ABSTRACT
Parkinson’s disease (PD) is one of the most prevailing aging diseases around the world. The present study was to investigate the potential effect of hydrogen sulfide (H2S) and silent mating type information regulation 2 homolog 1 (SIRT1) in MPP~+ induced SH-SY5Y cells and its underlying mechanisms in PD. SH-SY5Y cells were induced by MPP~+ and treated with the H2S donor NaHS to detect the effect of H2S on the molecular behaviors of MPP~+ induced SH-SY5Y cells. NaHS reduced the apoptosis rate and expressions of MDA, 4-HNE and p62, while increased cell viability, autophagy flux and expressions of LC3 II/I and Beclin1 in MPP~+ induced SH-SY5Y cells. Then, levels of autophagy-related proteins and inflammation-related proteins (TNF-α, IL-Iβ) were detected, indicating that Chloroquine and Sirtinol reversed the protective effect of H2S on SH-SY5Y cells induced by MPP~+. We further explored the particular function of H2S, SH-SY5Y cells treated with MPP~+, NaHS chloroquine, and SIRT1 inhibitor (Sirtinol). The results showed that H2S increased SIRT1 expression and sulfhydration. Finally, a PD mouse model verified the above results. In a word, H2S ameliorated SIRT1 activity through acceleration of SIRT1 sulfhydration to increase the autophagy flux and attenuate damage of SH-SY5Y cells induced by MPP~+. H2S and SIRT1 activator might be a target in the treatment of PD patients.
1. Introduction
Parkinson’s disease (PD) is one of the most prevailing neuro-degenerative motion disorders, which is tightly correlated with remarkable motor deficits [Citation1]. Its main motor symptoms include bradykinesia/muscular atrophy, rigidity, postural instability and tremor, but the clinical manifestations are other motor/non-motor disorders [Citation2]. It is estimated that 1% of the population over 60 y old worldwide is presently living with serious and even fatal PD [Citation3]. There are drugs that can increase or stabilize the brain dopamine, but they cannot block or slow down the PD progress [Citation4]. Autophagy is a highly conserved intracellular degradation process, which underlies various neurodegenerative disorders in the central nervous system [Citation5]. Promotion of autophagy maintains neuron survival in vivo and in vitro, and accelerates the elimination of protein aggregates in the models of PD and Alzheimer’s disease (AD) [Citation6]. In addition, it is suggested by different studies that mediation of autophagy might be a prospective therapeutic scheme for PD patients [Citation7,Citation8]. Besides, human SH-SY5Y cell line shows numerous dopaminergic neuron properties, which is widely employed to study toxicity induced by 1-methyl-4-phenylpyridinium (MPP~+), and a metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is extensively used as PD inducer in vitro [Citation4]. Therefore, we used SH-SY5Y cells treated with MPP~+ as the target of autophagy therapies in PD.
Hydrogen sulfide (H2S), a metabolic poison normally emitting malodor, participates in significant physiological functions in cancers, cardiovascular systems, inflammatory procedures and neuroprotection [Citation9]. Inhaled H2S or NaHS has been presented to exert neuroprotection in the rodent models of PD induced by neurotoxins [Citation10]. Besides, Sirtuins (SIRTs) are known as the nicotine adenine dinucleotide+-dependent deacetylases targeting forkhead transcription factor O3 a, one of the downstream factors of SIRT1 [Citation11]. SIRT1 belongs to the Sirtuin family, which partakes in different bioprocesses including oxidative stress, cell proliferation, cancer development, and pyroptosis [Citation12]. SIRT1 also extensively exists in adult brain and has been acknowledged to exert protective functions in neurodegenerative disorders, including PD [Citation13]. Interestingly, H2S upregulates SIRT1 and protects HT22 cells against neuronal senescence caused by high glucose through improving autophagy flux [Citation14]. By upregulating SIRT1, H2S can protect kidney cells from further damage of diabetes and reduce reactive oxygen species (ROS), fasting blood glucose and apoptosis, thus protecting renal cells against further damage led by diabetes mellitus [Citation15]. However, the relationship between H2S and SIRT1 in PD is rarely elucidated. Therefore, we established an SH-SY5Y cell model treated with MPP~+ to investigate the interaction of H2S and SIRT1 and their influence on autophagy.
2. Materials and methods
2.1. Ethics statement
All animal measurements were under the guidance on used animals and supervised by the ethic committee of The Second Hospital of Harbin Medical University. This study minimized the number and pain of experimental animals as much as possible.
2.2. Cell culture and grouping
Human neuroblastoma cell line SH-SY5Y was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagles medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin (ThermoFisher Scientific Inc., Rockford, IL, USA).
MPP~+ was used to simulate the microenvironment of PD [Citation16]. SH-SY5Y cells were treated with MPP~+ at different concentrations of 100, 200, 300, 400, 500, 600, and 700 μmoL/L (D048, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 24 h, and the optimal concentration was determined as 500 μmol/L. Then, cells were assigned into blank group (SH-SY5Y cells were cultured normally without any reagent treatment), MPP~+ group (SH-SY5Y cells were cultured with 500 μmol/L MPP~+), MPP~+ + NaHS groups (SH-SY5Y cells were respectively pre-treated with 50, 100, 200 or 400 μmol/L NaHS for 30 min and then treated with 500 μmol/L MPP~+ for 24 h), MPP~+ + NaHS + Chloroquine (CQ) group (SH-SY5Y cells were pre-treated with 200 μmol/L NaHS for 30 min and then treated with 500 μmol/L MPP~+ and CQ for 24 h), and MPP~+ + NaHS + Sirtinol group (SH-SY5Y cells were pre-treated with 200 μmol/L NaHS for 30 min and then treated with 500 μmol/L MPP~+ and 17 μg/mL Sirtinol) for 24 h. NaHS was purchased from Sigma-Aldrich, and CQ and Sirtinol were purchased from MedChemExpress (NJ, USA).
2.3. 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
The cells in logarithmic growth phase after different treatments were used for preparing the single cell suspension at the density of 1 × 105 cells/mL, and then the cells were seeded into the 96-well plate at the density of 200 μL/well. After 48 h of incubation, 20 μL of 5 mg/mL MTT solution (Sigma-Aldrich) was added to detect cell viability. Next, the cells were supplemented with 150 μL dimethyl sulfoxide followed by dissolving the crystals at room temperature for 15 min. The optical density (OD) of each well was measured at a wavelength of 490 nm using a microplate reader.
2.4. Flow cytometry
The cells in each group were seeded into the 12-well plate (8 × 104 cells/well). After proper treatment, the cells were treated following the instructions of annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit (Beyotime Biotechnology Co., Ltd., Shanghai, China). The cell apoptosis was detected using the flow cytometer.
2.5. Hoechst 33342 staining
The cells in each group were washed with phosphate buffered saline (PBS), fixed with 4% formaldehyde and stained with 10 μg/mL Hoechst 33342 for 15 min at room temperature. After washing with PBS, the cells were observed and recorded under the fluorescence microscope (Olympus, Tokyo, Japan).
2.6. Enzyme linked immunosorbent assay (ELISA)
The expressions of malondialdehyde (MDA) and 4-Hydroxynonenal (4-HNE) in SH-SY5Y cells were detected in strict accordance with the instructions of the human MDA ELISA kit (kt98244, MSK Biotechnology Co., Ltd., Wuhan, Hubei, China) and human 4-HNE ELISA kit (kt99767, MSK Biotechnology).
2.7. Monomeric red fluorescent protein (mRFP)-green fluorescent protein (GFP)-microtubule-associated protein1 light chain 3 (LC3) fusion protein tracing
mRFP-GFP-LC3 was purchased from HanBio Co., Ltd. (Shanghai, China). SH-SY5Y cells were cultured in the 24-well plate (1 × 105 cell/well). When cell confluence reached 70%-80%, the cells were incubated with adenovirus mRFP-GFP-LC3 (adjusting the virus multiplicity of infection to 100) for 2 h. After PBS washing, the cells were cultured in a complete culture medium overnight. The transfection efficiency of adenovirus mRFP-GFP-LC3 was observed and verified under the fluorescence microscope. Next, the cells were treated for subsequent experiments, and then observed and photographed under the fluorescence microscope (BX51, Olympus).
2.8. Western blot analysis
SH-SY5Y cells were lysed in cold radioimmunoprecipitation assay (RIPA) containing a mixture of protease inhibitors (Sigma-Aldrich) for 30 min. The lysate was centrifuged at 16000 g for 20 min at 4°C to collect the supernatant. Pierce bicinchoninic acid (BCA) protein assay kit (Beyotimea) was used to quantify protein concentration. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were sealed in 5% skimmed milk at room temperature for 2 h, incubated overnight with the primary antibodies at 4°C, then incubated with the secondary antibody for 1 h, and developed with chemiluminescence reagent using Bio-Rad Gel Dol EZ (Bio-Rad, Inc., Hercules, CA, USA). Image J software was used to analyze the gray value of the target band (National Institutes of Health, Bethesda, Maryland, USA). The antibodies used were as follows: p62 (1:1000, ab56416, Abcam, Cambridge, MA, USA), LC3B (1:1000, ab48394, Abcam), Beclin1 (1:1000, ab62557, Abcam), SIRT1 (1:1000, ab110304, Abcam), immunoglobulin G (IgG) (1:1000, ab205719, Abcam), β-actin (1:1500, ab8227, Abcam), nuclear factor kappa B (NF-κB) p65 (1:1000, ab16502, Abcam), and p53 (1:1000, ab26, Abcam).
2.9. Biotin switch assay
Cells were homogenized in HEN buffer [250 mM Hepes-NaOH (pH 7.7), 1 mM EDTA and 0.1 mM neocuproine] supplemented with 1% proteinase inhibitor cocktail and 100 µM deferoxamine. Next, the cells were centrifuged at 14000 × g for 15 min at 4°C. The samples were added with blocking buffer (HEN buffer adjusted to 2.5% SDS and 20 mM methyl methanethiosulfonate), and then incubated at 50°C for 20 min along with gentle and frequent vortexing devoid of foaming. Proteins were precipitated at −20°C for 1 h after the addition of acetone. After centrifugation at 2000 × g for 10 min, the acetone was removed. The protein pellets were re-suspended with the HEN buffer and incubated with 1% SDS and 30 mM biotin-disodium 1-hydroxypentyldiphosphonate for 3 h at 25°C. Afterward, the biotinylated proteins were precipitated by streptavidin-agarose beads, followed by the washes with HEN buffer and PBS solution. Subsequently, the biotinylated proteins were eluted by SDS-PAGE gel and analyzed by Western blot analysis with anti SIRT1 antibody.
2.10. Establishment of a PD model in mice
The establishment of a PD model in mice was based on the method in the literature [Citation17]. C57/BL6 mice (8 wk-old) were purchased from Shanghai Hengrui Pharmaceutical Co., Ltd. [SYXK (Hu) 2017–0015, Shanghai, China]. Twenty-four mice were randomly allocated into four groups (n = 6), namely control group (mice were injected with saline), PD group [mice were intraperitoneally injected with MPTP (14 mg/kg, m0896, Sigma-Aldrich) every 2 h within 8 h], NaHS group (mice were intraperitoneally injected with 5.6 mg/kg NaHS) and PD + NaHS group (mice were intraperitoneally injected with 5.6 mg/kg NaHS 30 min before MPTP injection). All mice were raised in specific pathogen-free (SPF) grade environment with food and water. Two weeks after MPTP injection, three mice in each group were randomly selected for behavioral tests. The remaining three mice were sacrificed by cervical dislocation. Brain tissues were taken out and ground into homogenate for ELISA or western blot analysis.
2.11. Behavioral tests
The rotarod test, beam walk test, and grid walking test were conducted to evaluate the mouse behaviors according to the methods as reported by Yuan et al. [Citation17].
2.12. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) 21.0 (IBM Corp. Chicago, IL, USA) was applied for data analysis. According to Kolmogorov–Smirnov detection, the data are in normal distribution and expressed as mean ± standard deviation. Differences between two groups were evaluated using the t-test while the differences among multiple groups were compared using one-way or two-way analysis of variance (ANOVA). Tukey’s multiple comparisons test was used for the pairwise comparison after ANOVA analysis. The p value was obtained by a two-tailed test and p < 0.05 indicated a significant difference.
3. Results
3.1. H2S protected SH-SY5Y cells induced by MPP~+
After the induction of SH-SY5Y cells by MPP~+ with different concentrations (100, 200, 300, 400, 500, 600, and 700 μmoL/L), the optimal concentration of MPP~+ was selected as 500 μmoL/L (). Then, it was found that H2S had protective effects on SH-SY5Y cells induced by MPP~+ after the pretreatment of different concentrations of H2S donor NaHS (50, 100, 200, and 400 μmoL/L). The optimal concentration of NaHS was 200 μmol/L (). After 24 h of MPP~+ treatment, SH-SY5Y cells had neural retraction and membrane blistering under microscope; NaHS could reduce the cell morphological changes, while NaHS treatment alone did not change the cell morphology (). In addition, NaHS treatment reduced the apoptosis rate of SH-SY5Y cells induced by MPP~+ () and the accumulation of cytotoxic substances MDA and 4-HNE (), while NaHS treatment alone had no effect on the apoptosis rate and the accumulation of MDA and 4-HNE in SH-SY5Y cells. These results showed that H2S could protect SH-SY5Y cells induced by MPP~+.
Figure 1. H2S protected SH-SY5Y cells from MPP~+ damages. (a). SH-SY5Y cell viability treated with different concentrations of MPP~+ detected by MTT assay; (b). SH-SY5Y cell viability treated with different concentrations of MPP~+ + NaHS detected by MTT assay; (c). SH-SY5Y morphology observed under an optical microscope: SH-SY5Y cells had neural retraction and membrane blistering; NaHS could reduce the cell morphological changes, while NaHS treatment alone did not change the cell morphology; (d). cell apoptosis detected by flow cytometry; (e). cell apoptosis detected by Hoechst 33342 staining; (f). MDA and 4-HNE levels detected by ELISA. ***p < 0.001 vs. the control group; #p < 0.05, ###p < 0.001 vs. the MPP~+ group. Data in panels (a), (b), and (d) were analyzed by one-way ANOVA and data in panel (f) were analyzed by two-way ANOVA, followed by Tukey’s multiple comparisons test for post hoc test. Repetitions = 3.
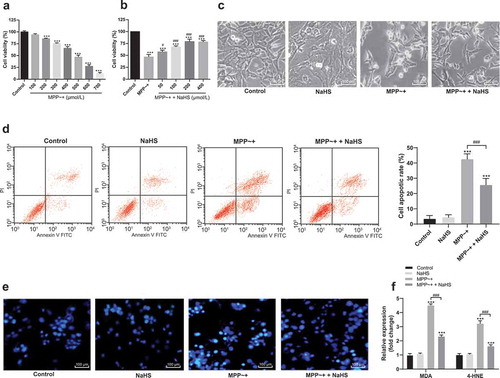
3.2. H2S increased autophagy flux of SH-SY5Y cells damaged by MPP~+
Autophagy is essential for obtaining cell homeostasis and related to a variety of neurodegenerative diseases [Citation5]. By tracing with MRFP-GFP-LC3, we found that autophagosome in SH-SY5Y cells induced by MPP~+ was significantly reduced and NaHS treatment reversed the trends (). Western blot analysis was used to detect the expressions of autophagy-related proteins (p62, LC3 and Beclin 1). The results showed that the expressions of LC3 II/I and Beclin1 decreased significantly (all p < 0.05) and the expression of p62 increased notably (p < 0.05) in SH-SY5Y cells induced by MPP~+, and these trends were reversed after NaHS treatment ().
Figure 2. H2S restored the damaged autophagy flux in SH-SY5Y cells induced by MPP~+. (a). mRFP-GFP-LC3 fluorescence tracing (LC3 fluorescence indicated the aggregation of autophagosome in cells); B. the expressions of autophagy-related proteins (p62, LC3 and Beclin 1) detected by Western blot analysis. **p < 0.01, ***p < 0.001 vs. the control group; ###p < 0.001 vs. the MPP~+ group. Data in panel B(i) were analyzed by one-way ANOVA and data in panel B(ii) were analyzed by two-way ANOVA, followed by Tukey’s multiple comparisons test for post hoc test. Repetitions = 3.
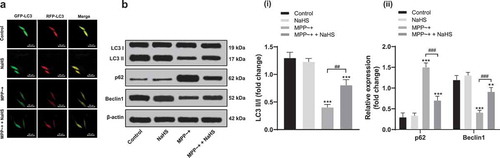
3.3. Blocking autophagy flux reversed the protective effect of H2S on SH-SY5Y cells induced by MPP~+
CQ is a lysosomal inhibitor. Under the action of CQ, the autophagosome of MPP~+ induced SH-SY5Y cells treated with NaHS reduced (), with decreased expressions of LC3 II/I and Beclin1 and increased p62 expression (all p < 0.05) (). Compared with MPP~+ induced SH-SY5Y cells treated with NaHS, CQ significantly increased the SH-SY5Y cell apoptosis rate (p < 0.05) (/D), as well as the contents of MDA and 4-HNE (p < 0.05) (). It was suggested that blocking autophagy could reverse the protective effect of H2S on MPP~+ induced SH-SY5Y cells.
Figure 3. CQ blocked autophagy in SH-SY5Y cells. (a). mRFP-GFP-LC3 fluorescence tracing (fluorescence point of LC3 in SH-SY5Y cells decreased under the effect of CQ); (b). the expressions of autophagy-related proteins in the SH-SY5Y cells after CQ treatment detected by Western blot analysis; (c). the SH-SY5Y cell apoptosis rate detected by flow cytometry; (d). the apoptosis detected by Hoechst 33342 staining (the stronger the blue fluorescence was, the higher the apoptosis rate); (e). the levels of the MDA and 4-HNE measured by ELISA. **p < 0.01, ***P < 0.001 vs. the MPP~+ + NaHS group. Data in panels B (i) and C were analyzed by t-test; data in panels B(ii) and E were analyzed by two-way ANOVA, followed by Tukey’s multiple comparisons test for post hoc test. Repetitions = 3.
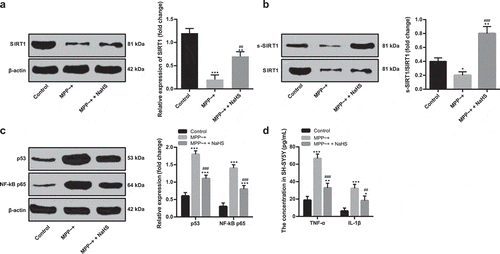
3.4. H2S increased the SIRT1 activity in SH-SY5Y cells induced by MPP~+ through mediation of SIRT1 sulfhydration
SIRT1 can improve autophagy flux, and the loss or mutation of SIRT1 can promote the pathological development of PD [Citation18]. The expression of SIRT1 in MPP~+ induced SH-SY5Y cells decreased markedly compared with that of untreated SH-SY5Y cells (p < 0.05), and increased after NaHS pretreatment (p < 0.05) (). The results of biotin switch assay revealed that H2S increased the sulfhydration of SIRT1 in SH-SY5Y cells (). NaHS inhibited the expression of target genes of SIRT1 (p53 and NF-kB p65) (), and reduced the levels of inflammatory factors in SH-SY5Y cells induced by MPP~+ ().
Figure 4. H2S increased SIRT1 sulfhydration in SH-SY5Y cells. (a/c). the expressions of SIRT1, p53, NF-kB p65 in the SH-SY5Y cells detected by Western blot analysis; (b). the degree of SIRT1 sulfhydration in SH-SY5Y cells detected by biotin switch assay; (d). the levels of TNF-α and IL-1β detected by ELISA. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the control group; ##p < 0.01, ###p < 0.001 vs. the MPP~+ group. Data in panels (a) and (b) were analyzed by one-way ANOVA; data in panels (c) and (d) were analyzed by two-way ANOVA, followed by Tukey’s multiple comparisons test for post hoc test. Repetitions = 3.
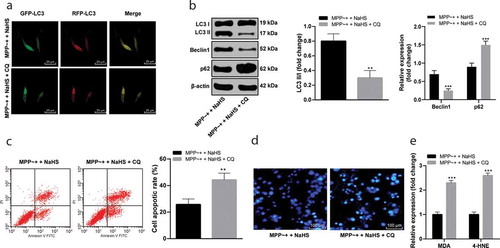
3.5. Sirtinol inhibited SIRT1 activity and reversed the effect of H2S on autophagy flux of SH-SY5Y cells induced by MPP~+
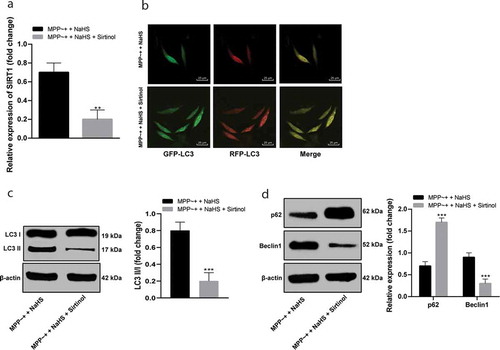
After NaHS pretreatment, Sirtinol (an inhibitor of SIRT1) was added to SH-SY5Y cells to inhibit the activity of SIRT1 (), which reversed the effect of NaHS treatment on the autophagy flux of SH-SY5Y cells induced by MPP~+ (), reduced the levels of LC3 II/I and Beclin1, and increased the expression of p62 (all p < 0.05) ().
Figure 5. Sirtinol suppressed SIRT1 activity and affected autophagy flux in SH-SY5Y cells. (a/c/d). the expressions of SIRT1 and autophagy-related proteins in SH-SY5Y cells induced by MPP~+ after Sirtinol treatment detected by Western blot analysis; (b). mRFP-GFP-LC3 fluorescence tracing (LC3 fluorescence point in SH-SY5Y cells decreased under the action of Sirtinol). **p < 0.01, ***p < 0.001 vs. the MPP~+ + NaHS group. Data in in panels (a) and (c) were analyzed by t-test; data in panel (d) were analyzed by two-way ANOVA, followed by Tukey’s multiple comparisons test for post hoc test. Repetitions = 3.
3.6. Sirtinol reversed the protective effect of H2S on SH-SY5Y cells induced by MPP~+
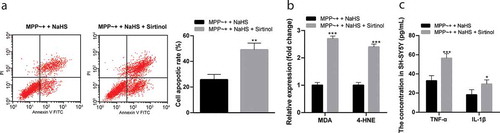
After SIRT1 inhibition, the apoptosis rate of SH-SY5Y cells induced by MPP~+ increased significantly (p < 0.05) (); the contents of MDA and 4-HNE in cells increased significantly (p < 0.05) (), and the levels of inflammatory-related factors increased (p < 0.05) (). It was suggested that Sirtinol reversed the protective effect of H2S on SH-SY5Y cells induced by MPP~+.
Figure 6. Sirtinol reversed the protective effect of H2S on SH-SY5Y cells induced by MPP~+. (a). cell apoptosis detected by flow cytometry; (b/c). the expressions of MDA, 4-HNE, TNF-α and IL-1β detected by ELISA. *p < 0.05, **p < 0.01 vs. the MPP~+ + NaHS group. Data in panel (a) were analyzed by t-test; data in panels (b) and (c) were analyzed by two-way ANOVA, followed by Tukey’s multiple comparisons test for post hoc test. Repetitions = 3.
3.7. H2S increased the SIRT1 activity in SH-SY5Y cells induced by MPP~+ through sulfhydration to ameliorate PD in vivo
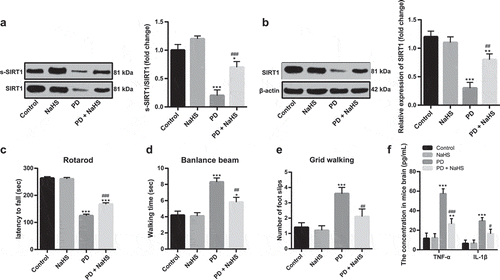
Later, a PD mouse model was established. After intraperitoneal injection of NaHS, the SIRT1 sulfhydration in the brain tissues of mice and SIRT1 activity increased (). Various behavior tests of mice showed that NaHS injection alleviated clinical symptoms of PD (-E), and reduced the levels of inflammatory factors in the brain tissues of PD mice ().
Figure 7. NaHS injection alleviated clinical symptoms of PD. (a). the degree of sulfhydration in brain tissues of PD mice detected by biotin switch assay; (b). SIRT1 expression in brain tissues of PD mice detected by Western blot analysis; (c–e). results of rotarod test, beam walk test, and grid walking test; (f). TNF-α and IL-1β expression in brain tissues of PD mice detected by ELISA. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. the PD group. Data in panels (a–e) were analyzed by one-way ANOVA and data in panel F were analyzed by two-way ANOVA, followed by Tukey’s multiple comparisons test for post hoc test. n = 3.
4. Discussion
PD is the most ubiquitous neurodegenerative disease that influences the motor system with the incidence ranging from 5 to over 35 per 100,000 people worldwide [Citation19]. H2S has been widely considered as a toxic gas in the scientific community for over 300 y, but understanding of this small molecule has changed since H2S is found to involve in the physiological and pathological mechanism of the brain [Citation20]. SIRT1 was suggested to suppress the synuclein aggregate formation in PD and it was also correlated with H2S in neuronal senescence and diabetes [Citation14,Citation15,Citation21]. Herein, this study aims to investigate the roles of H2S and SIRT1 in PD, and drew a conclusion that H2S ameliorated SIRT1 activity through acceleration of SIRT1 sulfhydration to increase the autophagy flux and attenuate damage of SH-SY5Y cells induced by MPP~+.
Generally speaking, there are two releasing models of H2S donors: instant-releasing and slow-releasing [Citation22]. Our study uses NaHS as the donor of H2S, belonging to the follower of the first model which has quite low relevance to a physiological situation. NaHS, an H2S donor, was found to protect PC12 cells against MPP~+-induced apoptosis and cytotoxicity [Citation23]. First of all, we found that H2S protected SH-SY5Y cells induced by MPP~+ through increasing autophagy flux. NaHS reduced the apoptosis rate and the accumulation of cytotoxic substances MDA and 4-HNE, while increased the autophagy flux and expression of LC3 II/I and Beclin1 in SH-SY5Y cells induced by MPP~+. Exogenous application of H2S protected the SH-SY5Y cells against 6-hydroxydopamine-induced or rotenone-induced cell apoptosis and death [Citation24]. In addition, inhaled NaHS or H2S was presented to show neuroprotection in rodent models of PD induced by neurotoxins [Citation10]. Exogenous H2S increased the protein expression of Beclin1 and the ratio of LC3 II/I so as to promote autophagy blocked by oleic acid [Citation25]. Shefa U et al. suggested that H2S protects neurons against oxidative stresses through downregulation of ROS and the aggregation of lipid peroxidation products [Citation26]. There was a study also indicating that H2S neuroprotection is, partly because of its anti-inflammation, antioxidation, and anti-apoptosis properties [Citation27]. Now, more and more sulfhydration targets are proved which provides new direction to known effects of H2S by shedding deeper sights on its biological functions [Citation28]. Chloroquine, a potent inhibitor of autophagy, has been employed in human patients for a variety of purposes [Citation29]. CQ blocks autophagy by preventing lysosomal acidification and autophagosome such as Beclin1 and LC3II/LC3I degradation and fusion [Citation30,Citation31]. The results in this study also revealed that CQ reversed the protective effect of H2S on SH-SY5Y cells induced by MPP~+.
H2S is a special SIRT1 activator through direct sulfhydration, and SIRT1 sulfhydration contributes to H2S inhibition of endothelial inflammation [Citation32]. Then, this study discovered that H2S increased the SIRT1 activity in SH-SY5Y cells induced by MPP~+ through mediation of SIRT1 sulfhydration. Upregulation of hippocampal SIRT1 improved the role of H2S in the cognitive function of rats treated by homocysteine, which also associated with inhibition of the endoplasmic reticulum stress [Citation33–Citation35]. Upregulation of SIRT1 also upregulated of antioxidant enzyme and improve the resistance of NaHS on oxidative stress injury induced by H2O2 [Citation36]. Through promotion of signal transducers and activators of transcription 3 (STAT3) deacetylation regulated by SIRT1, H2S could inhibit inflammatory hepatic hepcidin [Citation37]. H2S exerts a direct anti-apoptosis function in H9c2 cardiomyocytes through the SIRT1 signaling pathway [Citation38]. H2S is related to aging by suppressing free-radical reactions and activating SIRT1, and H2S was revealed to own therapeutic potential in age-correlated diseases [Citation39].
Besides, SIRT1 expression was reduced in SH-SY5Y cells induced by MPP~+ and PD mice. Inhibition of SIRT1 reversed the protective effect of H2S on SH-SY5Y cells induced by MPP~+. SIRT1 is an effective mediator of autophagy degradation and mitotic signaling pathways, thereby blocking the α-synuclein toxicity in PD [Citation40]. A study suggested that microRNA-200a induced down-regulation of SIRT1 to accelerate apoptosis of DA neurons, and contributed to pathogenesis of PD [Citation41]. SIRT1 expression markedly reduced not only in primary culture neurons treated by neurotoxin MPP~+, environmental factor PD models, and MPTP-treated mice but also in the genetic factor PD models [Citation42]. Reliable research indicates that SIRT1 expresses in a lower level in dopaminergic neurons in PD model and protects neurons against neurotoxicity [Citation13]. Autophagy can be triggered by SIRT1 overexpression, which is vital for nerve regeneration for its promotion of functional nerve reinnervation [Citation43]. What’s more, in vivo experiments were conducted. The results indicated that intraperitoneal injection of NaHS increased the activity of SIRT1 in the brain of PD mice. Various behavioral tests of mice showed that NaHS injection could alleviate clinical symptoms of PD.
In summary, it was uncovered that H2S promoted SIRT1 activity through acceleration of SIRT1 sulfhydration to increase the autophagy flux and ameliorate damage of SH-SY5Y cells induced by MPP~+. Collectively, this article provides an overview of the physiological functions and effects of H2S and SIRT1 on PD, and proposes the potential health and therapeutic benefits of H2S and SIRT1. The main function of SIRT1 is acetylation, but the theme of our research is hydrosulfidation. We will conduct a more in-depth and comprehensive research on SIRT1 acetylation in the future. On the other hand, there are certain limitations in using NaHS as the donor of H2S. For example, the instant-releasing of H2S fails to simulate the slow and continuous generation process of H2S in vivo [Citation44]. The instant-releasing of H2S may result in inaccurate results and harmful effects on human body [Citation45]. We can’t use slow-releasing H2S donors to perform our research in a short period of time due to the limitation of research conditions. Hence, the in-depth research on slow-releasing H2S donors is also deemed essential in the future.
Disclosure statement
All authors declare that there is no conflict of interests in this study.
Additional information
Funding
References
- Coundouris SP, Terrett G, Laakso L, et al. A meta-analytic review of prospection deficits in Parkinson’s disease. Neurosci Biobehav Rev. 2019;108:34–47.
- Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2019;27:27–42.
- Jiang X, Jin T, Zhang H, et al. Current progress of mitochondrial quality control pathways underlying the pathogenesis of Parkinson’s disease. Oxid Med Cell Longev. 2019;2019:4578462.
- Limboonreung T, Tuchinda P, Chongthammakun S. Chrysoeriol mediates mitochondrial protection via PI3K/Akt pathway in MPP(+) treated SH-SY5Y cells. Neurosci Lett. 2020;714:134545.
- Balke D, Tatenhorst L, Dambeck V, et al. AAV-mediated expression of dominant-negative ULK1 increases neuronal survival and enhances motor performance in the MPTP mouse model of Parkinson’s disease. Mol Neurobiol. USA: Humana Press, Inc.; 2019. p. 685–697.
- Heras-Sandoval D, Perez-Rojas JM, Pedraza-Chaverri J. Novel compounds for the modulation of mTOR and autophagy to treat neurodegenerative diseases. Cell Signal. 2020;65:109442.
- Hunn BHM, Vingill S, Threlfell S, et al. Impairment of macroautophagy in dopamine neurons has opposing effects on Parkinsonian pathology and behavior. Cell Rep. 2019;29:920–31 e7.
- Liang Y, Zheng D, Peng S, et al. Rifampicin attenuates rotenone-treated microglia inflammation via improving lysosomal function. Toxicol In Vitro. 2019;63:104690.
- Ali R, Pal HA, Hameed R, et al. Controlled release of hydrogen sulfide significantly reduces ROS stress and increases dopamine levels in transgenic C. elegans. Chem Commun (Camb). 2019;55:10142–10145.
- Hou X, Yuan Y, Sheng Y, et al. GYY4137, an H2S slow-releasing donor, prevents nitrative stress and alpha-synuclein nitration in an MPTP mouse model of Parkinson’s disease. Front Pharmacol. 2017;8:741.
- Ruankham W, Suwanjang W, Wongchitrat P, et al. Sesamin and sesamol attenuate H2O2 -induced oxidative stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling pathway. Nutr Neurosci. 2019;22:1–12.
- Zhao MW, Yang P, Zhao LL. Chlorpyrifos activates cell pyroptosis and increases susceptibility on oxidative stress-induced toxicity by miR-181/SIRT1/PGC-1alpha/Nrf2 signaling pathway in human neuroblastoma SH-SY5Y cells: implication for association between chlorpyrifos and Parkinson’s disease. Environ Toxicol. 2019;34:699–707.
- Wang Z, Sun L, Jia K, et al. miR-9-5p modulates the progression of Parkinson’s disease by targeting SIRT1. Neurosci Lett. 2019;701:226–233.
- Wu L, Chen Y, Wang CY, et al. Hydrogen sulfide inhibits high glucose-induced neuronal senescence by improving autophagic flux via up-regulation of SIRT1. Front Mol Neurosci. 2019;12:194.
- Ahmed HH, Taha FM, Omar HS, et al. Hydrogen sulfide modulates SIRT1 and suppresses oxidative stress in diabetic nephropathy. Mol Cell Biochem. 2019;457:1–9.
- Zhang X, Jin J, Xie A. Laquinimod inhibits MMP+ induced NLRP3 inflammasome activation in human neuronal cells. Immunopharmacol Immunotoxicol. 2020;42:264–271.
- Yuan YQ, Wang YL, Yuan BS, et al. Impaired CBS-H2S signaling axis contributes to MPTP-induced neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav Immun. 2018;67:77–90.
- Xu J, Jackson CW, Khoury N, et al. Brain SIRT1 mediates metabolic homeostasis and neuroprotection. Front Endocrinol (Lausanne). 2018;9:702.
- Ammal Kaidery N, Ahuja M, Thomas B. Crosstalk between Nrf2 signaling and mitochondrial function in Parkinson’s disease. Mol Cell Neurosci. 2019;101:103413.
- Kumar M, Sandhir R. Hydrogen sulfide in physiological and pathological mechanisms in brain. CNS Neurol Disord Drug Targets. 2018;17:654–670.
- Rana P, Franco EF, Rao Y, et al. Evaluation of the common molecular basis in Alzheimer’s and Parkinson’s diseases. Int J Mol Sci. 2019;20.
- Sun X, Wang W, Dai J, et al. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci Rep. 2017;7:3541.
- Sarukhani M, Haghdoost-Yazdi H, Sarbazi Golezari A, et al. Evaluation of the antiparkinsonism and neuroprotective effects of hydrogen sulfide in acute 6-hydroxydopamine-induced animal model of Parkinson’s disease: behavioral, histological and biochemical studies. Neurol Res. 2018;40:523–531.
- Sarookhani MR, Haghdoost-Yazdi H, Sarbazi-Golezari A, et al. Involvement of adenosine triphosphate-sensitive potassium channels in the neuroprotective activity of hydrogen sulfide in the 6-hydroxydopamine-induced animal model of Parkinson’s disease. Behav Pharmacol. 2018;29:336–343.
- Wang H, Zhong P, Sun L. Exogenous hydrogen sulfide mitigates NLRP3 inflammasome-mediated inflammation through promoting autophagy via the AMPK-mTOR pathway. In: Biol Open. UK: Company of Biologists Ltd.; 2019. p. 8.
- Shefa U, Kim MS, Jeong NY, et al. Antioxidant and cell-signaling functions of hydrogen sulfide in the central nervous system. Oxid Med Cell Longev. 2018;2018:1873962.
- Gong QH, Shi XR, Hong ZY, et al. A new hope for neurodegeneration: possible role of hydrogen sulfide. J Alzheimers Dis. 2011;24(Suppl 2):173–182.
- Zhang X, Bian JS. Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci. 2014;5:876–883.
- Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729.
- Ma K, Huang MY, Guo YX, et al. Matrine-induced autophagy counteracts cell apoptosis via the ERK signaling pathway in osteosarcoma cells. Oncol Lett. 2016;12:1854–1860.
- Maycotte P, Aryal S, Cummings CT, et al. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 2012;8:200–212.
- Du C, Lin X, Xu W, et al. Sulfhydrated Sirtuin-1 increasing its deacetylation activity is an essential epigenetics mechanism of anti-atherogenesis by hydrogen sulfide. Antioxid Redox Signal. 2019;30:184–197.
- Liu SY, Li D, Zeng HY, et al. Hydrogen sulfide inhibits chronic unpredictable mild stress-induced depressive-like behavior by upregulation of Sirt-1: involvement in suppression of hippocampal endoplasmic reticulum stress. Int J Neuropsychopharmacol. 2017;20:867–876.
- Tang YY, Wang AP, Wei HJ, et al. Role of silent information regulator 1 in the protective effect of hydrogen sulfide on homocysteine-induced cognitive dysfunction: involving reduction of hippocampal ER stress. Behav Brain Res. 2018;342:35–42.
- Wang CY, Zou W, Liang XY, et al. Hydrogen sulfide prevents homocysteineinduced endoplasmic reticulum stress in PC12 cells by upregulating SIRT1. Mol Med Rep. 2017;16:3587–3593.
- Liu AJ, Li B, Yang M, et al. Sirtuin 1 mediates hydrogen sulfide-induced cytoprotection effects in neonatal mouse cardiomyocytes. Chin Med J (Engl). 2017;130:2346–2353.
- Xin H, Wang M, Tang W, et al. Hydrogen sulfide attenuates inflammatory hepcidin by reducing IL-6 secretion and promoting SIRT1-mediated STAT3 deacetylation. Antioxid Redox Signal. 2016;24:70–83.
- Wu D, Hu Q, Liu X, et al. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide. 2015;46:204–212.
- Zhang Y, Tang ZH, Ren Z, et al. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol. 2013;33:1104–1113.
- Chen C, Xia B, Tang L, et al. Echinacoside protects against MPTP/MPP(+)-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metab Brain Dis. 2019;34:203–212.
- Salimian N, Peymani M, Ghaedi K, et al. Modulation in miR-200a/SIRT1axis is associated with apoptosis in MPP(+)-induced SH-SY5Y cells. Gene. 2018;674:25–30.
- Zhang Q, Zhang P, Qi GJ, et al. Cdk5 suppression blocks SIRT1 degradation via the ubiquitin-proteasome pathway in Parkinson’s disease models. Biochim Biophys Acta Gen Subj. 2018;1862:1443–1451.
- Romeo-Guitart D, Leiva-Rodriguez T, Fores J, et al. Improved motor nerve regeneration by SIRT1/Hif1a-mediated autophagy. Cells. Switzerland: Multidisciplinary Digital Publishing Institute (MDPI); 2019. p. 8.
- Li L, Whiteman M, Guan YY, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360.
- Caliendo G, Cirino G, Santagada V, et al. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Med Chem. 2010;53:6275–6286.
