Mitochondria where ROS are generated are the powerhouse of the cell, providing most of the adenosine triphosphate (ATP) for cellular reactions. Also, the function of mitochondria is vulnerable to ROS. In addition, impaired mitochondria in oocytes is a primary contributor to declining egg and embryo quality, which ties to aneuploidy oocytes [Citation12]. Sirtuin 1 (SIRT1) is involved in regulating ROS homeostasis and exhibits an important player during oocyte development [Citation13].
Melatonin (N-acetyl-5-methoxytrypt amine), a well-known antioxidant, shows a protective role against oocytes impairment. For instance, melatonin could reduce the obesity-induced ROS level in oocytes [Citation14], delay ovarian aging [Citation15], improve the quality and fertilization rate of oocytes [Citation16,Citation17], and restore the benzo(a)pyrene-induced meiotic failure in porcine oocytes [Citation18]. In addition, melatonin could be generated by oocytes and detected in follicular fluid [Citation19–Citation22]. Meanwhile, there is evidence that melatonin is related with mitochondria function [Citation23,Citation24]. Autophagy has been proved to be beneficial to the maturation of oocytes [Citation25,Citation26]. Thus, all these evidences indicate a crosstalk among melatonin, ROS and autophagy in oocyte development.
In this study, we used a mouse model of chronic restraint stress to investigate the effects of melatonin on stress-induced meiotic defects of metaphase II (MII) oocytes. Here, we revealed that melatonin supplement could ameliorate chronic stress-induced elevated ROS level, spindle abnormalities, chromatin misalignment and mitochondria malfunction by regulating SIRT1 and autophagy in vivo. Furthermore, we document that the protective role of melatonin against ROS in oocyte was dependent on SIRT1 and autophagy regulation in vitro.
Methods and materials
Animal experiments
Female BALB/c 6-week-old mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd., and were housed at 25°C under controlled conditions (lights on from 0800 h to 1800 h) at the Department of Animal Experiments, Medical School of Shanghai Jiao Tong University. After a week of adaptation, 100 mice were randomly assigned to the following groups: control (treated with vehicle), control+M (treated with melatonin), stress (treated with vehicle and stress), and stress+M (treated with melatonin and stress). Restraint stress was induced as described previously [Citation27], but a new 3D-printed microcage was applied to replace the 50 mL conical centrifuge tubes in the study. Briefly, the control mice were categorized into groups and allowed contact with each other. Conversely, the stress mice were individually subjected to 6 h/day of immobilization stress in the cage for 28 days between 0900 h and 1500 h. Growth of follicles is a protracted process requiring about 3 weeks in mice [Citation28]. Hence, the mice were treated for 28 days in the current study. Our 3D-printed cage was typical of multiple holes that allowed for a close fit to the mice and maintained enough ventilation. During this period, all mice were provided with water and food. Melatonin was dissolved in 1% ethanol (in normal saline). For melatonin (M) treatment, control+M, and stress+M group mice were administered intraperitoneal injections of M (20 mg/kg/day, Sigma-Aldrich, St. Louis, MO, USA) before exposure to restraint stress every day (0900 h) for 28 days while the control and stress group mice were injected with an equal volume of vehicle. The dosage of melatonin is selected according to the previous studies [Citation29,Citation30]. The body weight and food and water intake of the animals in each group were recorded. All mice were euthanized after anesthesia by inhaling isoflurane (RWD Life Science Co., Ltd, Shenzhen, China). Serum and oocytes were harvested for further analysis.
All animal procedures were approved by the Institutional Animal Care and Use Committee of Shanghai and performed in accordance with the National Research Council Guide for Care and Use of Laboratory Animals. Efforts were made to minimize the suffering of the animals and limit the number of animals used in the study.
Collection of oocytes and maturation
Mature oocytes at MII were collected from the oviducts of females super-ovulated with Pregnant Mare Serum Gonadotropin (PMSG, Ningbo Sansheng Pharmaceutical co. LTD, Ningbo, China) and human Chorionic Gonadotropin (hCG, Ningbo Sansheng Pharmaceutical co. LTD, Ningbo, China) (10 IU of each given 48 hr apart). The expanded cumulus cells surrounding the MII oocytes were treated with hyaluronidase (300 U/ml, Sigma-Aldrich, St. Louis, MO, USA) in M2 medium (Sigma-Aldrich, St. Louis, MO, USA). Clean MII oocytes were collected in M2 medium drop after thorough washings. To collect fully grown germinal vesicle (GV) oocytes, cumulus-oocyte complexes were collected by manually rupturing of antral follicles from BALB/c 6-week-old mice 48 hr after 10 IU PMSG injection. Cumulus cells were removed by mouth-pipetting.
For in vitro maturation, GV oocytes were cultured in M16 medium supplemented by melatonin and Ex527 (MedChemExpress, Monmouth Junction, NJ, USA) at different concentrations under mineral oil at 37°C in a 5% CO2 incubator.
Oxidative stress, melatonin, Ex527 and Bafilomycin A1 (Baf A1) treatments in vitro
To induce oxidative stress, GV oocytes were incubated with 100 µM H2O2 (Beyotime Institute of Biotechnology, Hangzhou, China) for 10 min [Citation31] or 25 µM H2O2 for 30 min [Citation32] in previous studies. In the current study, 100 µM H2O2 for 10 min was chosen to induce oxidative stress in GV oocytes. Then, GV oocytes were cultured in M16 medium for 16 h. For in vitro melatonin supplement, GV oocytes were cultured in maturation medium containing 1 µM of melatonin, a concentration based on the previous studies [Citation14,Citation16]. Also, our preliminary experiments confirmed that melatonin at 1 µM can best rescue the meiotic defects of oocytes, which showed dose-dependency. To obtain inhibition of SIRT1 activity, GV oocytes were treated within M16 medium contained 20 µM Ex527. The concentration was selected according to reported literature [Citation13] and our preliminary test. 100 nM Baf A1 (Sangon Biotech Co., Shanghai, China) dissolved in dimethyl sulfoxide (DMSO) was added to the culture medium according to previous reports [Citation33]. For other parallel groups, oocytes were matured in the presence of 0.001% DMSO to evaluate a possible toxic effect of DMSO in which Baf A1 and Ex527 were dissolved.
Measurement of cytoplasmic ATP contents
ATP contents in the cytoplasm were measured by an ATP assay kit (Beyotime Institute of Biotechnology, Hangzhou, China) according to the manufacturer’s protocol. Briefly, fresh oocytes (50 oocytes from five mice per group) were added to 50 µl ATP-releasing reagent for 5 min on ice and centrifuged for 10 min at 12,000 g under 4°C to obtain the supernatant. Then, 100 µl reaction mixture was added in wells of 96-well plates and incubated for 5 min at room temperature to allow an initial chemiluminescence flash period. Finally, 20 µl supernatant of oocytes was added in the well and bioluminescence of each sample was measured using high-sensitivity luminometer. Sample ATP contents were calculated using a standard curve generated from 8 ATP gradient concentrations ranging from 0 µM to 10 µM.
Mitochondrial DNA copy number quantitation by quantitative polymerase chain reaction (qPCR)
Individual oocytes were washed three times in Ca2+- and Mg2+-free PBS and finally transferred into a PCR tube containing 10 µl of ddH2O. We lysed the cells by three rounds of freeze-thawing (heating at 99°C with immediate freezing in liquid nitrogen) as previously described [Citation34]. Then, the obtained template was used to assess the number of mtDNA copies using Q-PCR kit (Qiagen, Hilden, Germany) using primers of mtDNA and 18 S. 18 S was chosen as the internal control, correcting for genomic DNA. The primer sequence of mtDNA: a 205 base pair fragment primers of NADH dehydrogenase subunit I, Forward, CCCATTCGCGTTATTCTT; Reverse: AAGTTGATCGTAACGGAAGC.18 S, Forward, 5′- GTATGCTGGGAGCCGTGGC-3′; Reverse, 5′- CAGCGCCCACATAGGATGAC-3′.
Measurement of mitochondrial membrane potential (ΔΨm) by JC-1 staining
JC-1 is a potential-sensitive indicator that accumulates preferentially within mitochondria in the oocyte cytoplasm. Oocytes (50 oocytes from five mice per group) were incubated in M2 containing JC-1 (10 μg/ml, Yeasen Biotech Co., Ltd., Shanghai, China) at 37°C for 10 min. The distribution of JC-1 monomers (green fluorescence) and J-aggregate fluorescence (red fluorescence) was determined by Leica microscope (Leica TCS SP8) with fixed microscopic parameters. The captured images were processed using Image J software (version 1.50i, NIH, Bethesda, MD, USA). Mitochondrial membrane potentials were assessed by measuring the ratios of red to green fluorescence.
Immunofluorescence
Oocytes collected were washed two times in M2. The zona pellucida was removed by being incubated in Tyrode’s Solution, Acidic (Sigma-Aldrich, St. Louis, MO, USA) and then washed with 0.1% bovine serum albumin (BSA) in PBS. Oocytes were fixed in 4% paraformaldehyde for 5 min at room temperature followed by being washed in PBS containing 0.05% Tween-20 and 0.1% BSA for 5 min. Oocytes were permeabilized in PBS containing 0.2% Triton X-100 and 0.1% BSA for 15 min at room temperature and then blocked in blocking buffer containing 1% goat serum, 1% BSA and 0.05% Tween-20 in PBS overnight at 4°C. Blocked oocytes (50 oocytes from five mice per group) were incubated 90 min at room temperature with mouse anti-α-tubulin antibody at 1:200 (Sigma-Aldrich, St. Louis, MO, USA), and mouse anti-ATPase inhibitory factor 1 (ATPIF1) antibody at 1:200 (Life Technologies, Gaithersburg, MD, USA) in blocking buffer for 2 h. After being washed 3 times for 10 min each with PBS containing 0.05% Tween-20 and 0.1% BSA, oocytes were labeled with secondary antibody immunoglobulin (IgG) (Life Technologies, Gaithersburg, MD, USA) for 1 h at room temperature followed by 3 washes with PBS containing 0.05% Tween-20 and 0.1% BSA. Finally, oocytes were transferred into mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and covered by glass slides. Isotype IgGs for the first antibody were used as the negative controls. All the stained oocytes were observed with a Leica laser scanning confocal microscope using identical magnification and gain settings throughout experiments. The fluorescence intensity from each oocyte was analyzed using Image J software (version 1.50i, NIH, Bethesda, MD, USA).
Analysis of oocyte autophagy vacuoles
Autophagic vacuoles in live denuded oocytes were visualized using the Cyto-ID Autophagy Detection Kit (Enzo Life Sciences, Farmingdale, USA) according to the manufacturer’s instructions. Briefly, after washing once with 1× assay buffer, oocytes (50 oocytes from five mice per group) were incubated with a detection solution at 37°C in the dark. Then, oocytes were washed once with 1× assay buffer and imaged immediately using a Leica SP8 spectral scanning confocal microscope at identical magnification and gain settings throughout experiments. Green fluorescence intensity was determined as the sum total of fluorescence using Image J software (version 1.50i, NIH, Bethesda, MD, USA).
Western blotting
Oocytes were washed in PBS and lysed in cold loading buffer (Beyotime Institute of Biotechnology, Hangzhou, China) supplemented with protease inhibitor cocktail (TransGen Biotech, Beijing, China) at 1:100 for 30 min on ice. Proteins (50 oocytes per lane) were loaded and separated on 10% SDS-polyacrylamide gel and transferred to polyvinylidene fluoride membranes (PVDF) membranes (Millipore, Billerica, MA, USA). As for the experiment for LC3, 15% SDS-polyacrylamide gel was selected. The membranes were blocked with 5% nonfat milk and probed with specific primary antibodies including anti-ACTB at 1:5000 (Proteintech Group, Inc., Chicago, USA), anti-Atg5 at 1:1000 (Novus Biologicals, Inc.; Littleton, CO, USA), anti-LC3 at 1:1000 (Novus Biologicals, Inc.; Littleton, CO, USA), anti-p62/Sequestosome 1(SQSTM1) at 1:1000 (Cell Signaling Technology, Danvers, MA, USA) and anti-SIRT1 at 1:1000 (Millipore, Billerica, MA, USA) at 4°C overnight. After three washes in TBS containing 0.1% Tween-20 (TBST). For primary antibody detection, we used HRP-conjugated anti-rabbit secondary antibodies (Sigma-Aldrich, St. Louis, MO, USA). The signals were measured using an enhanced chemiluminescence (ECL) detection kit (NCM Biotech, Suzhou, China). The integrated density values were calculated by comparing the signals of target proteins to that of the housekeeping ACTB. The immunoreactive band intensities in Western blotting were quantified by ImageJ software (version 1.50i, NIH, Bethesda, MD, USA).
Assay for intra-oocyte ROS
In order to assess the ROS in individual oocytes, oocytes (50 oocytes from five mice per group) were incubated in 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (DCHFDA) (Sigma-Aldrich, St. Louis, MO, USA) for 10 min at 37°C. After being washed three times in M2, oocytes were observed under a Leica microscope. The fluorescence was obtained by excitation at 488 nm. All images were taken using fixed microscopic parameters, and the fluorescence intensity from each oocyte was analyzed using Image J software (version 1.50i, NIH, Bethesda, MD, USA).
Statistical analysis
All the values were presented as mean ± standard error of the mean (SEM). Ordinary one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to evaluate statistical differences via GraphPad Prism version 6. Differences were considered statistically significant when p-value was <0.05.
Results
Melatonin ameliorates spindle abnormality and chromosome misalignment in MII oocytes from stressed mice in vivo
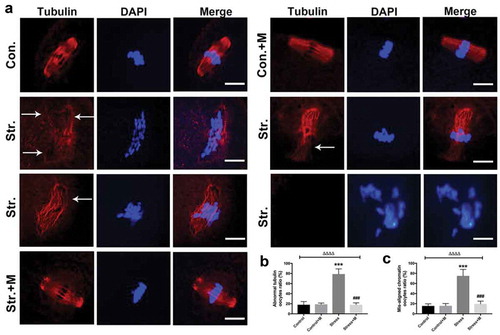
In the present study, restraint stress in female mice was utilized to mimic stress. In brief, mice were mobilized for 6 h/day in 3D-printed cage for 4 weeks which covers the whole period of follicle growth process in mice (Figure S1). The spindle morphology and chromosome alignment of oocytes were analyzed under a confocal microscope after fluorescent staining. Most of the spindles were barrel-shaped with chromosomes well congressed on the metaphase plate in MII oocytes of control and melatonin-treated control mice (). In contrast, the morphological shape of spindles was totally disrupted and abnormally dispersed chromosomes were observed in MII oocytes from stressed mice (), showing multipolar, asymmetric spindles or no spindles at all () and the corresponding chromosomes were displaced in the oocytes from stressed mice (). The images revealed that about 75% of MII oocytes from stressed mice had spindle abnormalities and disruption of chromosome alignment compared to only 15% of controls (,). However, melatonin almost rescued stress-induced spindle abnormality and chromosome misalignment in oocytes (with only 18% abnormal oocytes) (). Isotype IgGs for the first antibody were used as the negative controls (Figure S2). Altogether, our results demonstrate that melatonin ameliorates stress-induced meiotic defects including spindle abnormality and chromosome misalignment in MII oocytes.
Figure 1. Effects of melatonin (m) on restraint stress-induced spindle abnormality and chromatin misalignment in MII oocytes. (a) Representative examples of meiotic spindles and chromosomal misalignment in MII oocytes after labeling with α-tubulin antibody (red) and counterstaining of DNA with DAPI (blue) from control mice (Con.), control+M mice (Con.+M), stress mice model (Str.) and stress+M mice (Str.+M). The arrow shows the abnormal spindles (Bar = 10 μm). (b) Incidence of spindle abnormalities and (c) ratio of chromatin misalignment in MII oocytes (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ***P < 0.001 vs. control group; ###P < 0.001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
Melatonin improves ATP production, mtDNA copy number, mitochondria aggregation and heterogeneous distribution and mitochondrial membrane potential in MII oocytes from stressed mice in vivo
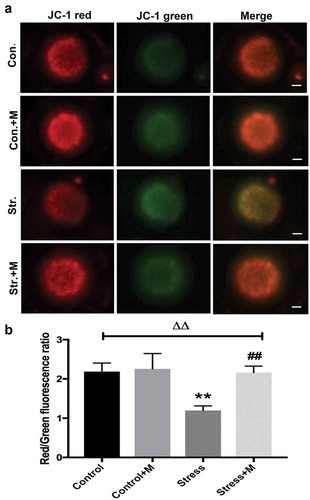
Many studies have proposed a link between insufficient ATP availability in eggs and defective chromosomal segregation – an outcome that probably ties to meiotic spindle abnormalities [Citation12,Citation35]. As the results showed, ATP content was significantly lower in oocytes from stressed mice ()). Considering that mitochondria as the powerhouse of cells to produce ATP, we further investigated the mitochondria function of oocytes. Firstly, mtDNA content remains to be a more reliable indicator of the mitochondrial capacity of a given oocyte, with each mitochondria containing one to ten copies of mtDNA [Citation35]. The number of mtDNA was quantitatively assessed in individual oocyte. Stress resulted in a dramatic decrease in oocyte mtDNA ()). In mammalian cells, ATPIF1 is located in the inner mitochondrial membrane and primarily regulates the function of the F1F0-ATP synthase [Citation36]. Then, to further investigate the number and distribution of mitochondria in oocytes, ATPIF1, an antibody raised against complex V Ox-Phos present in phosphorylated mitochondria [Citation37], was stained and analyzed in MII oocytes. Most of the control oocytes displayed a homogeneous mitochondrial distribution pattern ()), while clustered mitochondria, perinuclear and heterogeneous mitochondrial distribution pattern were found in about 80% oocytes from stressed mice ()). In addition, all the distributed mitochondria pattern was accompanied by misaligned chromatins in oocytes as pointed out by the white arrows ()). Meanwhile, the intensity of ATPIF1 protein in stressed oocytes was significantly lower than that of control oocytes ()). As the mitochondrial membrane potential is closely related to ATP generation [Citation38,Citation39], we then tested whether chronic stress affects the mitochondrial membrane potential of oocytes. As shown in the results, the average mitochondrial membrane potential in control oocytes was significantly higher than that in stressed oocytes (,)). Intriguingly, the ATP production decline, mtDNA copy number decrease, mitochondria aggregation and heterogeneous distribution pattern and mitochondrial membrane potential decrease were rescued by melatonin supplementation in stressed oocytes () and ,b)), but there was no significant change in oocytes between melatonin-treated control and control mice () and ,b)). Taken together, it is suggested that melatonin relieves mitochondria dysfunction in MII oocytes from stressed mice.
Figure 2. Effects of melatonin (m) and restraint stress on ATP content and mtDNA copy number, expression of ATPIF1 and mitochondria distribution in MII oocytes. (a) ATP content in MII oocytes (n = 50 oocytes from five mice per group). (b) Relative mtDNA copy number in MII oocytes (n = 50 oocytes from five mice per group). (c) ATPIF1 expression, distribution pattern and chromatin alignment in MII oocytes in MII oocytes from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+M) mice. The arrow shows the chromatin misalignment (Bar = 20 μm). (d) ATPIF1 relative expression (n = 50 oocytes from five mice per group). (e) Abnormal distributed mitochondria oocytes ratio (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ####P < 0.0001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
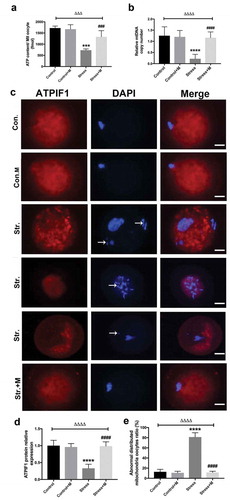
Figure 3. Effects of melatonin (m) and restraint stress on mitochondria membrane potential (ΔΨm). (a) Live oocytes stained with JC-1, where red fluorescence indicates high ΔΨm, and green indicates low ΔΨm from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+M) mice (Bar = 20 μm). (b) Red to green fluorescence ratio, an indicator of mitochondrial activity (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔP < 0.01 ANOVA; **P < 0.01 vs. control group; ##P < 0.01 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
Melatonin up-regulates autophagy and the expression of autophagy-related 5 (Atg5) and microtubule-associated protein 1 light chain 3(LC3)-II/LC3-I ratio in MII oocytes from stressed mice in vivo
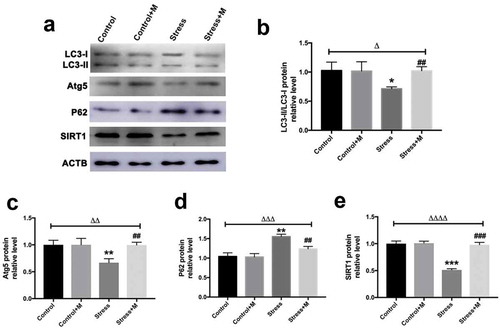
Autophagy is the main mechanism of mitochondrial turnover during development and under pathological conditions [Citation40], which controls the quality of mitochondria. As mtDNA number and mitochondria membrane potential were reduced in stressed oocytes, which suggested mitochondria may be damaged in stressed mice oocytes, we assessed the autophagy level of oocytes. By using an activity assay, we found that oocytes from stressed mice exhibited less autophagic vacuoles throughout the oocyte cytoplasm compared with the oocytes from control mice ()). Quantification assay confirmed that autophagy levels were 1.5-fold higher in oocytes from control mice compared with those of stressed mice ()). Furthermore, the melatonin treatment significantly upregulated autophagic activity in oocytes of stressed mice (,b)), whereas melatonin treatment had little impact on the autophagy levels in control oocytes (,b)). Atg5 and LC3 are markers of autophagy. SQSTM1/p62 plays an important role in the interaction between LC3 and ubiquitinated substrates and the amount of SQSTM1/p62 is inversely related to autophagic degradation [Citation41]. Western blotting showed that oocytes from stressed mice exhibited a reduction in Atg5 and LC3-II/LC3-I ratio and elevation in SQSTM1/p62 (). Markedly, melatonin elevated both the expression level of Atg5 and LC3-II/LC3-I ratio and reduced the level of SQSTM1/p62 in stressed oocytes, whereas there was little change in melatonin-treated control group (). Collectively, melatonin up-regulates the autophagy level in MII oocytes from stressed mice.
Figure 4. Effects of melatonin (m) and restraint stress on autophagy in MII oocytes. (a) Live oocytes were assessed for autophagic vacuoles, visualized as green fluorescence from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+M) mice. (Bar = 20 μm). (b) Autophagy levels were quantified as the sum total of green fluorescence within each oocyte (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ####P < 0.0001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
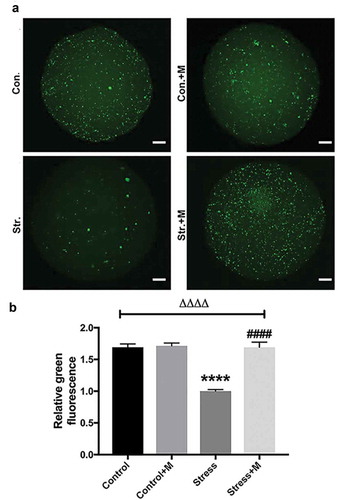
Figure 5. Effects of melatonin (m) and restraint stress on protein level of LC3-II/LC3-I ratio, Atg5, SQSTM1/p62 and SIRT1 in MII oocytes. (a) Western blots and (b-e) the relative expression level of LC3-II/LC3-I ratio, Atg5, SQSTM1/p62 and SIRT1 against ACTB of MII oocytes from per group (50 oocytes per lane, n = 3). ACTB was used as a loading control. All data are presented as mean ± SEM. ΔP < 0.05 ANOVA; ΔΔP < 0.01 ANOVA; ΔΔΔP < 0.001 ANOVA; ΔΔΔΔP < 0.0001 ANOVA; *P < 0.05 vs. control group; **P < 0.01 vs. control group; ***P < 0.001 vs. control group; ##P < 0.01; vs. stress group; ###P < 0.001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
Melatonin promotes the expression of SIRT1 in MII oocytes from stressed mice in vivo
SIRT1 is a potent inducer of autophagy [Citation42,Citation43]. To investigate whether SIRT1 could be the contributor which mediates the effects of stress on oocytes, SIRT1 was detected in oocytes. In quantity, the protein level of SIRT1 was significantly lower than that in control oocytes (). Amazingly, the expression of SIRT1 was rescued by melatonin supplementation in stressed oocytes (). However, melatonin posed no effects on the SIRT1 protein level in control oocytes (). Thus, melatonin promotes the expression of SIRT1 in MII oocytes from stressed mice.
Melatonin attenuates restraint stress-induced ROS in MII oocytes in vivo
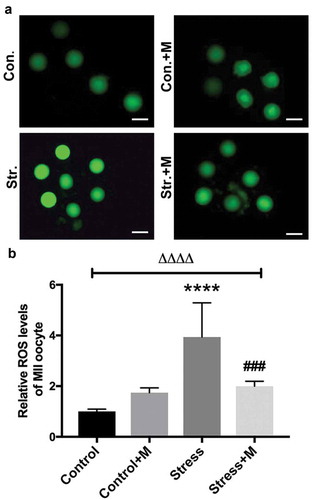
As a source of ROS, mitochondria are meanwhile a vulnerable target of ROS [Citation35], and mtDNA is a major target for oxidative attack, because of its location near the inner mitochondrial membrane sites where oxidants are formed, as well as its lack of both protective histones and DNA repair activity [Citation44]. In addition, the deficit of mitochondria-derived ATP during oxidative stress impairs mouse oocyte spindles [Citation35]. SIRT1 is also a regulator of ROS homeostasis [Citation13] and the positive role of melatonin has been reported to be dependent on scavenging ROS [Citation45], indicating ROS may mediate the aforementioned effects of chronic stress on oocytes. Therefore, we measured the intra-oocyte levels of ROS to test the effects of stress on the oxidative stress of oocytes. The results showed that ROS levels were significantly higher in stressed oocytes than that in control mice (). Expectedly, melatonin, as one of the antioxidants, alleviated the ROS production in stressed oocytes (). However, the ROS level in oocytes of melatonin-treated control mice was comparable with that of control mice (). Taken together, the chronic stress-induced excessive ROS production in oocytes could be attenuated by melatonin.
Figure 6. Effects of melatonin (m) and restraint stress on ROS level in MII oocytes. (a) Live oocytes were assessed for ROS, visualized as green fluorescence from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+D) mice. (Bar = 100 μm). (b) ROS levels were quantified as the sum total of green fluorescence within each oocyte (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ###P < 0.001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
SIRT1 inhibition abolishes the protective role of melatonin in restoring the H2O2-induced spindle defects in oocytes in vitro
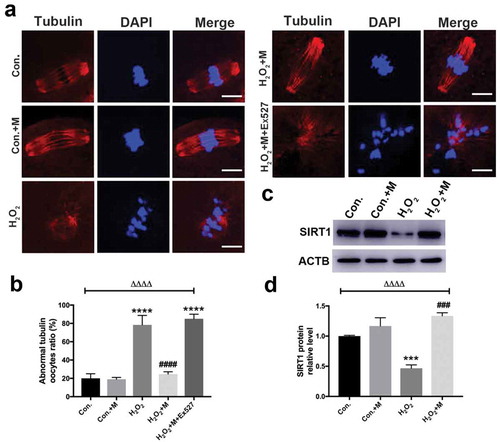
In order to investigate whether SIRT1 plays a role in mediating the protective effects of melatonin on oxidative spindle defects, GV oocytes were exposed to H2O2, melatonin and Ex527 in M16 medium and checked for the spindle formation 16 h later. Further analysis revealed that the spindle morphology was disrupted in 75% oocytes by 100 µM H2O2 for 10 min (), a similar proportion with that in our restraint stress mouse model. Thus, 100 µM H2O2 was chosen for the oxidative stress treatment in vitro. Melatonin significantly reduced the occurrence of H2O2-induced abnormal spindle formation in oocytes (). Furthermore, oxidative stress treatment indeed reduced the protein level of SIRT1 in vitro (). Consistently, the presence of Ex527, inhibitor of SIRT1, during in vitro maturation completely abolished the ability of melatonin to improve the oxidative meiotic defects of oocytes ().
Figure 7. Effects of melatonin and Ex527 treatment on H2O2-induced spindle abnormality and chromatin misalignment as well the protein level of SIRT1 in oocytes in vitro. (a) Micrographs of meiotic spindles and chromosomal misalignment in MII oocytes in control oocytes (Con.), in oocytes treated with melatonin (Con.+M), in oocytes exposed to H2O2 (H2O2), in oocytes treated with H2O2 and melatonin (H2O2 + M) and in oocytes treated with H2O2, melatonin and Ex527 (H2O2 + M+ Ex527) (Bar = 10 μm). (b) Incidence of spindle abnormalities in oocytes of each group (n = 50 oocytes per group). (c-d) Western blots and the relative expression level of SIRT1 against ACTB of oocytes in each group (50 oocytes per lane, n = 3). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ***P < 0.001 vs. control group; ****P < 0.0001 vs. control group; ###P < 0.001 vs. H2O2 group; ####P < 0.0001 vs. H2O2 group. Con., treated with vehicle; Con.+M, treated with melatonin; H2O2, treated with H2O2; H2O2 + M, treated with H2O2 and melatonin; H2O2 + M+ Ex527, treated with H2O2, melatonin and Ex527.
SIRT1 inhibition blocks the ability of melatonin to improve autophagy level in oocytes in vitro
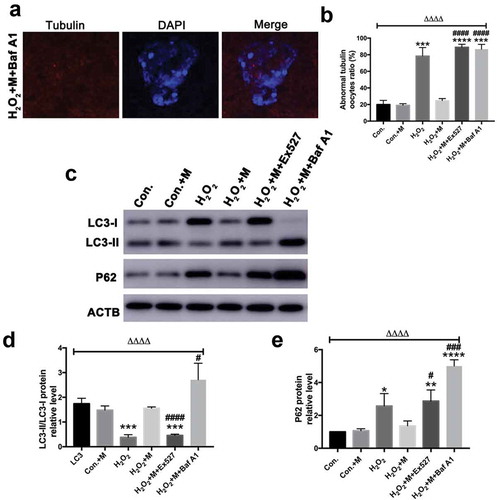
Following treatment with H2O2 in vitro, ATP content, mitochondrial membrane potential () and LC3-II/LC3-I ratio were downregulated in oocytes with the upregulation of SQSTM1/p62 (), which was reversed by melatonin administration ( and ). To investigate whether melatonin is modulating autophagy flux, Baf A1 was added to inhibit autophagy. Treatment of Baf A1 during in vitro maturation of GV oocytes completely blocked the ability of melatonin to improve H2O2-induced meiotic defects of oocytes (). In H2O2 + M+ BafA1 group, significant accumulation of both LC3 II/LC3-I and SQSTM was induced (). According to previous studies, SIRT1 can stimulate autophagy to be cytoprotective [Citation15,Citation46]. Consistently, treatment with SIRT1 inhibitor Ex527 blocked the ability of melatonin to improve autophagy level by reducing the level of LC3-II/LC3-I ratio and elevating the level of SQSTM1/p62 in oocytes (), suggesting the ability of melatonin to improve meiosis of oocytes and upregulate autophagy level is dependent on SIRT1.
Figure 8. Effects of melatonin and Ex527 treatment on H2O2-induced mitochondrial dysfunction in oocytes in vitro. (a) Live oocytes stained with JC-1, where red fluorescence indicates high ΔΨm, and green indicates low ΔΨm in control oocytes (Con.), in oocytes treated with melatonin (Con.+M), in oocytes exposed to H2O2 (H2O2), in oocytes treated with H2O2 and melatonin (H2O2 + M) and in oocytes treated with H2O2, melatonin and Ex527 (H2O2 + M+ Ex527) (Bar = 20 μm). (b) Red to green fluorescence ratio of JC-1, an indicator of mitochondrial activity (n = 50 oocytes per group). (c) ATP content in oocytes from each group (n = 50 oocytes per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ####P < 0.0001 vs. H2O2 group. Con., treated with vehicle; Con.+M, treated with melatonin; H2O2, treated with H2O2; H2O2 + M, treated with H2O2 and melatonin; H2O2 + M+ Ex527, treated with H2O2, melatonin and Ex527.
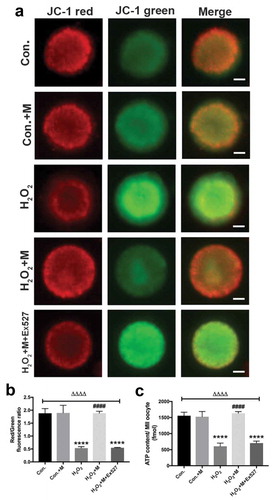
Figure 9. Effects of Baf A1 treatment on H2O2-induced spindle abnormality treated by melatonin and effects of melatonin, Ex527 and Baf A1 treatment on the expression of LC3-II/LC3-I ratio and SQSTM1/p62 in H2O2-treated oocytes. (a) Micrographs of meiotic spindles and chromosomal misalignment in MII oocytes in oocytes treated with H2O2, melatonin and Baf A1 (H2O2 + M+ Baf A1) (Bar = 10 μm). (b) Incidence of spindle abnormalities in oocytes of each group (n = 50 oocytes per group). (c) Western blots and (d-e) the relative expression level of LC3-II/LC3-I ratio and SQSTM1/p62 against ACTB of oocytes in each group (50 oocytes per lane, n = 3). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; *P < 0.05 vs. control group; **P < 0.01 vs. control group; ***P < 0.001 vs. control group; ****P < 0.0001 vs. control group; #P < 0.05 vs. H2O2 group; ###P < 0.001 vs. H2O2 group; ####P < 0.0001 vs. H2O2 group. Con., treated with vehicle; Con.+M, treated with melatonin; H2O2, treated with H2O2; H2O2 + M, treated with H2O2 and melatonin; H2O2 + M+ Ex527, treated with H2O2, melatonin and Ex527; H2O2 + M+ Baf A1, treated with H2O2, melatonin and Baf A1.
Discussion
Stress has been known as a risk to limit reproductive outcome [Citation1,Citation4,Citation7–Citation11]. Maternal restraint stress could increase oocyte aneuploidy by impairing spindle assembly [Citation8]. Consistently, our data demonstrate that chronic stress resulted in meiotic failure in mice MII oocytes including abnormal meiotic spindle assembly and chromatin misalignment, providing further evidence for that chronic stress could be a risk to impair oocyte meiotic process.
What’s more, excessive oxidative stress has deleterious effects on oocyte meiosis process [Citation35,Citation47]. Similarly, maternal restraint stress might also impair spindle assembly by inducing intra-oocyte oxidative stress [Citation8]. Likewise, meiotic defects were detected in stressed oocytes with elevated ROS levels in the current study. ROS are produced continuously in mitochondria along the electron transport chain, and mitochondria are also the main target of ROS. For example, mtDNA is vulnerable to oxidative attack due to its location as well as its lack of both protective histones and DNA repair activity [Citation44]. Oxidative stress reportedly led to a dissipation of mitochondrial membrane potential, and a decrease in cytoplasmic ATP levels [Citation35]. The oxidative phosphorylation within mitochondria provides a major source of ATP for oocytes [Citation35,Citation48]. Many studies have proposed a link between insufficient ATP availability in oocytes and meiotic failure [Citation12,Citation35]. However, it is not clear what effects chronic stress could pose on mitochondria in oocytes so far. Our findings revealed that mitochondrial defects such as less ATP production, lower mtDNA numbers, abnormal mitochondria distribution and aggregation and lower mitochondria membrane potential were considerably induced in MII oocytes from stressed mice, suggesting that chronic stress does impair the function of mitochondria in oocytes. Thus, these data suggest that meiotic failure in stressed oocytes might be attributed to the excessive ROS-induced mitochondria defects.
Melatonin is a well-characterized antioxidant and is protective against maternal obesity-, Bisphenol A-, BaP- and aging-associated quality defects in oocytes [Citation14,Citation15,Citation18,Citation49]. Accordingly, melatonin levels in follicular fluid serve as markers for IVF outcomes and predict ovarian reserve [Citation50]. Mitochondria could synthesize melatonin to ameliorate its function, improve mice oocyte’s quality [Citation22], significantly decrease the ROS level and inhibit apoptotic events of vitrified bovine oocytes to increase their developmental potential [Citation51]. Consistently, melatonin limits paclitaxel-induced mitochondrial dysfunction [Citation52]. Here, we also observed that the increased intra-oocyte ROS level and the defects of MII oocytes including meiotic abnormalities and the aforementioned mitochondrial defects could be relieved by melatonin in vivo. Also, our in vitro evidence demonstrated that melatonin efficiently relieved the oxidative stress-induced meiotic failure and mitochondrial malfunction. Cumulatively, the present study documents the beneficial effects of melatonin on oocyte quality from stressed mice and provides new insight for the understanding of oocyte maturation process. However, more studies are warranted to investigate whether melatonin could be utilized to promote maturation for stressed oocytes in vitro.
Autophagy is a predominantly cytoprotective process [Citation53,Citation54] as well as a cell survival program for female germ cells in the murine ovary [Citation26]. For example, the induction of autophagy during in vitro maturation improves the nuclear and cytoplasmic maturation of porcine oocytes [Citation25]. Autophagy is also a critical regulator of organellar homeostasis, particularly of mitochondria [Citation42]. Mitochondria that have been damaged could induce mitophagy to remove damaged mitochondria [Citation55] and initiate mitochondrial biogenesis and replace damaged mitochondria [Citation56,Citation57], contributing to autophagy-dependent mitochondrial quality control [Citation42,Citation58]. According to a previous study, autophagy has been demonstrated to be reduced by restraint in mice [Citation59]. Consistently, we observed that the autophagy-related proteins Atg5 and LC3-II/LC3-I ratio were downregulated by restraint here. Atg5 and LC3-II/LC3-I ratio are proteins that aid in the clearance of damaged organelles through autophagy [Citation60], so their downregulation confirmed the reduction of autophagy in stressed oocytes. SQSTM1/p62 is inversely related to autophagic degradation. Therefore, the upregulation of p62 induced by restraint and ROS also indicates the reduction of autophagy in stressed oocytes. It is generally accepted that a certain level of ROS may facilitate the autophagy process [Citation61,Citation62]. However, inhibition of autophagy is mediated by ROS as well, because free radicals can induce activation of mammalian target of rapamycin (mTOR) and inactivation of BECN1 (or Beclin1) by protein kinase B (PKB, or AKT) kinase signaling cascade to inhibit autophagy [Citation63]. For example, ROS-mediated autophagy inhibition in cystic fibrosis, inducing aggresome formation and lung inflammation [Citation64]. The inhibition of autophagy attenuated the protective role of melatonin in rescuing the spindle abnormality, suggesting that the role melatonin is sort of autophagy-dependent. Collectively, we speculate that ROS-mediated autophagy inhibition might contribute to the impairment in stressed oocytes.
SIRT1 has been proved to be a sensor of redox state and a protector against oxidative stress [Citation13] as well as a potent regulator of autophagy [Citation42,Citation43]. SIRT1 can stimulate autophagy by regulating Atg5 and LC3 [Citation65]. In addition, SIRT1 can induce autophagy to promote longevity [Citation66,Citation67]. Chronic stress has once been reported to decrease the expression levels of SIRT1 in the rat hippocampus [Citation68]. Consistently, our results showed that SIRT1 was downregulated in oocytes by chronic stress. Intriguingly, our data revealed that melatonin administration induced the expression of SIRT1 and autophagy proteins in stressed mouse oocytes both in vivo and in vitro, which was reversed by SIRT1 inhibitor Ex527 treatment, suggesting SIRT1 plays an important role in the beneficial effects of melatonin on oxidative stress-induced meiotic defects and mitochondrial dysfunction. The protective role of melatonin has been proved to be associated with the regulation of SIRT1 and autophagy in many tissues. For instance, melatonin improves mitochondrial function by regulating SIRT1 in cadmium-induced hepatotoxicity in vitro [Citation69]. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice [Citation70]. Melatonin treatment delays ovarian aging with the mRNA expression of SIRT1 and LC3 being enhanced [Citation15]. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy [Citation71]. Melatonin could impede cognitive decline in tau-related Alzheimer models by restoring the autophagic flux [Citation72]. Consistently, we also confirm that melatonin could promote the autophagic flux. Taken together, melatonin probably prevented the chronic stress-induced reduction of ATP production and meiotic defects in MII oocytes via regulation of SIRT1 and autophagy. However, there are several limitations in our study. First, the mechanism of how SIRT1 interacts with autophagy in stressed oocytes remains unclear. Whether the autophagy inhibition can block the role of melatonin was tested in vitro, whereas it is not verified in vivo. This would be the target of our future study. Although melatonin has been verified to enhance the autophagic flux, the direct mechanism of action of melatonin on autophagy restoration remains to be investigated. Second, the mechanism such as how stress and melatonin induce the regulation of SIRT1 in oocytes remains to be elucidated. AMP-activated protein kinase (AMPK)-dependent regulation of SIRT1 has been proved to be involved in ROS-induced molecular events [Citation73]. Hence, whether AMPK/SIRT1 signaling plays an important role in stress-induced effects in oocyte remains to be elucidated. Finally, whether by overexpressing SIRT1 in vivo could improve the oocyte quality from stressed mouse should be the target of future studies, which would provide new hope for SIRT1 as the therapeutic target of promoting oocytes maturation.
Altogether, the present study provides evidence demonstrating that melatonin could exert protective effects on oxidative meiotic defects in mice oocytes by regulating SIRT1 and autophagy. Our study has shed new light on the roles of chronic stress and melatonin in reproductive health as well as the potential mechanisms, providing new understanding for SIRT1 and melatonin as therapeutic targets to improve oocyte meiotic process in oxidative stress-induced female fertility decline.
Supplemental Material
Download MS Word (145.4 KB)Disclosure statement
The authors have no conflicts of interest to declare regarding the publication of this paper.
Supplementary material
The supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ebbesen SM, Zachariae R, Mehlsen MY, et al. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum Reprod. 2009;24(9):2173–2182.
- Skakkebaek NE, Jorgensen N, Main KM, et al. Is human fecundity declining? Int J Androl. 2006;29(1):2–11.
- Sharma R, Biedenharn KR, Fedor JM, et al. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11(1):66.
- Prasad S, Tiwari M, Pandey AN, et al. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23(1):36.
- Schroder AK, Katalinic A, Diedrich K, et al. Cumulative pregnancy rates and drop-out rates in a German IVF programme: 4102 cycles in 2130 patients. Reprod Biomed Online. 2004;8(5):600–606.
- Klonoff-Cohen H, Chu E, Natarajan L, et al. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76(4):675–687.
- Zhang SY, Wang JZ, Li JJ, et al. Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod. 2011;84(4):672–681.
- Zhou P, Lian HY, Cui W, et al. Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice. Biol Reprod. 2012;86(3):83.
- Liang B, Wei DL, Cheng YN, et al. Restraint stress impairs oocyte developmental potential in mice: role of CRH-induced apoptosis of ovarian cells. Biol Reprod. 2013;89(3):64.
- Wu XF, Yuan HJ, Li H, et al. Restraint stress on female mice diminishes the developmental potential of oocytes: roles of chromatin configuration and histone modification in germinal vesicle stage oocytes. Biol Reprod. 2015;92:13.
- Gao Y, Chen F, Kong QQ, et al. Stresses on female mice impair oocyte developmental potential: effects of stress severity and duration on oocytes at the growing follicle stage. Reprod Sci. 2016;23:1148–1157.
- Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17(6):838–850.
- Di Emidio G, Falone S, Vitti M, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29(9):2006–2017.
- Han L, Wang H, Li L, et al. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res. 2017;63(3):e12431.
- Tamura H, Kawamoto M, Sato S, et al. Long-term melatonin treatment delays ovarian aging. J Pineal Res. 2017;62(2):e12381.
- Dai X, Lu Y, Zhang M, et al. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum Reprod. 2017;32(3):598–606.
- Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–287.
- Miao Y, Zhou C, Bai Q, et al. The protective role of melatonin in porcine oocyte meiotic failure caused by the exposure to benzo(a)pyrene. Hum Reprod. 2018;33(1):116–127.
- Nakamura Y, Tamura H, Takayama H, et al. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80(4):1012–1016.
- Reiter RJ, Tamura H, Tan DX, et al. Melatonin and the circadian system: contributions to successful female reproduction. Fertil Steril. 2014;102(2):321–328.
- Reiter RJ, Rosales-Corral SA, Manchester LC, et al. Peripheral reproductive organ health and melatonin: ready for prime time. Int J Mol Sci. 2013;14(4):7231–7272.
- He C, Wang J, Zhang Z, et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci. 2016;17(6):939.
- Jou MJ, Peng TI, Hsu LF, et al. Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca 2+ -mediated permeability transition and beyond in rat brain astrocytes. J Pineal Res. 2010;48(1):20–38.
- Chuang JI, Pan IL, Hsieh CY, et al. Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J Pineal Res. 2016;61(2):230–240.
- Song BS, Kim JS, Kim YH, et al. Induction of autophagy during in vitro maturation improves the nuclear and cytoplasmic maturation of porcine oocytes. Reprod Fertil Dev. 2014;26(7):974–981.
- Gawriluk TR, Hale AN, Flaws JA, et al. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction. 2011;141(6):759–765.
- Wu L, Lu Y, Jiao Y, et al. Paternal psychological stress reprograms hepatic gluconeogenesis in offspring. Cell Metab. 2016;23(4):735–743.
- Clarke HJ, Vieux KF. Epigenetic inheritance through the female germ-line: the known, the unknown, and the possible. Semin Cell Dev Biol. 2015;43:106–116.
- Zhai M, Li B, Duan W, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63(2):e12419.
- Barberino RS, Menezes VG, Ribeiro A, et al. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol Reprod. 2017;96(6):1244–1255.
- Takahashi T, Takahashi E, Igarashi H, et al. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66(2):143–152.
- Choi JY, Kang JT, Park SJ, et al. Effect of 7,8-dihydroxyflavone as an antioxidant on in vitro maturation of oocytes and development of parthenogenetic embryos in pigs. J Reprod Dev. 2013;59(5):450–456.
- You SY, Park YS, Jeon HJ, et al. Beclin-1 knockdown shows abscission failure but not autophagy defect during oocyte meiotic maturation. Cell Cycle (Georgetown, Tex). 2016;15(12):1611–1619.
- Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet. 2008;40(12):1484–1488.
- Zhang X, Wu XQ, Lu S, et al. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16(10):841–850.
- Campanella M, Casswell E, Chong S, et al. Regulation of mitochondrial structure and function by the F1Fo-ATPase inhibitor protein, IF1. Cell Metab. 2008;8(1):13–25.
- Rigoglio NN, Fatima LA, Hanassaka JY, et al. Equine chorionic gonadotropin alters luteal cell morphologic features related to progesterone synthesis. Theriogenology. 2013;79(4):673–679.
- Wilding M, Dale B, Marino M, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16(5):909–917.
- Van Blerkom J, Cox H, Davis P. Regulatory roles for mitochondria in the peri-implantation mouse blastocyst: possible origins and developmental significance of differential DeltaPsim. Reproduction. 2006;131(5):961–976.
- Tolkovsky AM. Mitophagy. Biochim Biophys Acta. 2009;1793(9):1508–1515.
- Pi H, Xu S, Reiter RJ, et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy. 2015;11(7):1037–1051.
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695.
- Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci. 2013;14(2):3834–3859.
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–10778.
- Tamura H, Takasaki A, Taketani T, et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60(1):1–13.
- Chang C, Su H, Zhang D, et al. AMPK-dependent phosphorylation of GAPDH triggers sirt1 activation and is necessary for autophagy upon glucose starvation. Mol Cell. 2015;60(6):930–940.
- Perkins AT, Das TM, Panzera LC, et al. Oxidative stress in oocytes during midprophase induces premature loss of cohesion and chromosome segregation errors. Proc Natl Acad Sci U S A. 2016;113(44):E6823–E30.
- Dumollard R, Marangos P, Fitzharris G, et al. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131(13):3057–3067.
- Zhang M, Dai X, Lu Y, et al. Melatonin protects oocyte quality from Bisphenol A-induced deterioration in the mouse. J Pineal Res. 2017;62(3):e12396.
- Tong J, Sheng S, Sun Y, et al. Melatonin levels in follicular fluid as markers for IVF outcomes and predicting ovarian reserve. Reproduction. 2017;153(4):443–451.
- Zhao XM, Hao HS, Du WH, et al. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J Pineal Res. 2016;60(2):132–141.
- Galley HF, McCormick B, Wilson KL, et al. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J Pineal Res. 2017;63(4):e12444.
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–1010.
- Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12(9):842–846.
- Durcan TM, Fon EA. The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015;29(10):989–999.
- Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125(21):4963–4971.
- Rottenberg H, Hoek JB. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell. 2017;16(5):943–955.
- Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27(2):433–446.
- Duan WJ, Liu FL, He RR, et al. Autophagy is involved in the effects of resveratrol on prevention of splenocyte apoptosis caused by oxidative stress in restrained mice. Mol Nutr Food Res. 2013;57(7):1145–1157.
- Yue Z, Friedman L, Komatsu M, et al. The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochim Biophys Acta. 2009;1793(9):1496–1507.
- Rahman M, Mofarrahi M, Kristof AS, et al. Reactive oxygen species regulation of autophagy in skeletal muscles. Antioxid Redox Signal. 2014;20(3):443–459.
- Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11(4):777–790.
- Kaminskyy VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal. 2014;21(1):86–102.
- Luciani A, Villella VR, Esposito S, et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010;12(9):863–875.
- Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105(9):3374–3379.
- Morselli E, Maiuri MC, Markaki M, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1(1):e10.
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5(1):253–295.
- Sanchez-Hidalgo AC, Munoz MF, Herrera AJ, et al. Chronic stress alters the expression levels of longevity-related genes in the rat hippocampus. Neurochem Int. 2016;97:181–192.
- Guo P, Pi H, Xu S, et al. Melatonin Improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol Sci. 2014;142(1):182–195.
- Yang Y, Jiang S, Dong Y, et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res. 2015;58(1):61–70.
- Areti A, Komirishetty P, Akuthota M, et al. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J Pineal Res. 2017;62(3):e12393.
- Luengo E, Buendia I, Fernández‐Mendívil C, et al. Pharmacological doses of melatonin impede cognitive decline in tau-related Alzheimer models, once tauopathy is initiated, by restoring the autophagic flux. J Pineal Res. 2019;67:e12578.
- Li J, Yu S, Ying J, et al. Resveratrol prevents ROS-induced apoptosis in high glucose-treated retinal capillary endothelial cells via the activation of AMPK/Sirt1/PGC-1alpha pathway. Oxid Med Cell Longev. 2017;2017:7584691.
