ABSTRACT
Curcumin alleviates septic acute kidney injury (SAKI); however, the underlying mechanism remained unclear. To explore this, SAKI cell model and mice model were conducted by using LPS and cecal ligation and puncture (CLP), respectively. Cell counting kit-8 (CCK-8) and enzyme-linked immunosorbent assay (ELISA) assays indicated that LPS reduced the viability, but upregulated the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6, whereas Curcumin pretreatment had no effect on viability, but reduced the levels of TNF-α and IL-6. Further assays showed that Curcumin partly attenuated the LPS-induced injury as the viability was enhanced, TNF-α and IL-6 expressions and cell apoptosis rates were reduced. Western blot analysis indicated that Janus kinase (JAK) 2/signal transducer and activator of transcription (STAT) 3, p-65-NF-κB and cell apoptosis pathways were activated by LPS but suppressed by Curcumin. Mice SAKI model further indicated that the serum Cystatin C (Cys-C), creatinine (Cr) and blood urea nitrogen (BUN) were increased within 24 h of model construction while those indicators were decreased at 48 h. Pretreated with Curcumin, NF-κB inhibitor (PDTC) or JAK2 inhibitor (AG-490) could weaken the renal histological injury and the increased serum Cys-C, Cr and BUN, IL-6 and TNF-α induced by CLP. Moreover, PDTC, AG-490 and Curcumin all significantly reversed the previously increased expressions of p-JAK2/STAT3, p-p65 and proapoptotic proteins in the mice with AKI. The present study revealed that Curcumin attenuated SAKI through inhibiting NF-κB and JAK2/STAT3 signaling pathways, and proposed that Curcumin could be a potential therapeutic agent for treating SAKI.
Introduction
Sepsis is characterized by a systemic inflammation response and can lead to multiple organ failures [Citation1]. Acute kidney injury (AKI) is a common and serious complication of sepsis patients [Citation2], and about 50% of all sepsis patients are likely to develop sepsis-caused AKI [Citation3]. Though preventive strategies and supportive therapies have been constantly developed to manage sepsis acute kidney injury (SAKI), its incidence and mortality are still high. Some high-risk factors can trigger the occurrence of chronic kidney diseases and end-stage renal diseases to patients with a previous history of AKI [Citation4].
The synthesis and secretion of cortisol are regulated by local interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) expressions, and a higher cortisol concentration maintains the stability of the cardiovascular system and normal cell metabolism, also reduces the inflammatory response [Citation5]. The concept of relative adrenocortical dysfunction proposed by Annabe et al. [Citation6] refers to that sepsis patients have higher serum cortisol concentration than normal ones, but they still experience relative cortisol deficiency. For patients with sepsis or multi-organ failures, TNF-α is not only an important inflammatory indicative of disease severity and the risk of developing complications but also an important stimulator of the death receptor pathway to induce cell apoptosis [Citation7].
Signal transduction and transcriptional activator 3 (STAT3) of the Janus kinase (JAK)-STAT signaling pathway is expressed in a variety of cells and tissues, moreover, it is also a signal transductor and transcriptional activator of cytokines and growth factor receptors [Citation8]. Many cytokines including TNF-α and IL-6 of JAK2/STAT3 pathway are involved in immune responses [Citation9], and JAK2/STAT3 pathway is involved in renal disease [Citation10].
Early detection of kidney injury in patients with sepsis is particularly significant to the success of subsequent treatment and prognosis of the patients. In recent years, biological markers related to AKI have been gradually discovered [Citation11], for example, serum Cystatin C (Cys, C), creatinine (Cr) and blood urea nitrogen (BUN) indicative of renal function injury are also predictive of SAKI.
The anti-inflammatory, anti-viral, anti-bacterial, anti-fungal, anti-oxidant and anti-cancerous effects of Curcumin have been gradually confirmed [Citation12]. Researchers observed the effects of Curcumin on sepsis animal models injected with endotoxin or bacteria, and that Curcumin showed protective effects on septic shock and can improve the functions of the cardiovascular, renal, liver, small intestine and coagulation system [Citation13]. The current study investigated the effects of Curcumin on acute renal failure caused by sepsis and explored its molecular mechanisms.
Materials and methods
Chemicals and reagents
LPS (L2630, purity>99%), Curcumin (8203540010, purity>98%), hematoxylin and eosin, PDTC (p8765, purity>98%) and AG-490 (T3434, purity>98%) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). LPS was diluted and added into the cell medium at a final concentration of 0, 5, 10 and 100 μg/mL. Curcumin was diluted and added into the cell medium at a final concentration of 0, 5, 10 and 20 μmol/L as the stock solution. PDTC was diluted into DMSO at 100 mM as the stock solution. AG-490 was diluted into DMSO at 58 mg/mL as the stock solution.
HK-2 cells and treatment
Human kidney HK-2 cells (ATCC) were cultured in RPMI1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2 in saturated humidity. The cells were let grow to logarithmic phase and then collected for later use. The cell culture and treatment was according to one previous research [Citation14].
For SAKI cell model conduction, LPS at 0, 5, 10 and 100 μg/mL was treated with HK-2 cells at 37°C, respectively. To explore the effect of curcumin, HK-2 cells were treated with curcumin for 12 h (0, 5, 10 and 20 μmol/L) at 37°C. To explore the effect of curcumin on LPS-induced SAKI, HK-2 cells were pre-treated with curcumin (10 μmol/L) for 12 h and then induced with LPS (10 μg/mL) for 4 h at 37°C according to one previous report [Citation15]. After that, cell functions were assessed.
CCK-8
The HK-2 cells in logarithmic growth phase were digested by trypsin to prepare a single-cell suspension, and the cells were seeded in 96-well culture plates at a density of 4 × 103 (200 μL/well). After treating the cells by LPS or Curcumin, a 10 μL of CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to the cells and incubated at 37°C for 2 h. The absorbance at 450 nm was measured using a microplate reader (SpectraMax M2, Molecular Devices, Silicon Valley, CA, USA).
Animals
Adult male C57 mice (weighting 20–25 g) were purchased from the Shanghai SLAC Laboratory Animal Co., Ltd. Every 5 mice were housed as a group in a cage, and provided with free access to food and water at controlled room temperature (22 ± 2°C) and humidity (60–80%) under a 12 h/12 h light/dark cycle. All animal experiments were performed in accordance with the Guidelines of the Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine Animal Committee.
Establishment of CLP model mice
The mice were equally divided into sham operation, CLP+saline, CLP+Cur (100 mg/kg), CLP+Cur (200 mg/kg), CLP+PDTC (120 mg/kg, NF-κB inhibitor) and CLP+AG-490 (5 mg/kg, JAK2 inhibitor) groups.
The mice were anesthetized by intraperitoneal injection of chloric acid, and then ligated 1 cm away from the blind end, the vessel was punctured using a 25 G syringe head. After the cecal ligation, the cecum was carefully placed back into the abdominal cavity. After the model establishment, peripheral blood of mice was collected at 1 h, 12 h, 24 h and 48 h, and the kidney tissues of the mice were taken. The above drugs (saline, Cur, PDTC, AG-490) were used to pretreat the mice for 0.5 h before the surgery. The kidney tissues of the mice were taken, and the peripheral blood of the mice was collected 24 h after the modeling.
Enzyme-linked immunosorbent assay (ELISA)
The TNF-α (human, P01375; mouse, P06804; RayBiotech, Norcross, GA, United States) and IL-6 (human, P05231; mouse, P08505; RayBiotech) in the cell supernatant and mouse serum were determined by ELISA assay kit. Briefly, the cell supernatant sample was collected by centrifugation at 4°C, 500 g, 5 min. Mice serum was collected by centrifugation at 4°C, 2000 g, 10 min. One hundred-microliter sample was added into the 96-well plate and then the well was sealed with a membrane incubated at room temperature for 120 min. Then, biotinylated antibody, Horseradish peroxidase (HRP) labeled Streptavidin, and reaction reagent was added and incubated orderly at 25°C for a proper time. After that, 50 μL stop solution was added and the A450 value was measured immediately after blending using a microplate reader (SpectraMax M2, Molecular Devices, Silicon Valley, CA, USA). The assays were conducted in duplicate according to the manufacturer’s protocols.
Flow cytometry
An apoptosis detection kit (Annexin V-FITC/PI, Beyotime, China) was purchased for the assessment of cell apoptosis. Briefly, the cells were collected by trypsinization at 37°C for 2 min and centrifugation at 1000 x g, 4°C for 5 min. Then, 5 µL Annexin V and 10 µL PI mixed into 195 µL binding buffer (1x) were incubated with cells for 15 min for room temperature. Then, the fluorescence intensity was detected by a cytometer (BD Biosciences, USA). The apoptotic cells were then analyzed by FACSCanto™ system software v2.4 (cat: 646602, BD Biosciences, USA).
Quantitative polymerase chain reaction (qPCR)
The total RNA from cells was extracted by using a kit (RNA Easy Fast, DP451, Tiangen, China). All operations were conducted according to the instructions. Then, 1 µg RNA was used for reverse-transcription to obtain the total cDNA via cDNA Synthesis Kit (KR118, Tiangen, China). The cDNA was then used for PCR by using SYBR Green I Mix (FP215, Tiangen, China). The reaction condition for PCR was set as follows: 15 min at 95°C predegeneration, 40 cycles of 95°C (10 sec) and 60°C (20 sec), then melting/Dissociation Curve Stage. Real-Time PCR Detection System (CFX 96 Touch, Bio-Rad, China) was used to perform the PCR reaction. GAPDH was used as a reference gene. The 2-ΔΔCt method was applied to determine the relative expression. The forward primer for IL-6 (human): 5ʹ-ACTCACCTCTTCAGAACGAATTG-3ʹ and the reverse primer: 5ʹ-CCATCTTTGGAAGGTTCAGGTTG-3ʹ. The forward primer for TNF-α (human): 5ʹ-CTCGCTGCAGTTGCTTTTGT-3ʹ; and the reverse primer: 5ʹ-TGGAATGCCTGATCCACACC-3ʹ. The forward primer for IL-6 (mouse): 5ʹ-GAGACTTCCATCCAGTTGCCT-3ʹ; and the reverse primer: 5ʹ-TGGGAGTGGTATCCTCTGTGA-3ʹ. The forward primer for TNF-α (mouse): 5ʹ- GAGGCACTCCCCCAAAAGAT-3ʹ; and the reverse primer: 5ʹ- GAGGGAGGCCATTTGGGAAC −3ʹ.
Mouse serum Cys, C, Cr and BUN levels detection
Mice blood samples were collected via tail vein 1 h, 12 h, 24 h and 48 h after CLP, respectively. Then, the serum was obtained by centrifugation at 4°C, 3000 g for 15 min. Mouse serum creatinine (Cr), urea nitrogen (BUN) and cystatin C (Cys) were then measured using Roche automatic biochemical analyzers (cobas c311, Basel, Switzerland).
Hematoxylin and eosin (H&E) staining
Mouse kidney tissue was isolated and rinsed in 10% formalin for 24 h at room temperature. Sections (2 μm) were prepared after paraffin embedding. Then, the sections were dewaxed and hydrated by xylene and alcohol (70%, 90%, 100%, v/v) at room temperature. The sections were then stained by Hematoxylin for 5 min and then eosin for 1 min (Beyotime, China) at room temperature. After washed by 70% alcohol, the stained sections were observed under an optical microscope (TS100, Nikon, Japan).
Western blot
An RIPA lysate (Pierce; Thermo Fisher Scientific, Inc.) containing protease inhibitor and phosphatase inhibitor was used to extract total proteins from HK2 cells and mouse kidney tissues, and the protein concentration was detected by BCA kit. Thirty micrograms of proteins were separated on 10% SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes (Nanjing Institute of Bioengineering, China). The PVDF membranes were immersed in 5% nonfat milk for 2 h and then incubated with the following primary antibodies overnight at 4°C: anti-p-JAK2 (130 kDa; rabbit; 1:500; ab32101; abcam), anti-JAK2 (130 kDa; rabbit; 1:1000; ab108596; abcam), anti-p-STAT3 (88 kDa; rabbit; 1:1000; ab76351; abcam), anti-STAT3 (88 kDa; rabbit; 1:1000; ab68153; abcam), anti-p-p65 (60 kDa; rabbit; 1:1000; ab86299; abcam), anti-p65 (60 kDa; rabbit; 1:1000; ab16502; abcam) and anti-GAPDH (36 kDa; mouse; 1:5000; ab8245; abcam). After washing the membranes using TBS, horseradish peroxidase-labeled goat anti-rabbit or mouse IgG (1:5000, sc-516102/sc-2357; Santa Cruz Biotechnology, Inc. Dallas, TX, USA) was used to incubate with the target strips at 37°C for 2 h. The target strips were then developed by ECL kit (Biyuntian Biotechnology Co., Ltd.).
Statistical analysis
The results were shown as the mean + SD. Statistical significance was determined by analysis of variance between groups (ANOVA), followed by Tukey t test. The statistical analyses were all performed in GraphPad Prism (Graph-Pad Software Inc). P < 0.05 was considered statistically significant.
Results
LPS-induced inflammatory response of HK-2 cells
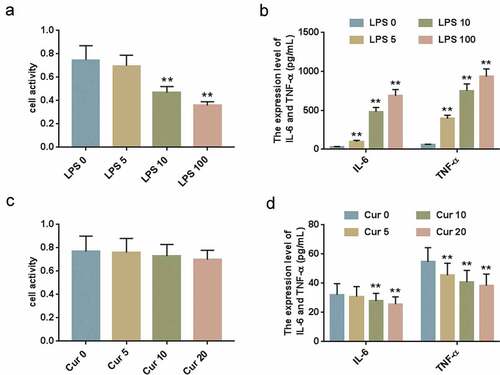
HK-2 cells were treated by LPS at different concentrations (0, 5, 10 and 100 μg/ml) and subjected to CCK-8 and ELISA. The results of CCK-8 assay showed that LPS at 10 μg/mL and 100 μg/mL obviously inhibited HK-2 cell viability ()). The ELISA data demonstrated that LPS significantly increased expression levels of IL-6 and TNF-ɑ ()). Next, the HK-2 cells were treated by different concentrations of Curcumin (0, 5, 10 and 20 μmol/L), and CCK-8 assay and ELISA were performed to determine the cell viability and IL-6 and TNF-ɑ expression levels. The results demonstrated that CCK-8 had no cytotoxicity on HK-2 cells ()), and that LPS inhibited IL-6 and TNF-ɑ expression levels ()). Therefore, in the subsequent experiments, the optimal treating concentration for LPS was determined to be 10 μg/mL and 10 μmol/L for Curcumin.
Figure 1. LPS-induced inflammatory response of HK-2 cells.
Curcumin ameliorated LPS-induced inflammatory response of HK-2 cells via JAK2/STAT3 and NF-κB
To investigate the effects of Curcumin on LPS-treated HK-2 cells, the cells were stimulated by LPS and Curcumin. CCK-8 assay data demonstrated that compared with the LPS group, Curcumin remarkably increased HK-2 activity ()). The inflammatory response of cell was evaluated according to TNF-ɑ and IL-6 expression levels, as shown in -c), Curcumin significantly reduced the expression levels of TNF-α and IL-6 in HK-2 cells previously increased by LPS. Flow cytometry analysis indicated that LPS induced the increase of cell apoptosis rate, which could be partly rescued by Curcumin ()).
Figure 2. Curcumin Ameliorated LPS-Induced inflammatory response of HK-2 cells via JAK2/STAT3 and NF-κB.
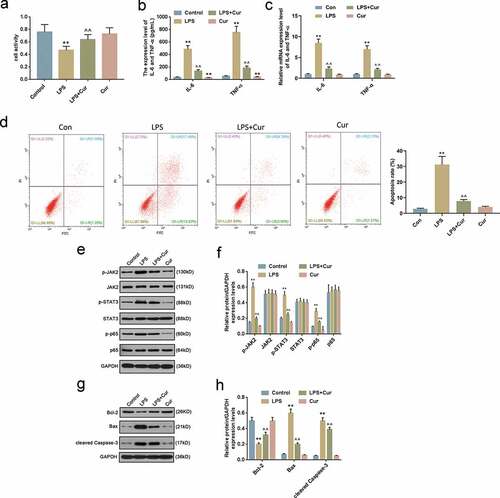
To explore the underlying mechanism, Western blot was performed to detect the expression levels of JAK2/STAT3 and NF-κB and apoptosis-associated proteins. The results showed that Curcumin markedly suppressed the expressions of JAK2, STAT3 and p65 phosphorylation (,)) previously increased by LPS, and partially reversed the LPS-induced protein expressions of Bcl-2, Bax and cleaved caspase-3 (,)).
Establishment of septic acute kidney injury (SAKI) model in the mice using CLP
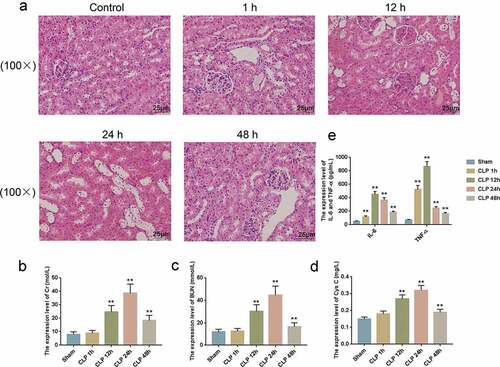
It has been demonstrated in vitro that Curcumin could protect kidney damage, but the effects of Curcumin on the SAKI model in mice remained unclear. In the current study, kidney tissues collected from the CLP-induced SAKI model mice were paraffin-embedded and sectioned. H&E staining was performed to observe the changes in renal physiological structures. We found that compared with the normal group, the kidney tissues of sepsis mice showed mild damage after 1 h, and the changes were more obvious after 6 h. At 24 h, the renal tissues of the sepsis model mice showed significant pathological changes; moreover, we observed infiltration of inflammatory cells, swelling of most renal tubular epithelial cells, and degeneration of vacuoles. However, 48 h after the induction of sepsis to the model mice, the renal histopathology of the mice was significantly improved, but inflammatory cell infiltration was still observed in the renal interstitium ()). Cr ()), BUN ()), and Cys C ()) are markers indicative of SAKI, and their expressions were determined. The results showed that the expression levels of Cr, BUN and Cys C of the model mice were increased by CLP treatment at 12, 24 and 48 h, also the expressions of IL-6 and TNF-α of the mice were increased by CLP ()).
Figure 3. Septic acute kidney injury (SAKI) mouse model was established using CLP.
Curcumin alleviated CLP-induced SAKI of the mice
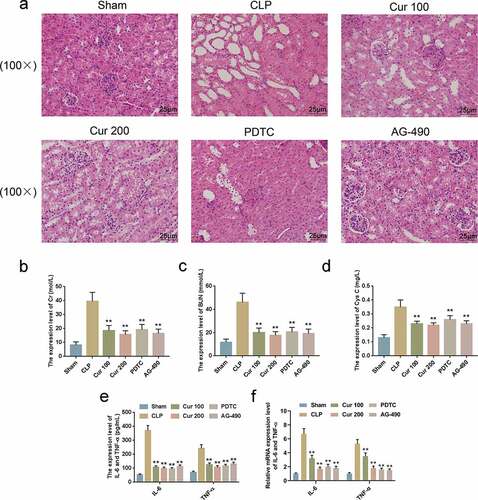
The model mice were pre-injected with Curcumin, NF-κB signaling pathway inhibitor PDTC and JAK2/STAT3 signaling pathway inhibitor AG-490, so as to investigate whether Curcumin had protective effects on CLP-induced SAKI of the mice and the role of NF-κB signaling pathway and JAK2/STAT3 signaling pathway in SAKI. H&E staining showed that kidney tissue damages were significantly alleviated by Curcumin, PDTC and AG-490 as compared with the CLP group ()). After pretreating the cells with Curcumin, PDTC and AG-490, the serum levels of Cr ()), BUN ()) and Cys C ()) of the mice were significantly lower after the establishment of the CLP model at 24 h. Furthermore, we also found that the levels of IL-6 and TNF-ɑ in the CLP-treated mice were reduced by Curcumin, PDTC and AG-490 (-f)).
Figure 4. Curcumin alleviated CLP-Induced SAKI of the mice.
Curcumin alleviated CLP-activated JAK2/STAT3 and NF-κB signaling pathways and reduced cell apoptosis of the mice
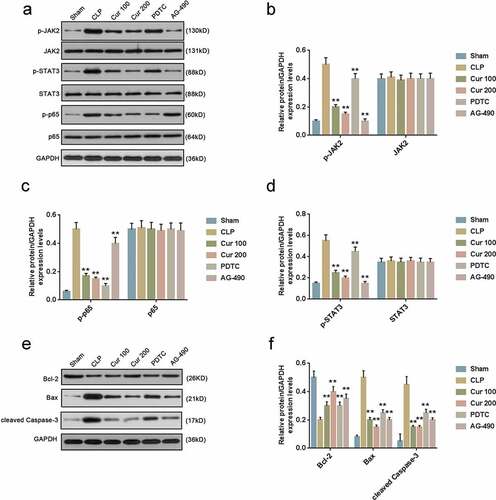
Western blot was performed to protein expressions in JAK2/STAT3 and NF-κB signaling pathways and cell apoptosis. The results showed that phosphorylation levels of JAK2, STAT3 and p65 were all inhibited by Curcumin, PDTC and AG-490 ()). Moreover, protein expressions of Bax and cleaved caspase-3 previously increased by CLP were partially reversed by Curcumin, PDTC and AG-490, and protein expressions of Bcl-2 previously reduced by CLP were partially reversed by Curcumin, PDTC and AG-490 (,)).
Figure 5. Curcumin alleviated CLP-activated JAK2/STAT3 and NF-κB signaling pathways and ERS of the mice.
Discussion
The current study found that SAKI induced by LPS or CLP was significantly ameliorated by pre-treatment of Curcumin through greatly reducing the secretion of inflammatory cytokines (IL-6 and TNF-ɑ). Curcumin ameliorated SAKI through reducing inflammation and cell apoptosis and suppressing JAK2/STAT3 and NF-κB pathway activation. Our research provided a potential clinical value of Curcumin for AKI.
Sepsis renal injury model was established by cecal perforation ligation (CLP) [Citation16] and is an effective model for studying SAKI mechanism. In the pathogenesis of sepsis, monocytes release a variety of cytokines including TNF-ɑ and IL-6, which are seen as key factors [Citation17]. The main basis for the current diagnosis of AKI is serum creatinine, BUN and Cys [Citation18]. In our study, the expressions of TNF-ɑ and IL-6 as well as the levels of BUN, serum creatinine and Cys-C were increased in LPS-treated HK2 cells, at the same time, obvious pathological damages were observed in the CLP-treated model mice. Those data indicated that the SAKI models were successfully set up in vitro and in vivo.
Many studies showed that Curcumin has significant anti-inflammatory and anti-apoptosis effects. Shuaijun Zhu et al. reported that Curcumin attenuated renal injury in rats with severe acute pancreatitis by inhibiting inflammatory cytokines [Citation19]. Curcumin also has multiple molecular targets. NF-κB is one of the targets for Curcumin. Curcumin inhibits the NF-κB signaling pathway, and reduces the release of proinflammatory factors in RAW246.7 cells treated by cigarette smoke extracts [Citation20]. Inhibition of NF-κB may be the main mechanism for the anti-inflammatory effect of Curcumin [Citation21]. The activation of NF-κB is relayed on the phosphorylation of p65 [Citation22]. Our research confirmed that the increased p65 phosphorylation caused by CLP could be reversed by Curcumin, PDTC or AG-490, suggesting that Curcumin might regulate the activation of NF-κB to play the protective effects on SAKI. Nevertheless, a combination of Curcumin and PDTC should be conducted to confirm this.
Evidence showed that the JAK/STAT pathway is involved in renal disease. IL-1β cerulean-stimulated pancreatic acinar cells are expressed under the activation of JAK2/STAT3 signaling [Citation16]. Moreover, it has been found that Curcumin, as an inhibitor of the JAK2/STAT3 pathway, has remarkable protective effects on hyperuricemia, renal endothelial dysfunction and myocardial ischemia and reperfusion [Citation23]. A previous study also demonstrated that Curcumin could slow down the growth of cysts in autosomal dominant polycystic kidney disease [Citation16]. It is also known that Curcumin suppresses the activation of JAK2/STAT3 pathway to protect against rat acute renal injury [Citation19]. In other inflammatory-related diseases, such as neuropathic pain and cerebral ischemia, similar results have been reported [Citation24,Citation25]. Our findings showed that Curcumin attenuated renal injury via the inhibition of JAK2/STAT3 signaling pathway in vitro and in vivo, which was consistent with the previous researches. Nevertheless, the further mechanism on how Curcumin modulates the activation of JAK2/STAT3 signaling pathways remains unknown.
Curcumin supplementation could reduce the expressions of IL-8, TNF-α and IL-6 in the rats with diabetes [Citation26]. Besides, cell apoptosis can be regulated by Curcumin despite the contrary conclusion that has been found in different cell models. For instance, apoptosis of preadipocyte and castration-resistant prostate cancer cells could be induced by Curcumin [Citation27,Citation28]. Different from those results, in chondrocytes, Curcumin is found to inhibit the apoptosis progress through modulating ERK1/2 pathways [Citation29]. In the present study, we found that Curcumin attenuated renal injury in vitro and in vivo through the inhibition of apoptosis-related proteins and inflammatory cytokines. A flow cytometry analysis also indicated that apoptosis could be significantly attenuated by Curcumin.
It is noted that differentially expressed genes, non-coding RNAs and related pathways had been analyzed in other inflammatory-related diseases, such as retinitis pigmentosa and cerebral cavernous malformations using RNA sequencing analysis [Citation30–Citation34]. However, an RNA sequencing analysis of differentially expressed genes and pathways in Curcumin treated mice with AKI has not been reported. In the future, the role of NF-κB and JAK2/STAT3 signaling pathways in Curcumin treated AKI will be further analyzed by the RNA sequencing analysis.
In conclusion, the present study demonstrated the protective effects of Curcumin in cell and mouse models of SAKI. The underlying mechanism through which Curcumin realized such effects was partly through reducing inflammatory cytokine secretion by inhibiting the activation of JAK2/STAT3 and TNF-α pathways. The current findings revealed that Curcumin to be used in the prevention and treatment of SAKI.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Bullock B, Benham MD. Bacterial Sepsis. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2019.
- Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010 Feb;21(2):345–352. PubMed PMID: 20019168; PubMed Central PMCID: PMCPMC2834549. eng.
- Bagasha P, Nakwagala F, Kwizera A, et al. Acute kidney injury among adult patients with sepsis in a low-income country: clinical patterns and short-term outcomes. BMC Nephrol. 2015 Jan 16;16:4. PubMed PMID: 25592556; PubMed Central PMCID: PMCPMC4361197. eng. DOI:10.1186/1471-2369-16-4
- Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009 May;4(5):891–898. PubMed PMID: 19406959; PubMed Central PMCID: PMCPMC2676192. eng.
- Hietbrink F, Koenderman L, Rijkers G, et al. Trauma: the role of the innate immune system. World J Emerg Surg. 2006 May;20(1):15. PubMed PMID: 16759367; PubMed Central PMCID: PMCPMC1481567. eng
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. Jama. 2002 Aug 21;288(7):862–871. PubMed PMID: 12186604; eng.
- Morrell ED, Kellum JA, Pastor-Soler NM, et al. Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Crit Care. 2014 Sep 2;18(5):501. PubMed PMID: 25575158; PubMed Central PMCID: PMCPMC4729166. eng.
- Lu X, Zhu Z, Jiang L, et al. Matrine increases NKG2D ligand ULBP2 in K562 cells via inhibiting JAK/STAT3 pathway: a potential mechanism underlying the immunotherapy of matrine in leukemia. Am J Transl Res. 2015;7(10):1838–1849. PubMed PMID: 26692928; PubMed Central PMCID: PMCPMC4656761. eng
- Yang X, He G, Hao Y, et al. The role of the JAK2-STAT3 pathway in pro-inflammatory responses of EMF-stimulated N9 microglial cells. J Neuroinflammation. 2010 Sep;9(7):54. PubMed PMID: 20828402; PubMed Central PMCID: PMCPMC2945324. eng
- Horiguchi A, Asano T, Kuroda K, et al. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer. 2010 May 25;102(11):1592–1599. PubMed PMID: 20461084; PubMed Central PMCID: PMCPMC2883159. eng.
- Pang M, Ma L, Gong R, et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010 Aug;78(3):257–268. PubMed PMID: 20520592; eng.
- Constantin J-M, Futier E, Perbet S, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. 2010 Mar;25(1):176.e1-6. PubMed PMID: 19781900; eng.
- Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–243. PubMed PMID: 17569214; eng
- Donato L, Scimone C, Rinaldi C, et al. Stargardt phenotype associated with two ELOVL4 promoter variants and ELOVL4 downregulation: new possible perspective to etiopathogenesis? Invest Ophthalmol Vis Sci. 2018 Feb 1;59(2):843–857. PubMed PMID: 29417145; eng.
- Zhong F, Chen H, Han L, et al. Curcumin attenuates lipopolysaccharide-induced renal inflammation. Biol Pharm Bull. 2011;34(2):226–232. PubMed PMID: 21415532; eng
- Yu JH, Kim KH, Kim H. Suppression of IL-1beta expression by the Jak 2 inhibitor AG490 in cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol. 2006 Nov 30;72(11):1555–1562. PubMed PMID: 16934228; eng
- Copeland S, Warren HS, Lowry SF, et al. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005 Jan;12(1):60–67. PubMed PMID: 15642986; PubMed Central PMCID: PMCPMC540200. eng.
- Kozan R, Sare M, Yilmaz TU, et al. Effectiveness of new parameters in the evaluation of pneumoperitoneum-related acute kidney injury in rats. Turk J Med Sci. 2018 Dec 12;48(6):1278–1284. PubMed PMID: 30542596; eng.
- Zhu S, Zhang C, Weng Q, et al. Curcumin protects against acute renal injury by suppressing JAK2/STAT3 pathway in severe acute pancreatitis in rats. Exp Ther Med. 2017 Aug;14(2):1669–1674. PubMed PMID: 28810635; PubMed Central PMCID: PMCPMC5526141. eng.
- Li N, Liu TH, Yu JZ, et al. Curcumin and curcumol inhibit NF-kappaB and TGF-beta 1/Smads signaling pathways in CSE-treated RAW246.7 cells. Evid Based Complement Alternat Med. 2019;2019:3035125. PubMed PMID: 31007701; PubMed Central PMCID: PMCPMC6441512. eng
- Ukil A, Maity S, Karmakar S, et al. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003 May;139(2):209–218. PubMed PMID: 12770926; PubMed Central PMCID: PMCPMC1573841. eng.
- Peng J, Ren X, Lan T, et al. Renoprotective effects of ursolic acid on ischemia/reperfusion‑induced acute kidney injury through oxidative stress, inflammation and the inhibition of STAT3 and NF‑κB activities. Mol Med Rep. 2016 Oct;14(4):3397–3402. PubMed PMID: 27573738; eng.
- Zhang J, Tang L, Li GS, et al. The anti-inflammatory effects of curcumin on renal ischemia-reperfusion injury in rats. Ren Fail. 2018 Nov;40(1):680–686. PubMed PMID: 30741618; PubMed Central PMCID: PMCPMC6282432. eng.
- Wu Y, Xu J, Xu J, et al. Study on the mechanism of JAK2/STAT3 signaling pathway-mediated inflammatory reaction after cerebral ischemia. Mol Med Rep. 2018 Apr;17(4):5007–5012. PubMed PMID: 29393445; PubMed Central PMCID: PMCPMC5865961. eng.
- Liu S, Li Q, Zhang MT, et al. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep. 2016 Jul;6(6):28956. PubMed PMID: 27381056; PubMed Central PMCID: PMCPMC4933926. eng
- Jain SK, Rains J, Croad J, et al. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009 Feb;11(2):241–249. PubMed PMID: 18976114; PubMed Central PMCID: PMCPMC2933148. eng.
- Wu LY, Chen CW, Chen LK, et al. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients. 2019 Sep 28;11(10). PubMed PMID: 31569380; PubMed Central PMCID: PMCPMC6836120. eng. DOI:10.3390/nu11102307.
- Yang C, Ma X, Wang Z, et al. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des Devel Ther. 2017;11:431–439. PubMed PMID: 28243065; PubMed Central PMCID: PMCPMC5317247. eng
- Li X, Feng K, Li J, et al. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients. 2017 Apr 21;9(4). PubMed PMID: 28430129; PubMed Central PMCID: PMCPMC5409753. eng. DOI:10.3390/nu9040414.
- Donato L, Scimone C, Nicocia G, et al. Role of oxidative stress in retinitis pigmentosa: new involved pathways by an RNA-Seq analysis. Cell Cycle (Georgetown, Tex). 2019 Jan;18(1):84–104. PubMed PMID: 30569795; PubMed Central PMCID: PMCPMC6343708. eng.
- Donato L, Scimone C, Rinaldi C, et al. Non-coding RNAome of RPE cells under oxidative stress suggests unknown regulative aspects of retinitis pigmentosa etiopathogenesis. Sci Rep. 2018 Nov 9;8(1):16638. 10.1038/s41598-018-35086-z. PubMed PMID: 30413775; PubMed Central PMCID: PMCPMC6226517. eng
- Donato L, Bramanti P, Scimone C, et al. miRNAexpression profile of retinal pigment epithelial cells under oxidative stress conditions. FEBS Open Bio. 2018 Feb;8(2):219–233. PubMed PMID: 29435412; PubMed Central PMCID: PMCPMC5794457. eng.
- Donato L, Scimone C, Nicocia G, et al. GLO1 gene polymorphisms and their association with retinitis pigmentosa: a case-control study in a Sicilian population. Mol Biol Rep. 2018 Oct;45(5):1349–1355. 10.1007/s11033-018-4295-4. PubMed PMID: 30099685; eng
- Rinaldi C, Bramanti P, Famà A, et al. GLYOXALASE I A111E, PARAOXONASE 1 Q192R AND L55M POLYMORPHISMS IN ITALIAN PATIENTS WITH SPORADIC CEREBRAL CAVERNOUS MALFORMATIONS: A PILOT STUDY. J Biol Regul Homeost Agents. 2015 Apr-Jun;29(2):493–500. PubMed PMID: 26122242; eng
