ABSTRACT
This study aimed to determine the WNT2 expression in patients with severe preeclampsia and to explore the function of WNT2 dysregulation on the biological behaviors of trophoblast cells. The WNT2 and β-catenin expression in the patients with early-onset and late-onset severe preeclampsia and normal controls was determined. Subsequently, WNT2 was overexpressed and knocked down in HTR8 cells and WNT2 signaling pathway in regulating trophoblast cell proliferation, migration, invasion, and apoptosis were evaluated in vitro. The mRNA and protein expression levels of WNT2 and β-catenin were decreased in patients with preeclampsia, especially early-onset severe preeclampsia. Overexpression of WNT2 promoted trophoblast cell proliferation, migration, and invasion and inhibited apoptosis in vitro, whereas knockdown of WNT2 had opposite effects. The findings of this study reveal that WNT2 and β-catenin were decreased expressed in patients with preeclampsia. Decreased expression of WNT2 may inhibit trophoblast cell proliferation, migration, and invasion but induced apoptosis. WNT2 may serve as a promising biomarker for early detection of preeclampsia.
Introduction
Preeclampsia is a disorder in which there are high blood pressure and other signs of organ injury, according to the criteria of the American Congress of Obstetricians and Gynecologists (ACOG) [Citation1]. It can occur during pregnancy or after childbirth and is characterized by hypertension, proteinuria, and other symptoms that cause adverse health effects in both mother and fetus [Citation2]. It is reported that preeclampsia results from the reduced invasion of trophoblasts into the endometrium and myometrium during early placentation and failed remodeling of the maternal spiral artery during pregnancy [Citation3]. Early-onset preeclampsia can lead to an elevated perinatal mortality rate and results in severe neonatal morbidity [Citation4,Citation5]. Moreover, preeclampsia is likely to progress to eclampsia due to delayed diagnosis and treatment [Citation6]. Although great efforts have been made, the pathogenesis of preeclampsia remains poorly understood [Citation7,Citation8]. Therefore, to improve the clinical outcome of preeclampsia, it is still imperative to deepen understand the key mechanisms underlying this disease.
Preeclampsia is considered as a pregnancy complication of placental origin because the clinical symptoms rapidly disappear after the delivery of placenta [Citation9]. The placenta is shown to participate in the initiation and progression of preeclampsia [Citation10]. The trophoblast cells, the major cell type in the placenta, play crucial roles in both placental and fetal development [Citation11], and dysfunction of trophoblast cells may result in preeclampsia [Citation12]. Secreted glycoprotein WNT2 is one of the canonical Wnt ligands, whose expression is detected during placenta development [Citation13]. WNT2 gene is found to be decreased expressed in the preeclampsia placentas [Citation14]. A recent study also confirms that reduced WNT2 expression may be implicated in the development of early-onset preeclampsia [Citation15]. Furthermore, increasing studies have revealed the key roles of the Wnt pathway in multiple physiological and pathological processes, such as proliferation, apoptosis, differentiation, and migration [Citation16,Citation17]. WNT2 signaling pathway is found involved in placental vasculogenesis and function [Citation18]. Our previous study has also reported that aberrant activation of the WNT2 signaling pathway may participate in preeclampsia pathogenesis [Citation18]. However, the function of WNT2 in the development of preeclampsia has not been fully clarified.
In this study, we determined the WNT2 and β-catenin expression in patients with early-onset and late-onset severe preeclampsia. Subsequently, WNT2 was overexpressed and knocked down in HTR8 cells, and the effects of overexpression and knockdown of WNT2 on trophoblast cell proliferation, migration, invasion, and apoptosis were evaluated in vitro. Our findings will provide new insight into the early diagnosis or efficient treatment of preeclampsia.
Materials and methods
Patients studied
Between January 2018 and December 2018, 30 patients with early-onset (defined as onset before 34 weeks of pregnancy) severe preeclampsia (ZPE group), 30 patients with late-onset severe preeclampsia (WPE group), and 30 normal controls that presented for delivery at term (≥ 37 weeks gestation) (NC group) were enrolled in the study. These patients underwent a cesarean section in the Department of Gynecology and Obstetrics, Binzhou Medical University Hospital (Binzhou, Shandong, China). Severe preeclampsia was diagnosed according to the diagnosis criteria [Citation19]. All patients had regular menstruation and knew the exact time of last menstruation. The patient was confirmed to be intrauterine single pregnancy by ultrasound, and the gestational week was confirmed by ultrasound according to the first trimester.
Patients with multiple pregnancies, chronic hypertension, renal disease, thyroid dysfunctions, or pregnancy complications such as gestational diabetes were excluded. Patients had blood and urine biochemical abnormalities that could not be explained by preeclampsia were also excluded.
The study was approved by the local ethics committee of Binzhou Medical University Hospital, and all patients provided written informed consents.
Sample collection
Within 30 min after delivery from the uterus, masses of placental villous tissues (0.5 × 0.5 × 0.5 cm) were collected from the maternal side of the placenta and then washed with sterile PBS. Some samples were fixed with formalin and then embedded in paraffin. The remaining samples were immediately snap-frozen in liquid nitrogen and stored at a refrigerator at – 80°C for later use.
Real-time quantitative PCR (qPCR)
Total RNA was extracted using the Trizol reagent (TaKaRa, Japan). After evaluating the concentration and purity of the RNA, reverse transcription for cDNA synthesis was conducted using 1 μg RNA as a template. Subsequently, real-time qPCR was carried out using the SYBR ExScript qRT-PCR Kit (Takara, Japan) in an Mx3000PTM RealTime PCR System (Stratagene, La Jolla, CA, USA). GAPDH was used as the internal control, and the relative expression levels of WNT2 and β-catenin were evaluated using the 2−ΔΔCT method. The sequences of primers were synthesized at Sangon Biotech (Shanghai, China) and shown in .
Table 1. The sequences of primers used in this study.
Western blot assay
Total proteins were extracted on ice using 1 × RIPA buffer (50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and 1 mM EDTA-Na2) containing phenylmethylsulfonyl fluoride (PMSF, Sigma, USA). The concentrations of extracted protein were then examined by bicinchoninic acid (BCA) protein assay kit (Invitrogen, Carlsbad, CA, USA). Protein extracts (20 µg per lane) were then resolved by 10%SDS-PAGE electrophoreses and transferred to polyvinylidene difluoride (PVDF) membranes (Roche, Switzerland). After blocking for 60–70 min, the target protein was probed with rabbit anti-human primary antibodies (WNT2 (dilution, 1:5000; 66656-1-Ig; PTG, Chicago, IL), β-catenin (dilution, 1:5000; 51067-2-AP; PTG), Bax (dilution, 2:1000; 50599-2-lg; PTG), BCL-2 (dilution, 1:1000; AF6139; Affinity Biosciences, Inc., Cincinnati, OH, USA), Cleaved-Caspase 3 (dilution, 1:1000; AF6311; Affinity Biosciences, Inc.), E-Cadherin (dilution, 1:1000; 20874-1-AP; PTG), N-Cadherin (dilution, 1:1000; 22018-1-AP; PTG), Vimentin (dilution, 1:1000; AF7013; Affinity Biosciences, Inc.)), and mouse anti-human GAPDH primary antibody (dilution, 1:5000; 60004-1-Ig; PTG) overnight at 4°C, and incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (dilution, 1: 5000; S0001; Affinity Biosciences, Inc.) or anti-mouse IgG (dilution, 1: 5000; S0002; Affinity Biosciences, Inc.) secondary antibodies for 1 h at room temperature. The protein bands in membranes were developed with enhanced chemiluminescence (ECL) reagents (Millipore, Billerica, MA, USA).
Immunohistochemistry
Placental villous tissue sections (3 μm) cut from paraffin-embedded samples were deparaffinized and rehydrated. The sections were then incubated with citrate buffer for antigen retrieval. After rinsing with PBS, 3% H2O2 was added to block endogenous peroxidases for 10 min. Subsequently, the sections were incubated with rabbit anti-human WNT2 primary antibody (dilution, 1:50; A5864; ABclonal Technology, MA, USA) and β-catenin primary antibody (dilution, 1:50; 51067-2-AP; PTG) overnight at 4°C, and probed with HRP-labeled goat anti-rabbit IgG secondary antibody (dilution, 1: 200; GB23303; Servicebio, Inc., Woburn, MA) at 37°C for 30 min. After rinsing thrice with PBS, the color was developed using 3, 3ʹ-diaminobenzidine (DAB, Servicebio, Inc.). Sections were then counterstained with hematoxylin, dehydrated, and finally covered with a coverslip. Each slide was photographed by a microscope (XSP-C204, COIC, Chongqing, China), and two pictures (200× magnification) were randomly selected from each slide. The integrated optical density (IOD) and the area of pictures were measured using Image-Pro Plus 6.0 software, and the mean density was calculated as IOD/area in each image. The higher the mean density, the higher the level of positive WNT2 protein expression.
Cell culture and transfection
Human trophoblast HTR8 cell line (Cell Bank of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS) and then maintained at a 37°C, 5% CO2 incubator.
HTR8 cells were plated into 6-well plates and grown until 80% confluency. The short hairpin RNA (shRNA) targeting WNT2 (WNT2-shRNA), WNT2-overexpression vectors, and their negative controls (NC) were transfected into HTR8 cells by means of Lipofectamine 2000 (Invitrogen). After 48 h of transfection, cells were harvested for the subsequent experiments.
Enzyme linked immunosorbent assay (ELISA)
After different transfection, the WNT2 concentration in the supernatants of cell cultures in different groups was measured using a human WNT2 ELISA kit (Jianglaibio. Inc., Shanghai, China) following the manufacturer’s instructions.
CCK8 assay
HTR8 cells (1500 cells) were seeded onto 96-well plates. After different transfection, cell viability of different groups was detected using the CCK-8 kit (CK04, Dojindo, Japan) following the manufacturers’ recommended protocols. Briefly, at various times of treatments, 10 μl of CCK8 solution was added to incubate the cells for 1.5 h at 37°C. With a microplate reader (Epoch, BioTek, USA), absorbance at 450 nm was detected to calculate cell viability.
Colony-forming assay
After 24 h of transfection, HTR8 cells of different groups were digested with 0.25% trypsin, resuspended, and counted. Then, 500 cells were inoculated into a 60 mm culture dish containing 5 mL of preheated complete medium and maintained a 37°C, 5% CO2 incubator for 7–14 d until a visual clone appeared. After fixation with 4% paraformaldehyde, the colonies were stained with 0.1% crystal violet and counted.
Detection of cell apoptosis
After 24 h of transfection, the HTR8 cells of different groups were starved for 24 h with a serum-free medium. The cells were harvested, washed with pre-chilled PBS, and then resuspended in 1 × binding buffer with a density of 1 ~ 5 × 106 cells/mL. Afterward, 100 μL cell suspension was stained with 5 μL Annexin V-FITC and 10 μL propidium iodide (PI) away from light. Cell apoptosis was then detected by a FACS can (Beckman Coulter, Fullerton, CA, USA), and analyzed by using FlowJo software (Tree Star Software, San Carlos, California, USA).
Scratch wound healing assay
A marker pen was used to draw five lines on the back of the culture dishes. Approximately 5 × l05 HTR8 cells of different groups were grown in culture dishes. After 24 h of incubation, cells formed a confluent monolayer. A straight “scratch” of the cell monolayer was created using a sterile pipette tip and the debris after the scratch was removed. After incubation with a serum-free medium at a 37°C, 5% CO2 incubator for 24 h, the migrated cells were observed and photographed.
Transwell assays
Transwell assays were performed to assess cell migration and invasion. Briefly, at 24 h post-transfection, HTR8 cells were suspended in serum-free media and then seeded into the upper chamber of an insert (8-mm pore size; Millipore). Notably, the insert was coated with Matrigel for the invasion assays. The lower chamber was filled with medium containing 10% FBS as a chemoattractant. After 24 h of incubation, the migrated or invaded cells through the membrane were fixed with 4% paraformaldehyde for 15 min, stained with 0.1% crystal violet for 5 min, and counted microscopically.
Statistical analysis
All experiments were repeated at least three times independently. The data were presented as the mean ± standard deviation (SD) and tested for the normal distribution. If the measurement data conformed to the normal distribution, a significant difference between groups was compared using a two-tailed Student’s t-test. If not, a significant difference between groups was analyzed by the Mann-Whitney U test. All statistical analyses were conducted using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was interpreted as statistically significant.
Results
Clinical characteristics of pregnancies with and without preeclampsia
The clinical data of pregnancies in ZPE, WPE, and NC groups were retrospectively analyzed (). As a result, there were significant differences between WPE and NC groups in the gestational age at delivery, SBP, DBP, proteinuria, neonatal birthweight, and 1 min Apgar score, as well as between ZPE and NC groups (P < 0.05). Moreover, significant differences existed between ZPE and WPE groups in the gestational age at delivery, neonatal birthweight, and 1 min Apgar score (P < 0.05). No significant difference existed in other clinical features.
Table 2. Clinical data of pregnancies presented for delivery at term (NC group), with early-onset severe preeclampsia (ZPE group), and with late-onset severe preeclampsia (WPE group).
WNT2 and β-catenin were decreased expressed in patients with early-onset severe preeclampsia
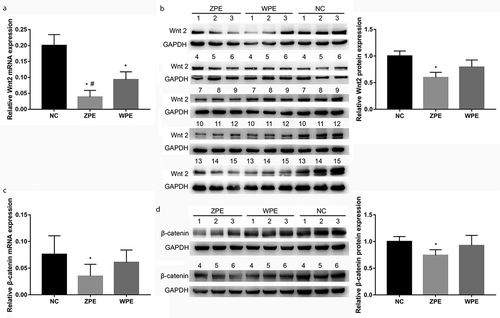
Consistent results of qPCR and western blot assays were obtained that the mRNA and protein expression levels of the WNT2 gene were all significantly decreased in the placental villous tissues of the ZPE group compared to NC group (P < 0.05, ). The mRNA expression levels of the WNT2 gene were also markedly decreased in the placental villous tissues of the WPE group compared to the NC group (P < 0.05, )), while the WNT2 protein expression was slightly decreased without significant difference (P > 0.05, )). These data indicated a possible association between WNT2 expression with the pathogenesis of preeclampsia. Moreover, the mRNA expression levels of the WNT2 gene in the placental villous tissues of the ZPE group were clearly lower than the WPE group (P < 0.05, )). In addition, the results of qPCR and western blot assays showed that the mRNA and protein expression levels of β-catenin were also dramatically down-regulated in the placental villous tissues of ZPE groups relative to NC group (P < 0.05, ). There were no significant differences in the mRNA and protein expression levels of β-catenin between WPE and NC groups as well as between ZPE and WPE groups (P > 0.05, ).
Figure 1. The mRNA and protein expression levels of WNT2 were decreased in the placental villous tissues of patients with early-onset severe preeclampsia (ZPE group) or late-onset severe preeclampsia (WPE group) compared to that in normal controls (NC group). A: qPCR assay showed the WNT2 mRNA expression levels in the three groups (N = 6 each group). B: Western blot assay showed the WNT2 protein expression levels in the three groups (N = 15 each group). C: qPCR assay showed the β-catenin mRNA expression levels in the three groups (N = 6 each group). D: Western blot assay showed the β-catenin protein expression levels in the three groups (N = 6 each group). All experiments were conducted with three biological replicates. Data were expressed as mean ± standard deviation (SD). * P < 0.05 compared to NC group. # compared to the WPE group.
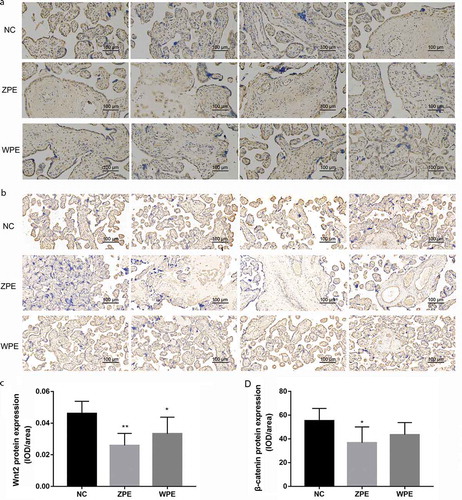
Furthermore, the immunohistochemical staining intensity of WNT2 and β-catenin protein in the placental villous tissues was the strongest in the NC group, followed by WPE and ZPE groups ()). According to the mean density (IOD/area value) in each image, the WNT2 protein expression levels in the placental villous tissues of both ZPE and WPE group were also remarkably lower than NC group (P < 0.05, )), while the β-catenin protein expression was only significantly down-regulated in the placental villous tissues of ZPE group (P < 0.05, )). No significant differences in the expression of WNT2 and β-catenin were obtained between ZPE and WPE groups (P > 0.05, ).
Figure 2. Immunohistochemical staining showed the localization of WNT2 and β-catenin protein in the placental villous tissues of patients with early-onset severe preeclampsia (ZPE group) or late-onset severe preeclampsia (WPE group) compared to that in normal controls (NC group) (200× magnification). A-B: Immunohistochemical staining showed the localization of WNT2 and β-catenin protein in the three groups (N = 6 each group), respectively. C-D: Quantitative analysis of the WNT2 and β-catenin protein expression levels in the three groups according to the mean density (IOD/area value) in each image (N = 6 each group). All experiments were conducted with three biological replicates. Data were expressed as mean ± SD. * P < 0.05 compared to NC group.
WNT2 was overexpressed and knocked down in HTR8 cells
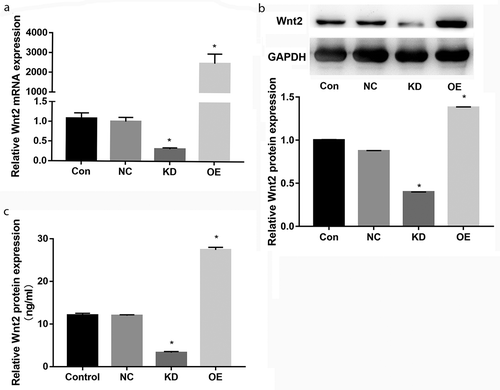
To investigate the role of WNT2 in preeclampsia, the WNT2 gene was overexpressed and knocked down in HTR8 cells by transfection. In comparison with the NC group, the mRNA and protein expression levels of the WNT2 gene were all significantly up-regulated in the overexpression (OE) group and remarkably down-regulated in knockdown (KD) group (P < 0.05, ). Moreover, the ELISA assay showed that the concentration of WNT2 protein was also dramatically increased in the OE group and obviously decreased in the KD group (P < 0.05, )). These data suggested that the transfection efficiency was high and could be used in the following experiments.
Figure 3. WNT2 was overexpressed and knocked down in HTR8 cells by transfection. A: qPCR assay showed the WNT2 mRNA expression levels in blank control (Con), negative control (NC), overexpression (OE) and knockdown (KD) groups. B: Western blot assay showed the WNT2 protein expression levels in the four groups. C: ELISA showed the WNT2 protein expression levels in the four groups. All experiments were conducted with three biological replicates. Data were expressed as mean ± SD. * P < 0.05 compared to NC group.
Overexpression of WNT2 promoted the malignant behaviors of trophoblast cells in vitro, whereas knockdown of WNT2 had opposite effects
The effects of WNT2 overexpression and knockdown on the proliferation of trophoblast cells were firstly investigated. The results of the CCK8 assay showed that, compared to the NC group, the HTR8 cell viability in the OE group was significantly increased, but was markedly decreased in the KD group (P < 0.05, )). Also, the colony-forming assay confirmed the colony-forming ability of HTR8 cells in the OE group was significantly stronger than the NC group, while that in the KD group was obviously weaker than the NC group (P < 0.05, ). These data indicated that overexpression of WNT2promoted trophoblast cell proliferation, whereas knockdown of WNT2 had opposite effects.
Figure 4. Overexpression of WNT2 promoted trophoblast cell proliferation, migration, and invasion and inhibited apoptosis in vitro, whereas knockdown of WNT2 had opposite effects. A: CCK8 assay showed cell viability in blank control (Con), negative control (NC), overexpression (OE), and knockdown (KD) groups. B-C: The colony-forming assay showed the colony-forming ability of HTR8 cells in the four groups. D-E: The scratch wound healing assay showed that the closed wound area of HTR8 cells in the four groups. F and I: Transwell assay that the migration and invasion number of HTR8 cells in the four groups. J: Western blot assay determined the expression of epithelial-mesenchymal transition (EMT) markers in the four groups. K-L: Flow cytometry showed that the percentage of apoptosis cells in the four groups. M: Western blot assay determined the expression of apoptosis-related proteins in the four groups. All experiments were conducted with three biological replicates. Data were expressed as mean ± SD. * P < 0.05 compared to NC group.
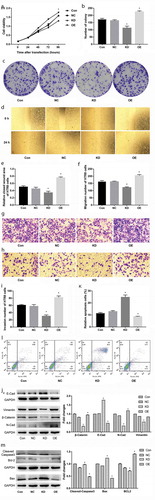
Moreover, the effects of WNT2 overexpression and knockdown on the migration and invasion of trophoblast cells were detected. The results of the scratch wound healing assay showed that the closed wound area of HTR8 cells in the OE group was remarkably larger than that of the NC group, while that in the KD group was dramatically smaller than the NC group (P < 0.05, ). Similar results were also verified by Transwell assay that the migration and invasion number of HTR8 cells in the OE group was significantly increased compared to the NC group, while distinctly decreased in the KD group (P < 0.05, )). In addition, western blot assay was performed to detect the expression of epithelial-mesenchymal transition (EMT) markers. The results showed that overexpression of WNT2 in the OE group resulted in the down-regulation of E-cadherin expression and up-regulation of the expression of N-cadherin, Vimentin, and β-catenin, whereas knockdown of WNT2 in KD group led to the inverse expression changes of these proteins (P < 0.05, )). These data indicated that overexpression of WNT2 promoted trophoblast cell migration and invasion, whereas knockdown of WNT2 had opposite effects.
Furthermore, the effects of WNT2 overexpression and knockdown on trophoblast cell apoptosis were explored. The results of flow cytometry showed that, in comparison to the NC group, the percentage of apoptosis cells was clearly decreased in the OE group, but visibly increased in the KD group (P < 0.05, ). Further western blot analysis showed that overexpression of WNT2 markedly enhanced Bcl-2 expression and inhibited the expression of Bax and cleaved-caspase-3; and knockdown of WNT2 exhibited the inverse expression changes of these proteins (P < 0.05, )). These data indicated that overexpression of WNT2 inhibited trophoblast cell apoptosis, whereas knockdown of WNT2 had opposite effects.
Discussion
The present study revealed that WNT2 and β-catenin were decreased expressed in patients with early-onset severe preeclampsia. Subsequent in vitro experiments showed that overexpression of WNT2 promoted trophoblast cell proliferation, migration, and invasion and inhibited apoptosis, whereas knockdown of WNT2 had opposite effects. These results suggested the role of WNT2 signaling in preeclampsia and merited further discussion.
In previous studies, the secretory glycoprotein WNT2 has been reported to function as a key regulator in regulating the malignant behavior of tumor cells in various human cancers, including colorectal, pancreatic, esophageal, gastric, and non-small cell lung cancers [Citation20–Citation23]. In addition, exogenous WNT2 has been found to function as an angiogenic factor to promote endothelial cell proliferation [Citation24], and angiogenic imbalance is considered as a contributor to the development of preeclampsia [Citation25]. Furthermore, Wnt/β-catenin signaling pathway plays an important role in the physiological processes of human trophoblasts [Citation26]. β-catenin is shown to function an essential role in the Wnt/β-catenin pathway. When the Wnt ligands are absent (off-state), there are low levels of free β-catenin in the cytoplasm. Extracellular Wnt proteins can bind to the Fzd receptor and subsequent Disheveled to recruit axin and other associated kinases, thus leading to the accumulation of β-catenin in the cytoplasm, which is able to enter the nucleus and consequently results in the abnormal cellular proliferation, apoptosis, and/or other biological effects [Citation27]. In this study, we found that WNT2 and β-catenin were decreased expressed in patients with early-onset severe preeclampsia. Overexpression of WNT2 promoted trophoblast cell proliferation, migration, invasion, and inhibited apoptosis in vitro, whereas knockdown of WNT2 had opposite effects. These data promote us to further speculate that WNT2 may regulate placental development and function by altering β-catenin levels to influence the biological behavior of trophoblast cells, thus contributing to preeclampsia development.
Furthermore, it is reported that changes in Bcl-2 and Bax expression indirectly reflect cell apoptosis level [Citation28], and berberine may alleviate preeclampsia development via regulating the Bcl-2/Bax expression [Citation29]. Moreover, caspase-3 is found to be enhanced expressed in the villous trophoblasts of patients with preeclampsia, indicating that increased placental apoptosis contributes to preeclampsia development [Citation30]. On the other hand, E-cadherin is a cell-cell adhesion protein that plays a crucial role in controlling the invasive phenotype of placenta accrete extravillous trophoblasts [Citation31]. A previous study confirmed the high expression of E-cadherin in the preeclamptic placenta [Citation32]. N-cadherin is also a key EMT marker involved in trophoblast invasion [Citation33]. Knockdown of N-cadherin results in trophoblast invasion failure [Citation34]. In this study, dysregulation of WNT2 markedly altered the expression of apoptosis-related proteins and EMT markers in trophoblast cells. It can, therefore, be speculated that the down-regulation of WNT2 may contribute to preeclampsia development via inducing placental apoptosis and inhibiting the migration and invasion of trophoblast cells.
In conclusion, our findings reveal that WNT2 and β-catenin expression was decreased in patients with preeclampsia, especially early-onset severe preeclampsia. Decreased expression of WNT2 may inhibit trophoblast cell proliferation, migration, and invasion, and induced apoptosis. WNT2 may serve as a promising biomarker for early detection of preeclampsia.
Acknowledgments
Thanks to The Binzhou Medical University Hospital hosted the research. Thanks to the Research Ethics Committee, Department of obstetrics and gynecology, and Department of pathology for their support and guidance in this study.
Disclosure statement
The authors declare that they have no competing interests.
References
- Obstetricians ACo, Gynecologists. Preeclampsia and high blood pressure during pregnancy. cited 2018 Dec 9.
- Laganà AS, Vitale SG, Sapia F, et al. miRNA expression for early diagnosis of preeclampsia onset: hope or hype? J Matern Fetal Neonatal Med. 2018;31:817–821.
- Phipps E, Prasanna D, Brima W, et al. Preeclampsia: updates in Pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;6:1102–1113.
- Van Esch JJA, van Heijst AF, de Haan AFJ, et al. Early-onset preeclampsia is associated with perinatal mortality and severe neonatal morbidity. J Matern Fetal Neonatal Med. 2017;23:2789–2794.
- Phipps EA, Thadhani R, Benzing T, et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15:275–289.
- Shamshirsaz AA, Hall NR, Malvasi A, et al. Eclampsia. Management and therapy of late pregnancy complications. Cham: Springer; 2017. p. 95–113.
- Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol. 2017;37:386–397.
- Gormley M, Ona K, Kapidzic M, et al. Preeclampsia: novel insights from global RNA profiling of trophoblast subpopulations. Obstetric Anesthesia Dig. 2018;38: 79.
- Phipps E, Prasanna D, Brima W, et al. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;11:1102–1113.
- Fisher SJ, McMaster M, Roberts JM. The placenta in normal pregnancy and preeclampsia. In: Taylor RN, Roberts JM, Cunningham FG, et al., editors. Chesley’s hypertensive disorders in pregnancy 4th ed. San Diego: Academic Press; 2015. p. 81–112.
- Staud F, Karahoda R. Trophoblast: the central unit of fetal growth, protection and programming. Int J Biochem Cell Biol. 2018;105:35–40.
- Chen H, Zhou X, Han T-L, et al. Decreased IL-33 production contributes to trophoblast cell dysfunction in pregnancies with preeclampsia. Mediators Inflamm. 2018;2018:1–11.
- Situmorang PC, Ilyas S. Study of preeclampsia in placenta, kidney, and hepatic dieases. Asian J Pharm Clin Res. 2018;11:21–28.
- Wang X, Zhang Z, Zeng X, et al. Wnt/β-catenin signaling pathway in severe preeclampsia. J Mol Histol. 2018;49:317–327.
- Zhang L, Leng M, Li Y, et al. Altered DNA methylation and transcription of WNT2 and DKK1 genes in placentas associated with early-onset preeclampsia. Clin Chim Acta. 2019;490:154–160.
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473.
- Tayyar AT, Karakus R, Eraslan Sahin M, et al. Wnt signaling pathway in early- and late-onset preeclampsia: evaluation with Dickkopf-1 and R-Spondin-3 glycoproteins. Arch Gynecol Obstet. 2019;299:1551–1556.
- Liu Y, Ma Y. Promoter methylation status of WNT2 in placenta from patients with preeclampsia. Med Sci Monit. 2017;23:5294.
- Xing X, WenLi G. Obstetrics and gynecology. 8th edition ed. [M]. Beijing: People’s Medical Publishing House; 2013.
- Kramer N, Schmöllerl J, Unger C, et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene. 2017;36:5460.
- Huang C, Ma R, Xu Y, et al. Wnt2 promotes non-small cell lung cancer progression by activating WNT/β-catenin pathway. Am J Cancer Res. 2015;5:1032.
- Xu Y, Li H, Huang C, et al. Wnt2 protein plays a role in the progression of pancreatic cancer promoted by pancreatic stellate cells. Med Oncol. 2015;32:97.
- Zhang Z, Wang J, Dong X. Wnt2 contributes to the progression of gastric cancer by promoting cell migration and invasion. Oncol Lett. 2018;16:2857–2864.
- Unterleuthner D, Neuhold P, Schwarz K, et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159–177.
- Meeme A, Buga GA, Mammen M, et al. Angiogenic imbalance as a contributor to the pathophysiology of preeclampsia among black African women. J Matern Fetal Neonatal Med. 2017;30:1335–1341.
- Zhang Z, Wang X, Zhang L, et al. Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia. Mol Med Rep. 2017;16:1007–1013.
- Zhang Z, Wang X, Zhang L, et al. Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review). Mol Med Rep. 2017;16:1007–1013.
- Mendilcioglu I, Karaveli S, Erdogan G, et al. Apoptosis and expression of Bcl-2, Bax, p53, caspase-3, and Fas, Fas ligand in placentas complicated by preeclampsia. Clin Exp Obstet Gynecol. 2011;38:38–42.
- Wang A, Liu Q, Zhang J, et al. Berberine alleviates preeclampsia possibly by regulating the expression of interleukin-2/interleukin-10 and Bcl-2/Bax. Int J Clin Exp Med. 2015;8:16301.
- Cali U, Cavkaytar S, Sirvan L, et al. Placental apoptosis in preeclampsia, intrauterine growth retardation, and HELLP syndrome: an immunohistochemical study with caspase-3 and bcl-2. Clin Exp Obstet Gynecol. 2013;40:45–48.
- Duzyj C, Buhimschi IA, Motawea H, et al. The invasive phenotype of placenta accreta extravillous trophoblasts associates with loss of E-cadherin. Placenta. 2015;36:645–651.
- İrtegun S, Tekin MA, Alpaycı R. Increased expression of E-cadherin, endothelin-1, and CD68 in preeclamptic placentas. Erciyes Med J/Erciyes Tip Dergisi. 2016;38:149–152.
- Peng B, Zhu H, Leung PC. Gonadotropin-releasing hormone regulates human trophoblastic cell invasion via TWIST-induced N-cadherin expression. J Clin Endocrinol Metab. 2015;100:E19–E29.
- Multhaup A, Huppertz B, Göhner C, et al. N-cadherin knockdown leads to disruption of trophoblastic and endothelial cell interaction in a 3D cell culture model–New insights in trophoblast invasion failure. Cell Adh Migr. 2018;12:259–270.
