ABSTRACT
Although chemotherapy is one of the effective means of treating gastric cancer, the resistance of chemotherapeutic drugs has followed. And the mechanisms of resistance are not completely clear. The main aim of this article was to develop a kind of drug that could reduce the resistance of cisplatin on gastric cancer cells.
The MGC-803 and MGC-803/DDP cells were treated by cisplatin for 48 h and Lidocaine (Lido) for 24 h. Cell viability, apoptosis, migration and invasion were tested by cell counting kit-8 (CCK-8) assay, apoptosis assay, western blot, migration and invasion assay. After MGC-803/DDP cells were transfected for 48 h, the expression of microRNA-10b (miR-10b) were detected by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Activation of AKT/mTOR and β-catenin pathways was tested by western blot.
Cisplatin caused MGC-803 and MGC-803/DDP cell apoptosis, and MGC-803/DDP cells possessed higher cisplatin resistance than MGC-803 cells. Lido reduced the cisplatin resistance of MGC-803/DDP cells. Besides, Lido inhibited MGC-803/DDP cell migration and invasion. In addition, Lido declined cisplatin resistance by down-regulating miR-10b. Lido also repressed AKT/mTOR and β-catenin pathway by down-regulating miR-10b.
This article explained the role of Lido in cisplatin resistance in MGC-803/DDP cells. Furthermore, Lido weakened the cisplatin resistance in MGC-803/DDP cells at least in part through decreasing the expression of miR-10b.
Introduction
It is well known that gastric cancer is a kind of common cancers. There is an extremely high incidence every year [Citation1,Citation2]. The pathogenesis of gastric cancer is not fully understood until, but its mechanism comes close to other cancers. All the time, excessive cancer cell growth and migration, as well as the decreased cell apoptosis are regarded as the main features of cancers[Citation3]. So, it is aimed to treat cancers via inhibiting cell proliferation and promoting cell apoptosis. Currently, the effective ways of gastric cancer treatment include chemical, radiative and molecular-targeted therapies[Citation4]. Although chemotherapy is the most widely used, the resistance of drugs comes along. Cisplatin is a common drug of chemotherapy[Citation5], but the mechanism of cisplatin resistance is not still fully realized in the treatment of gastric cancer. So, it is important to find new drugs that can reduce the cisplatin resistance.
Lidocaine (Lido) is a common clinical local amide anesthetic[Citation6]. It was widely employed in different aspects with outstanding functions on the treatment of pain[Citation7]. In addition to this, Lido also has a remarkable anti-cancer effect. Studies have suggested Lido can suppress cell proliferation and migration in gastric[Citation8], lung[Citation7] and breast[Citation9] cancer cells. Meanwhile, the study showed Lido could improve the ability of cisplatin-repressed breast cancer cell cytotoxicity[Citation9]. But the function of Lido on cisplatin resistance in gastric cancer was not reported still.
MicroRNAs (miRNAs) acted as not only an important regulator of managing cell growth and development but also a key controller of determining the fate of cancer cells. A report has released that miRNAs have an important influence in the conduction of gastric cancer cells[Citation10]. At the same time, studies have verified that the expression of miR-10b is up-regulated in gastric cancer cells[Citation10]. The higher level of miR-10b also promoted gastric cancer cell proliferation and invasion[Citation11]. Additionally, the expression of miR-10b was tested as a diagnostic and prognostic biomarker of gastric cancer[Citation12]. Since the miR-10b plays an important role in the pathogenesis of gastric cancer, it is necessary to deeply study the role of miR-10b in cisplatin resistance for the treatment of gastric cancer.
In short, the role of miR-10b in cisplatin resistance reduced by Lido was explored in MGC-803/DDP cells. This article brought evidence to elucidate how Lido alleviated cisplatin resistance in gastric cancer MGC-803/DDP cells.
Methods
Cell culture and treatment
The MGC-803 and MGC-803/DDP cells were gained from the Academy of Military Medical Science (Beijing, China). Dulbecco’s modified eagle medium (DMEM) was purchased from Gibco Life Technologies (Waltham, MA, USA). The DMEM was supplemented by 10% fetal bovine serum (FBS) (Gibco Life Technologies, Waltham, MA, USA) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). MGC-803 and MGC-803/DDP cells cultured with complete DMEM were placed in a chamber (Thermo Fisher, Waltham, MA, USA) 37°C containing 5% CO2.
Cisplatin (item number, 479,306; ≥99.9%, HPLC) and Lido (item number, 39,778; ≥98.0%, HPLC) was received from Sigma-Aldrich. MGC-803 and MGC-803/DDP cells were treated with cisplatin (0, 10, 20, 30, 40, 50 µg/mL) for 48 h and Lido (0, 25, 50, 100, 200 µM) for 24 h, according to previous studies [Citation13,Citation14]. Cisplatin and Lido were dissolved in dimethyl formamide (DMF, Sigma-Aldrich) to get stock solution. Then, stock solution was diluted into different experimental concentrations.
Cell transfection
MiR-10b mimic (5ʹ- UACCCUGUAGAACCGAAUUUGUG-3ʹ) and the respective negative control (NC) mimic (5ʹ-TTCTCCGAACGTGTCACGT-3ʹ) were synthesized by GenePharma Co. (Shanghai, China). The transfection was done by using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) for 48 h, on the basis of the manufacturers’ information.
Cell counting kit-8 (CCK-8) assay
The MGC-803 and MGC-803/DDP cells were, respectively, seeded in the 96-well plate. The CCK-8 solution (Dojindo Molecular Technologies, Gaithersburg, MD, USA) was added into every well. After cells were cultured with CCK-8 solution for 1 h, the absorbance of every well was measured at a wavelength of 450 nm by a Microplate Reader (Bio-Rad, Hercules, CA, USA).
Apoptosis assay
Cell apoptosis assay was achieved by choosing propidium iodide (PI) and fluorescein isothiocyanate (FITC)-conjugated Annexin V staining. After all cells were treated by different drugs, cells were washed by using phosphate-buffered saline (PBS) (Sigma-Aldrich). Then, cells were stained in 100 µL PI/FITC-Annexin V (Sangon Biotech, Shanghai, China). Flow cytometry test was done, according to the instruction of FACS can (Beckman Coulter, Fullerton, CA, USA). All results were calculated by FlowJo software (Treestar, San Carlos, CA, USA).
Migration and invasion assay
Cell migration assay was performed through a reconstituted transwell (Sigma-Aldrich). The upper layer was filled with cells with serum-free medium. The lower layer was supplemented with serum medium. After incubation at 37°C, cells were fixed by methanol. Then, cells were stained by 0.5% crystal violet (Sigma-Aldrich) for 10 min. Ultimately, cells’ number was calculated that migrated to the underside of the membrane.
Cell invasion assay was similar to the cell migration assay. However, in cell invasion assay, the upper layer was pre-coated with 8 µm size of matrigel (Millipore, Bedford, MA, USA). The remaining steps were same as the cell migration assay.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA) was chosen to acquire cell RNA. Then, RNA was reversed by miScript Reverse transcription kit (Qiagen GmbH, Hilden, Germany). The qRT-PCR was completed by adopting the Taqman Universal Master Mix II (Takara Bio, Otsu, Japan) for miRNA. U6 was regarded as the control of miRNA. The 2-ΔΔCt method was adopted to perform data analysis. The forward and reverse primer sequences of miR-10b and U6 were as follows:
MiR-10b
Forward primer: 5ʹ-GGGTACCCTGTAGAACCG-3ʹ;
Reverse primer: 5ʹ-AACTGGTGTCGTGGAGTCGGC-3ʹ.
U6
Forward primer: 5ʹ-CTCGCTTCGGCAGCACATATACT-3ʹ;
Reverse primer: 5ʹ-ACGCTTCACGAATTTGCGTGT-3ʹ.
Western blot
The proteins in MGC-803 and MGC-803/DDP cells were obtained via using a protein extraction kit (Sigma-Aldrich). The concentration of protein was counted by adopting BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). All proteins were quantitated, according to the concentration of various proteins. A Bio-Rad Bis-Tris Gel system was used to separate proteins in line with the manufacturer’s instructions. The membranes (Millipore) were covered by 5% Bovine Serum Albumin (BSA) (Sigma-Aldrich) in Tris Buffered saline Tween (TBST) (Sigma-Aldrich). Primary antibodies against Bax (ab32503, Abcam, Cambridge, UK), cleaved-caspase-3 (ab2302), p-AKT (ab38449), t-AKT (ab8805), t-mTOR (ab2732), β-catenin (ab32572), β-actin (ab8227), cleaved-PARP (AB3620, Sigma-Aldrich, St. Louis, MO, USA), MMP-2 (MAB13405), TIMP-1 (WH0007076M1), MMP-9 (sc-21,733, Santa Cruz Biotechnology, Santa Cruz, CA, USA), p-mTOR (#5536, Cell Signaling Technology, Beverly, MA, USA) were chosen. Two kinds of second antibodies (goat anti-rabbit (ab6940, Abcam, Cambridge, UK) and goat anti-mouse (ab97035)) were used. Different proteins were estimated by Image Lab™ Software (Bio-Rad, Shanghai, China).
Statistical analysis
Three experiments were accomplished to repeat every data. All data were shown as mean ± standard deviation (SD) or mean + SD. We adopted Graphpad statistical software 6.0 to analyze our data. The p-values were calculated with t-test and a one-way analysis of variance (ANOVA). p < 0.05 was regarded as a meaningful result.
Results
Cisplatin-repressed cell proliferation and accelerated cell apoptosis in MGC-803 and MGC-803/DDP cells
Cisplatin (0, 10, 20, 30, 40, 50 µg/mL) was selected to treat MGC-803 and MGC-803/DDP cells for 48 h. When the concentration of cisplatin was 30 µM, the viability of MGC-803 and MGC-803/DDP cell was significantly decreased compared with 0 µM cisplatin group (p < 0.01 or p < 0.001). As the concentration of cisplatin increased, those two cell viability displayed markedly decreased trends. So, 30 µM cisplatin was used as the following experimental concentration. The rate of cell apoptosis was both strikingly increased by cisplatin. (p < 0.001, )). The expression levels of Bax, cleaved-caspase-3 and cleaved-PARP protein were also tested. As depicted in -), those three protein levels were increased by cisplatin in the above two cell lines (p < 0.001). These data suggested that cisplatin suppressed cell proliferation and promoted apoptosis in two cell lines, and MGC-803/DDP cells had higher resistance than MGC-803 cells.
Figure 1. Cisplatin-repressed cell proliferation and accelerated cell apoptosis in MGC-803 and MGC-803/DDP cells. MGC-803 and MGC-803/DDP cells were treated by 0, 10, 20, 30, 40, 50 µg/mL of cisplatin for 48 h respectively. (a) Cell viability was tested by CCK-8 assay. ** denotes p < 0.01 and *** denotes p < 0.001 vs. the MGC-803 group. MGC-803 and MGC-803/DDP cells were treated by 30 µg/mL of cisplatin. (b) Cell apoptotic rate was measured by apoptosis assay, (c-f) the levels of Bax, cleaved-caspase-3, and cleaved-PARP protein were tested by western blot. *** denotes p < 0.001 vs. control (Ctrl) group.
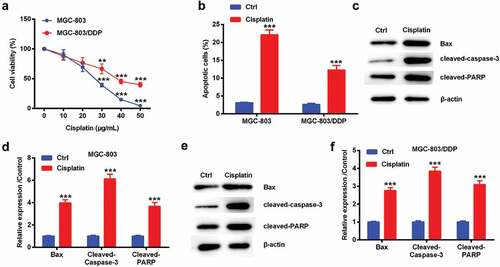
Lido reduced cisplatin resistance in MGC-803/DDP cells
After MGC-803/DDP cells were treated by 0–200 µM of Lido, cell viability was monitored by CCK-8 assay. Lido of 0–200 µM cannot significantly change cell viability in MGC-803/DDP cells ()). When 200 µM of Lido treated MGC-803/DDP cells, cell viability exhibited a decreased trend, suggesting this concentration of Lido may have a toxic effect on MGC-803/DDP cells. So, we chose 100 µM of Lido with no effect on the cells for the following experiments. Cisplatin-driven cell viability was further decreased by Lido (p < 0.01 or p < 0.001, )). Cisplatin-driven cell apoptotic number was further increased by Lido (p < 0.001, )). Cisplatin-induced the above apoptosis-associated protein expression levels were further up-regulated by Lido in MGC-803/DDP cells (p < 0.01 or p < 0.001, -)). The above results suggested that Lido reduced cisplatin resistance in MGC-803/DDP cells.
Figure 2. Lido reduced cisplatin resistance in MGC-803/DDP cells. MGC-803/DDP cells were treated by 0, 25, 50, 100, 200 µM of Lido for 24 h. (a) Cell viability was tested by CCK-8 assay. MGC-803/DDP cells was treated by cisplatin (0, 10, 20, 30, 40, 50 µg/mL) alone for 48 h or in combination with Lido (100 µM). (b) Cell viability was tested by CCK-8 assay. ** denotes p < 0.01 and *** denotes p < 0.001 vs. MGC-803/DDP + cisplatin group. MGC-803/DDP cells were treated by cisplatin (30 µg/mL) and Lido (100 µM) or only cisplatin (30 µg/mL). (c) Cell apoptotic rate was measured by apoptosis assay, (d-e) Bax, cleaved-caspase-3, and cleaved-PARP protein levels in were tested by western blot. ** denotes p < 0.01 and *** denotes p < 0.001 vs. control (Ctrl) group. ## denotes p < 0.01 and ### denotes p < 0.001 vs. cisplatin group.
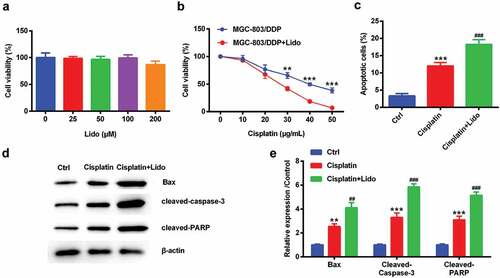
Lido suppressed the migration and invasion of MGC-803/DDP cells
MGC-803/DDP cells were treated by 100 µM Lido for 24 h. The percentage of cell migration and invasion was obviously declined by Lido (p < 0.01, -)). The expression levels of MMP-2 and MMP-9 protein were lowered by Lido (p < 0.01 or p < 0.001, -)). However, TIMP-1 expression was increased by Lido (p < 0.001, -)). So, we made a conclusion that Lido inhibited the MGC-803/DDP cell migration and invasion.
Figure 3. Lido suppressed the migration and invasion of MGC-803/DDP cells. MGC-803/DDP cells were treated by 100 µM of Lido for 24 h. (a) The rate of cell migration was determined by migration assay, (b) the rate of cell invasion was determined by invasion assay, (c-d) MMP-2, MMP-9 and TIMP-1 protein levels were measured by western blot. ** denotes p < 0.01 and *** denotes p < 0.001 vs. control (Ctrl) group.
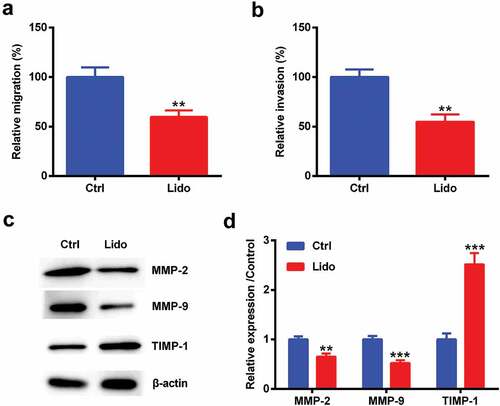
Lido inhibited miR-10b level in MGC-803/DDP cells
MiR-10b expression was measured in MGC-803 and MGC-803/DDP cells. MiR-10b was expressed in the two kinds of cells, but miR-10b expression in MGC-803/DDP cells was higher than the MGC-803 cells (p < 0.001, )). Meanwhile, Lido decreased miR-10b expression (p < 0.001, )). These results released that Lido inhibited miR-10b expression in MGC-803/DDP cells.
Figure 4. Lido inhibited miR-10b level in MGC-803/DDP cells. (a) The expression levels of miR-10b in MGC-803 and MGC-803/DDP cells were determined by qRT-PCR. *** denotes p < 0.001 vs. the MGC-803. (b) MGC-803/DDP cells were treated 100 µM of Lido for 24 h, and then miR-10b expression levels were determined by qRT-PCR. *** denotes p < 0.001 vs. control (Ctrl) group.
Lido reduced cisplatin resistance in MGC-803/DDP cells through down-regulating miR-10b expression
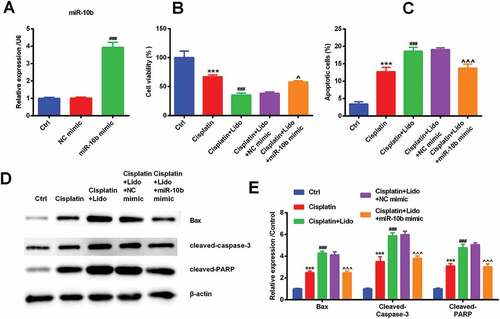
MiR-10b mimic and NC mimic were transfected to confirm the effect of miR-10b on cisplatin resistance reduced by Lido. After transfection of miR-10b mimic, the level of miR-10b was markedly increased (p < 0.001, )). This showed that our transfection was successful. The transfection of miR-10b mimic could reverse that Lido repressed cell viability and increased cell apoptotic number (p < 0.05 or p < 0.001, -)). In addition to this, Lido-evoked up-regulation of Bax, cleaved-caspase-3 and cleaved-PARP protein was also suppressed by the transfection of miR-10b mimic (p < 0.001, -)). So, we made a guess that Lido reduced cisplatin resistance in MGC-803/DDP cells via down-regulating miR-10b level.
Figure 5. Lido reduced cisplatin resistance in MGC-803/DDP cells through down-regulating miR-10b expression. NC mimic and miR-10b mimic were transfected in MGC-803/DDP cells for 48 h. (a) MiR-10b expression levels were determined by qRT-PCR. ### denotes p < 0.001 vs. NC mimic group. After transfection with NC mimic or miR-10b mimic, MGC-803/DDP cells were treated by cisplatin (30 µg/mL) in combination with Lido (100 µM). (b) Cell viability was determined by CCK-8 assay, (c) cell apoptotic rate was determined by apoptosis assay, (d-e) Bax, cleaved-caspase-3, and cleaved-PARP protein expression levels were determined by western blot. *** indicates p < 0.001 vs. control (Ctrl) group. ### denotes p < 0.001 vs. cisplatin group. ^ denotes p < 0.05 and ^^^ denotes p < 0.001 vs. cisplatin + Lido + NC mimic group.
Lido repressed AKT/mTOR and β-catenin pathways via down-regulating miR-10b level
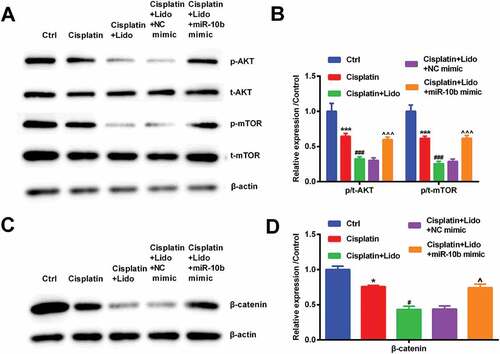
After the miR-10b mimic and NC mimic were transfected, and then the activation of AKT/mTOR and β-catenin pathways was tested by western blot. The phosphorylation levels of AKT and mTOR were declined by cisplatin (p < 0.001). The down-regulated AKT and mTOR phosphorylation levels in cisplatin-treated cells were further decreased by Lido (p < 0.001). After transfection of miR-10b mimic, the declined phosphorylation levels of AKT and mTOR were reversed (p < 0.001, -)). Furthermore, the β-catenin expression was down-regulated by cisplatin (p < 0.05). The down-regulated β-catenin expression level in cisplatin-treated cells was further decreased by Lido (p < 0.05). After transfection of miR-10b mimic, the declined β-catenin expression was reversed (p < 0.05, -)). Above all results demonstrated that Lido repressed AKT/mTOR and β-catenin pathways via declining miR-10b expression.
Figure 6. Lido repressed AKT/mTOR and β-catenin pathways via down-regulating miR-10b level. After transfection with NC mimic or miR-10b mimic, MGC-803/DDP cells were treated by cisplatin (30 µg/mL) in combination with Lido (100 µM). (a-b) The phosphorylation levels of AKT and mTOR were determined by western blot, (c-d) the β-catenin levels were also determined by western blot. * denotes p < 0.05 and *** denotes p < 0.001 vs. control (Ctrl) group. # denotes p < 0.05 and ### denotes p < 0.001 vs. cisplatin group. ^ denotes p < 0.05 and ^^^ denotes p < 0.001 vs. cisplatin + Lido + NC mimic group.
Discussion
Gastric cancer is one of the most typical cancer [Citation15–17]. Gradually, gastric cancer is widely valued by people. At the same time, the ways of gastric cancer treatment have developed widely now. As a means of treatment, chemotherapy is extensively applied [Citation18,Citation19]. Cisplatin is an effective drug of chemotherapy[Citation20]. Although cisplatin could inhibit the proliferation of tumor cell and promote apoptosis to a certain degree, the resistance of cisplatin should be taken seriously by people[Citation21]. So, the effect of cisplatin resistance in MGC-803/DDP cells is fairly important for the effective treatment of gastric cancer. In our study, cisplatin could suppress proliferation and promote apoptosis in MGC-803 and MGC-803/DDP cells. But MGC-803/DDP cells possessed higher resistance than MGC-803 cells by cisplatin. Furthermore, Lido relieved the cisplatin resistance in MGC-803/DDP cells. Lido also inhibited MGC-803/DDP cell migration and invasion. Moreover, Lido also decreased miR-10b expression. It was tested that Lido reduced cisplatin resistance and migration in MGC-803/DDP cells via down-regulating miR-10b. We also certificated that Lido repressed AKT/mTOR and β-catenin pathways via decreasing miR-10b.
The inhibition of cell viability and the promotion of apoptosis are effective means of gastric cancer treatment [Citation22,Citation23]. In our study, MGC-803 and MGC803/DDP cell viability was markedly suppressed by cisplatin. And apoptosis was obviously promoted by cisplatin. However, MGC803/DDP cells showed significant resistance to cisplatin. Bax, cleaved-caspase-3 and cleaved-PARP were regarded as factors of regulating cell apoptosis[Citation24]. Our experimental results showed that the above protein levels were markedly up-regulated by cisplatin in MGC-803 and MGC-803/DDP cells. Our experimental data were similar to the data from other studies. Other researches have confirmed that cisplatin as a chemotherapeutic drug not only degrades cell viability and promotes apoptosis but also exhibits resistance to repression of cell proliferation and promotion of cell apoptosis in gastric cancer cells [Citation25,Citation26]. So, it is necessary to find a new drug that alleviates cisplatin resistance.
Currently, countless studies explained the truth that Lido not just inhibited cancer cell growth and sped cell apoptosis, but also suppressed cell migration and invasion[Citation14]. Our study has released that Lido further decreased cell growth and promotes apoptosis induced by cisplatin in MGC-803/DDP cells. MMP-2, MMP-9 and TIMP-1 are key regulators of migration and invasion[Citation27]. We additionally found that Lido decreased MGC-803/DDP cell migration and invasion. In addition, Lido down-regulated MMP-2 and MMP-9 level, while promoted TIMP-1 expression. Our data were consistent with the previous results. But previous studies mainly focused on other cancer, like liver[Citation28], breast[Citation9] and lung cancer[Citation7].
As crucial regulators of cell growth and development, miRNAs are involved in controlling the proliferation, apoptosis and invasion of various cancer cells, including gastric cancer[Citation29]. Recently, it was suggested that miR-10b expression was increased in gastric cancer and down-regulation of miR-10b was induced to treat gastric cancer [Citation30,Citation31]. Our results indicated that the miR-10 level in MGC-803/DDP cells was higher than in MGC-803 cells. Moreover, Lido could down-regulate miR-10b expression. So, we made a guess that Lido could reduce cisplatin resistance through declining miR-10b level. Further results showed the up-regulation of miR-10b inhibited the decrease of cell viability and the increase of cell apoptotic number induced by Lido. Besides, high expression of miR-10b reversed the changes of apoptosis-associated protein induced by Lido. Above all results prompted a message that Lido reduced cisplatin resistance by decreasing miR-10b expression.
The AKT/mTOR and β-catenin pathways are two key signaling pathways that are closely related to cancer. A previous study has verified that gastric cancer cell apoptosis was modulated by the AKT/mTOR signaling pathway[Citation32]. Furthermore, a research also explained that the stimulation of mTOR was induced by the activation of AKT[Citation33]. In addition to this, excessive researches showed that the β-catenin signaling pathway controlled the development and metastasis of gastric cancer cells[Citation34]. A report also explicated that some miRNAs participated in cell proliferation and migration via regulating AKT/mTOR and β-catenin pathways in gastric cancer cells. For instance, the up-regulation of miR-501-5b induced the activation of β-catenin pathway to induce gastric cancer cell proliferation[Citation35]. Also, a study explained that high expression of miR-375 inhibited the activation of AKT/mTOR pathway to repress cell proliferation and migration in gastric cancer cells[Citation36]. Although countless researches were reported that miRNAs played an important role in gastric cancer, different miRNAs had different effects on gastric cancer cell growth and development through various pathways. Moreover, it was not reported if AKT/mTOR and β-catenin signaling pathways controlled gastric cancer cell growth through miR-10b expression. In our research, cisplatin inhibited AKT/mTOR and β-catenin signaling pathways. Furthermore, Lido repressed those two signaling pathways through down-regulation of miR-10b. Above all data illustrated that we could decrease the miR-10b level to repress signaling pathways and relieve cisplatin resistance in gastric cancer.
Conclusion
As a whole, this article described the role of Lido in MGC-803/DDP cells. This study pointed out that Lido alleviated cisplatin resistance in MGC-803/DDP cells. What is more, Lido reduced cisplatin resistance and repressed AKT/mTOR and β-catenin signaling pathways in MGC-803/DDP cells through down-regulation of miR-10b. Our experimental data lays a solid theoretical foundation for cisplatin resistance on chemotherapy in gastric cancer.
Disclosure statement
The authors declare that there are no conflicts of interest.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Sun Z, Wang Q, Yu X, et al. Risk factors associated with splenic hilar lymph node metastasis in patients with advanced gastric cancer in northwest China. Int J Clin Exp Med. 2015;8:21358–21364.
- Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713.
- Yao Z, Luo J, Hu K, et al. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 2017;11:422–437.
- Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626.
- Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53.
- Sprung CL, Marcial EH, Garcia AA, et al. Prophylactic use of lidocaine to prevent advanced ventricular arrhythmias during pulmonary artery catheterization. Prospective double-blind study. Am J Med. 1983;75:906–910.
- Sun H, Sun Y. Lidocaine inhibits proliferation and metastasis of lung cancer cell via regulation of miR-539/EGFR axis. Artific Cells Nanomed Biotechnol. 2019;47:2866–2874.
- Sui H, Lou A, Li Z, et al. Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC Cancer. 2019;19:233.
- Li K, Yang J, Han X. Lidocaine sensitizes the cytotoxicity of cisplatin in breast cancer cells via up-regulation of RARbeta2 and RASSF1A demethylation. Int J Mol Sci. 2014;15:23519–23536.
- Bakhshi M, Asadi J. Increased expression of miR-146a, miR-10b, and miR-21 in cancer stem-like gastro-spheres. J Cell Biochem. 2019;120:16589–16599.
- Wang YY, Li L, Ye ZY, et al. MicroRNA-10b promotes migration and invasion through Hoxd10 in human gastric cancer. World J Surg Oncol. 2015;13:259.
- Jamali L, Tofigh R, Tutunchi S, et al. Circulating microRNAs as diagnostic and therapeutic biomarkers in gastric and esophageal cancers. J Cell Phys. 2018;233:8538–8550.
- Li Z, Wu C, Wu J, et al. Synergistic antitumor effects of combined deguelin and cisplatin treatment in gastric cancer cells. Oncol Lett. 2014;8:1603–1607.
- Yang Q, Zhang Z, Xu H, et al. Lidocaine alleviates cytotoxicity-resistance in lung cancer A549/DDP cells via down-regulation of miR-21. Mol Cell Biochem. 2019;456:63–72.
- Wang D, Ye F, Sun Y, et al. Protein signatures for classification and prognosis of gastric cancer a signaling pathway-based approach. Am J Pathol. 2011;179:1657–1666.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1211–1259.
- Huang T, Zhou F. Reactive oxygen species are involved in the development of gastric cancer and gastric cancer-related depression through ABL1-mediated inflammation signaling pathway. Oxidat Med Cell Long. 2019;2019:5813985.
- Qiu H. Complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases: results of a propensity score matching analysis from France. Cancer Commun (Lond). 2019;39:45.
- Reutovich MY, Krasko OV, Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol. 2019;45(12):2405–2411.
- Sasaki K, Onodera S, Otsuka K, et al. Validity of neoadjuvant chemotherapy with docetaxel, cisplatin, and S-1 for resectable locally advanced gastric cancer. Med Oncol. 2017;34:139.
- Zhai J, Shen J, Xie G, et al. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43.
- Song C, Han Y, Luo H, et al. HOXA10 induces BCL2 expression, inhibits apoptosis, and promotes cell proliferation in gastric cancer. Cancer Med. 2019;8(12):5651–5661.
- Ni T, Wang H, Li D, et al. Huachansu Capsule inhibits the proliferation of human gastric cancer cells via Akt/mTOR pathway. Biomed Pharmacother. 2019;118:109241.
- Zhang Y, Zhang W, Tao L, et al. Quercetin protected against isoniazide-induced HepG2 cell apoptosis by activating the SIRT1/ERK pathway. J Biochem Molecul Toxicol. 2019;33:e22369.
- Han X, Zhang JJ, Han ZQ, et al. Let-7b attenuates cisplatin resistance and tumor growth in gastric cancer by targeting AURKB. Cancer Gene Ther. 2018;25:300–308.
- Yan R, Li K, Yuan DW, et al. Downregulation of microRNA-4295 enhances cisplatin-induced gastric cancer cell apoptosis through the EGFR/PI3K/Akt signaling pathway by targeting LRIG1. Int J Oncol. 2018;53:2566–2578.
- Bayramoglu A, Gunes HV, Metintas M, et al. The association of MMP-9 enzyme activity, MMP-9 C1562T polymorphism, and MMP-2 and −9 and TIMP-1, −2, −3, and −4 gene expression in lung cancer. Genet Test Mol Biomarkers. 2009;13:671–678.
- Xing W, Chen DT, Pan JH, et al. Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiology. 2017;126:868–881.
- Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20:10432–10439.
- Wang YY, Ye ZY, Zhao ZS, et al. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum Pathol. 2013;44:1278–1285.
- Liu Z, Zhu J, Cao H, et al. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int J Oncol. 2012;40:1553–1560.
- Yang ZJ, Chee CE, Huang S, et al. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–1541.
- Fu L, Kim YA, Wang X, et al. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–8976.
- Yang XH, Zhuang MK, Xie WH, et al. 12-Lipoxygenase promotes epithelial-mesenchymal transition via the Wnt/beta-catenin signaling pathway in gastric cancer cells. Onco Targets Ther. 2019;12:5551–5561.
- Fan D, Ren B, Yang X, et al. Upregulation of miR-501-5p activates the wnt/beta-catenin signaling pathway and enhances stem cell-like phenotype in gastric cancer. J Exp Clin Cancer Res. 2016;35:177.
- Yuan KT, Li BX. Deregulation of MicroRNA-375 inhibits proliferation and migration in gastric cancer in association with autophagy-mediated AKT/mTOR signaling pathways. Technol Cancer Res Treat. 2018;17:1533033818806499.
