ABSTRACT
MiR-34a is associated with diabetic retinopathy (DR). This article aims to demystify the role of miR-34a in DR. We established a DR model by streptozocin injection. Rat retinal vascular endothelial cells (RVECs) were treated with high glucose (HG) to induce DR. The pathological changes of retinal tissues and blood-retinal vascular barrier permeability of DR rats were assessed by HE staining and Evans-Blue leak test. The expression of gene and protein was evaluated by quantitative real-time PCR or western blot. MTT assay and flow cytometry were performed to detect proliferation and apoptosis. The relationship between miR-34a and SIRT1 was evaluated using luciferase reporter assay. MiR-34a was up-regulated and SIRT1 was down-regulated in retinal tissues of DR rats and HG-induced RVECs. MiR-34a silencing improved DR by regulating apoptosis and VEGF expression in DR rats. Furthermore, miR-34a interacted with SIRT1 and suppressed SIRT1 expression. MiR-34a overexpression inhibited proliferation and promoted apoptosis of RVECs, which was effectively abolished by SIRT1 up-regulation. In summary, our data demonstrate that miR-34a promotes apoptosis of RVECs by targeting SIRT1 in DR rats. Our findings suggest that miR-34a/SIRT1 axis could be a valuable target for DR therapies.
Introduction
Diabetic retinopathy (DR) is a progressive neurodegenerative disease [Citation1]. DR is the most common complication of ophthalmology in diabetes mellitus (DM) patients, and its incidence is extremely high in DM patients. In recently, as the increase of DM patients, the number of patients with DR has also increased, causing widespread concern. Under the pathological micro-environment of DM, there are abnormalities in the structure and function of various cells in the retina, including apoptosis of retinal ganglion cells, retinal vascular endothelial cells (RVECs) disorders, and abnormal cell metabolism. DR ultimately leads to irreversible blindness, which seriously affects the quality of life of DR patients. Thus, the prevention and treatment of DR has become a major public health problem at home and abroad, and it has been become the research focus of ophthalmologists.
MicroRNAs (miRNAs) are broadly defined as RNA molecules that have 19–25 nucleotides that appear to lack protein-coding potential. MiRNAs regulate gene expression mainly at the post-transcriptional level. Previous studies have shown that abnormal miRNA expression is closely associated with the occurrence and development of DR [Citation2]. Surveys such as those conducted by Liu show that miR-133b is down-regulated in DR rats. And miR-133b overexpression promotes cell proliferation and represses apoptosis of RVECs by inhibiting AGT expression [Citation3]. MiR-384-3p expression is decreased in DR rats, and miR-384-3p overexpression inhibits cell proliferation and angiogenesis of RVECs in DR rats [Citation4]. Vascular endothelial growth factor (VEGF) and roundabout 4 (Robo 4) have vital role in the progression of DR, and these two genes are co-expressed in fibrovascular membranes of DR patients. However, miR-15a ameliorates DR by repressing the expression of VEGF and Robo4 [Citation5]. Compared with normal mice, miR-451a is down-regulated in DM mice. MiR-451a up-regulation notably suppresses cell proliferation and migration and promotes mitochondrial function by repressing ATF2 expression in retinal pigment epithelial cells [Citation6]. High glucose (HG)-treated retinal pigment epithelial cells exhibit a decrease of miR-455-5p expression, and miR-455-5p ameliorates apoptosis, oxidative stress, and inflammatory by targeting SOCS3 in HG-treated retinal pigment epithelial cells [Citation7]. Recent research has reported that miR-34a is highly expressed in HG-induced retina epithelial cells, and up-regulation of miR-34a significantly inhibits cell proliferation and migration of retina epithelial cells [Citation8]. However, the mechanism of action of miR-34a in DR and RVECs has not been reported.
Silent information regulator 1 (SIRT1) is closely associated with the development of DR. SIRT1 expression is decreased in HG-induced RVECs and DR models [Citation9]. Studies have shown that SIRT1 inhibits NF-κB via regulating deacetylation of NF-κB p65, thereby inhibiting inflammatory responses [Citation10]. SIRT1 also inhibits apoptosis of retinal cells through regulating p53, Bax, or other pathways [Citation11]. SIRT1 represses the occurrence and development of DR by inhibiting the synthesis of VEGF. Existing researches have proved that miR-34a targeting SIRT1. We suspected that whether the mechanism of action of miR-34a/SIRT1 axis existed in RVECs. Therefore, this work aims to explore the relationship between miR-34a and SIRT1 in DR, and focus on the role of miR-34a/SIRT1 signaling pathway in DR.
Materials and methods
Experimental animals
Sprague Dawley (SD) rats weighing 180–200 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The rats were housed under standardized conditions. SD rats were given 60 mg/kg of streptozocin (STZ, Sigma, St. Louis, MO, USA) by a single intraperitoneal injection to establish DR model. Then, the blood glucose level in the tail vein was detected by a Contour glucose meter (Roche, Basel, Switzerland) after 72 h. The glucose level of rats more than 300 mg/dL was considered as DR rats. The SD rats intraperitoneally injected sodium citrate buffer with the same dosage was served as control. Blood glucose results showed that the blood glucose level of rats exceeded 300 mg/dL three d after STZ injection (Supplementary Figure 1), indicating that DR rat models had been successfully established. DR rats were still alive until 12 weeks. Subsequently, DR rats were injected with antagomiR-34a or antagomiR-NC (RIBOBIO, Guangzhou, China) by cauda vein. The rats were killed after 12 weeks, and the retinal tissues of the rats were isolated. All protocols were authorized by the Ethics Committee of Affiliated First Hospital of USTC.
Cell culture and treatment
Rat RVECs were obtained from Shanghai Xuanya Biotechnology CO. Ltd (XY-XB-5566, Shanghai, China). RVECs were cultured in RPMI-1640 medium (Sangon Biotech, Shanghai, China) supplemented with 10% fetal bovine serum (Sangon Biotech) and 1% penicillin/streptomycin. RVECs were subcultured to the third passages. Then, RVECs were incubated with 5 mM glucose (normal glucose, NG) or 25 mM glucose (HG) for 48 h in a humidified atmosphere at 37°C and 5% CO2.
Cell transfection
The miR-34a mimics, inhibitor, and the corresponding negative control (NC) were synthesized by RIBOBIO. The cDNA encoding SIRT1 was PCR amplified and then subcloned into the vector pcDNA3.1 (Invitrogen, Carlsbad, CA, USA), generating the vector pcDNA3.1-SIRT1. The empty pcDNA3.1 vector was used as control. Plasmids were transfected into the cells using Lipofectamine 2000 Transfection Reagent (Invitrogen) following the manufacturer’s protocol. The transfected cells were harvested after 48 to 72 h.
Quantitative real-time PCR (qRT-PCR)
QRT-PCR was used to measure the expression intensity of different genes. Total RNA was extracted from tissues and cells using TRIzol reagent (Invitrogen). The purity of RNA was detected using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The quantity of RNA was determined by agarose gel electrophoresis. The RNA was reversely transcribed to complementary DNA using PrimeScript™ RT Reagent Kit (Takara, Tokyo, Japan). QRT-PCR was carried out using SYBR Green PCR Mix Kit (Takara) according to the instruction. The results were analyzed using the ∆∆CT (cycle threshold) method for quantification.
Western blot (WB)
Total protein was extracted from retinal tissues or cells using Tissue or Cell Total Protein Extraction Kit (Sangon Biotech). Equivalent protein from different samples was separated by protein electrophoresis, following by transformation onto PVDF membranes (Merck Millipore, Billerica, MA, USA). The membranes were incubated with the anti-rabbit SIRT1, Ac-p53, p53, Bax, Bcl-2, or VEGF antibodies (1:1000 dilution, Abcam, Cambridge, MA, USA) at 4°C overnight after immersed into sealed liquid. After the membranes were washed with TBST for several times, goat anti-mouse IgG antibody (1:5000, Abcam) labeled with horseradish peroxidase were incubated with the membranes as a secondary antibody. Anti-mouse GAPDH antibody (1:5000 dilution, Abcam) was used as a reference protein for normalization. The gray level of the protein bands were examined by Image J software.
HE staining
Fresh retinal tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Five-micron sections were obtained after deparaffin and rehydration. Then, sections were stained using HE staining kit (Solarbio, Beijing, China) to observe the pathological changes of retinal tissues.
Evans-blue (EB) leak test
Blood retinal vascular barrier permeability of rats was measured by EB leak test. The rats were anesthetized with sodium pentobarbital, and then the rats were injected with 45 mg/kg EB solution (Sangon Biotech) by cauda vein. After 2 h, the retinal tissue of the rats was isolated. Retinal tissues were dried at 4°C overnight and weighed. Then, the retinal tissues were incubated with 150 μL formamide at 70°C for 18 h. The absorbance of supernatant was detected at 620 and 740 nm wavelength using enzyme-labeled instrument (Thermo Fisher Scientific). Calculated the net absorbance value and normalized the EB content with dry weigh of retinal tissues.
MTT assay
The MTT assay was performed to estimate the cell viability. The cells at log phase were seeded into 96-well plates (5 × 103/well). After that, MTT reagent (20 μL) was added into each well, and the cells cultured for 4 h in an incubator at 37°C. Then, supernatant was discarded and DMSO reagent (100 μL) was added into each well. The absorbance of samples was detected at 570 nm wavelength using an enzyme-labeled instrument (Thermo Fisher Scientific).
Flow cytometry
The apoptosis of the cells was measured by flow cytometry. The cells were collected by centrifugation and washed with pre-cooling PBS for 2 times after treated with doxorubicin (2 μg/mL) for 48 h. The cells were then resuspended in the Annexin V Binding buffer. The cell suspension was dyed with Annexin V-FITC and PI and plunged into darkness at room temperature for 15 min. Then, the cell suspension was mixed with Annexin V Binding buffer and placed on ice. The apoptosis rate of cells was determined by flow cytometry in an hour. The assay was performed according to the instruction of Annexin V-FITC/PI Cell Apoptosis Detection Kit (TransGen Biotech, Beijing, China).
Luciferase reporter assay
SIRT1 containing the predicted miR-34a binding sites were cloned into pGL3-SIRT1-Wt (wild-type) and pGL3-SIRT1-Mut (mutant-type) vectors (RIBOBIO). The Wt (Mut) 3′ untranslated region (UTR) of SIRT1 vector and miR-34a mimic or mimic NC were co-transfected into 293 cells using Lipofectamine 2000 Transfection Reagent (Invitrogen). The luciferase activity of the cells was detected after 48 hours of transfection using the luciferase assay system (Ambion, Austin, TX, USA).
Statistical analysis
All values were exhibited as mean ± standard deviation and analyzed by SPSS 22.0 statistical software (IBM, Armonk, NY, USA). For comparison of two groups, a two-tailed Student’s t test was used. Comparison of multiple groups was made using a one- or two-way ANOVA. Difference was considered statistically significant at P < 0.05.
Results
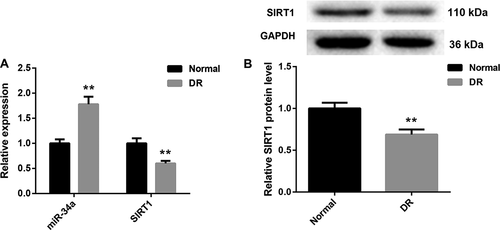
MiR-34a is up-regulated and SIRT1 is down-regulated in the retinal tissues of DR rats
To investigate the involvement of miR-34a in DR, we constructed DR model rats. And we compared the expression of miR-34a and SIRT1 in the retinal tissues between normal and DR rats. As shown in Figure 1a, miR-34a is significantly increased in the retinal tissues of DR rats with respect to normal rats. However, SIRT1 expression was notably lower in the retinal tissues of DR rats than that in normal rats (). Then, we explored the protein expression of SIRT1 in the retinal tissues of normal and DR rats. Compared with normal rats, the retinal tissues of DR rats exhibited a decrease in SIRT1 protein expression. These data show that miR-34a and SIRT1 are associated with DR. MiR-34a is up-regulated and SIRT1 is down-regulated in the retinal tissues of DR rats.
Figure 1. MiR-34a is up-regulated and SIRT1 is down-regulated in the retinal tissues of DR rats
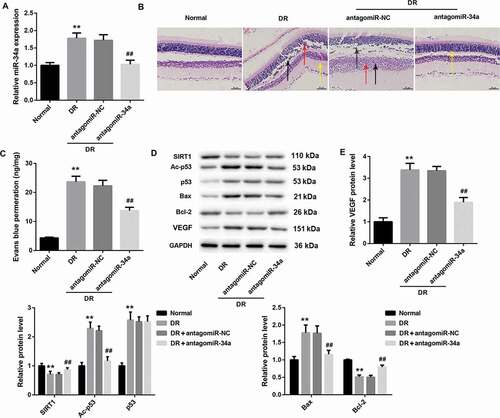
MiR-34a silencing inhibits the progression of DR in rats by regulating apoptosis and VEGF expression
To further verify the role of miR-34a in DR, we silenced miR-34a in DR rats. QRT-PCR assay showed that miR-34a was up-regulated in the retinal tissues of DR rats. And miR-34a silencing caused a decrease of miR-34a expression in the retinal tissues of DR rats (). Then, HE staining was performed to observe the structure of retinal tissues of the rats. Normal rats did not show significant pathological changes in the retinal tissues. However, DR rats had evident retinal damage, which could be effectively improved by miR-34a silencing (). Furthermore, blood-retinal vascular barrier permeability of rats was measured by EB. The content of EB permeability in the retinal tissues of DR rats was significantly higher than that in normal rats. MiR-34a silencing led to a notable decrease in the content of EB permeability in the retinal tissues of DR rats (). In addition, WB was performed to measure the expression of SIRT1, Ac-p53, p53, Bax, Bcl-2, and VEGF in retinal tissues of the rats. As shown in , DR rats have an obvious increase of VEGF expression in retinal tissues, which could be effectively abolished by miR-34a silencing. And DR rats exhibited a decrease of SIRT1 expression. The expression of SIRT1 was suppressed in DR rats after injected with antagomiR-34a. Ac-p53 and p53 expression were obviously enhanced in DR rats. MiR-34a silencing led to a decrease of Ac-p53 expression in the retinal tissues of DR rats, whereas p53 expression did not show significant changes in the retinal tissues. Bax expression was significantly enhanced in DR rats, and the expression of Bax was suppressed by miR-34a silencing. However, Bcl-2 expression exhibited the opposite results (). Taken together, these data suggest that miR-34a silencing inhibits the progression of DR in rats by regulating apoptosis and VEGF expression.
Figure 2. MiR-34a silencing inhibits the progression of DR in rats by regulating apoptosis and VEGF expression
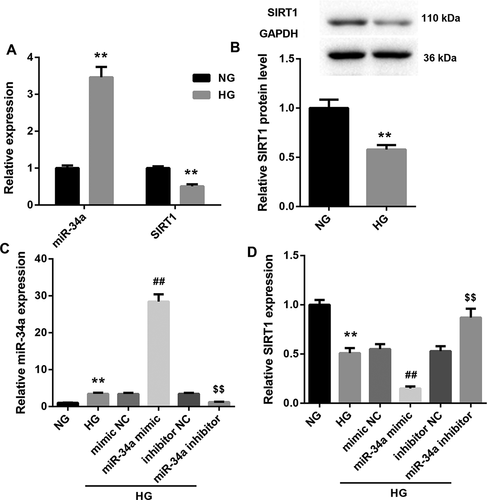
MiR-34a overexpression inhibits SIRT1 expression in HG-induced RVECs
We further investigated the expression of miR-34a and SIRT1 in HG-induced RVECs in vitro. We first explored the gene or protein expression of miR-34a and SIRT1 in HG-induced RVECs by qRT-PCR and WB. Compared with NG-induced RVECs, miR-34a expression was increased in HG-induced RVECs, whereas SIRT1 gene and protein expression were severely down-regulated in HG-induced RVECs (). Then, we constructed miR-34a-overexpressed RVECs and miR-34a-silenced RVECs by cell transfection. And the modified RVECs were treated with HG. HG-induced RVECs showed a boost of miR-34a expression as compared with NG-induced RVECs. The expression of miR-34a was facilitated in HG-induced RVECs after transfected with miR-34a mimic. And the expression of miR-34a was inhibited in HG-induced RVECs after transfected with miR-34a inhibitor (). Moreover, SIRT1 expression was obviously decreased in HG-induced RVECs with respect to NG-induced RVECs. And miR-34a overexpression significantly inhibited the expression of SIRT1, miR-34a silencing facilitated SIRT1 expression in HG-induced RVECs (). Therefore, these results reveal that miR-34a overexpression inhibits SIRT1 expression in HG-induced RVECs in vitro.
Figure 3. MiR-34a overexpression inhibits SIRT1 expression in HG-induced RVECs
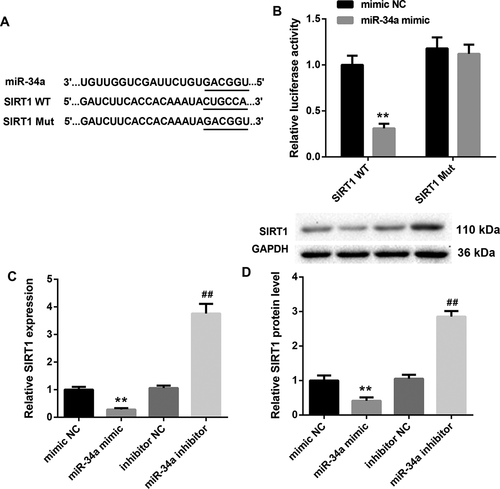
MiR-34a interacts with the 3'UTR of SIRT1 and suppresses SIRT1 expression
To investigate the relationship between miR-34a and SIRT1, we construed Wt (Mut) 3′ UTR of SIRT1 luciferase reporter gene vector (). Then, we performed luciferase reporter assay, showing that miR-34a interacted with the 3′UTR of SIRT1 (). In addition, we constructed miR-34a-overexpressed RVECs and miR-34a-silenced RVECs to explore the effect of miR-34a on SIRT1 expression. MiR-34a up-regulation severely inhibited the gene and protein expression of SIRT1, whereas MiR-34a silencing notably promoted the gene and protein expression of SIRT1 in RVECs ( and ). Thus, these results indicate that miR-34a interacts with the 3′UTR of SIRT1 and suppresses SIRT1 expression.
Figure 4. MiR-34a interacts with the 3'UTR of SIRT1 and suppresses SIRT1 expression
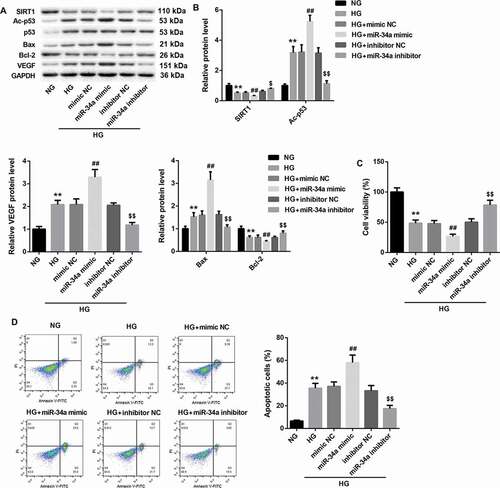
MiR-34a overexpression inhibits cell proliferation and promotes apoptosis of RVECs
To explore the role of miR-34a in RVECs, RVECs were transfected with miR-34a mimic or miR-34a inhibitor. And these modified RVECs were treated with HG. As shown in and , we have found that HG-induced RVECs exhibit a decrease of SIRT1 expression, which could be inhibited by miR-34a overexpression. And the expression of SIRT1 was notably up-regulated in HG-induced RVECs after transfected with miR-34a inhibitor. Moreover, HG-induced RVECs exhibited a boost of Ac-p53 and VEGF expression, which could be promoted by miR-34a up-regulation. And miR-34a silencing inhibited Ac-p53 and VEGF expression in HG-induced RVECs. Bax expression was significantly enhanced in HG-induced RVECs as compared with NG-induced RVECs. MiR-34a overexpression promoted Bax expression, whereas miR-34a silencing repressed Bax expression in HG-induced RVECs. However, Bcl-2 expression exhibited the opposite results ( and ). In addition, the effect of miR-34a on cell proliferation and apoptosis of RVECs was assessed by MTT assay and flow cytometry. HG-induced RVECs displayed a decrease in cell proliferation with respect to NG-induced RVECs. MiR-34a up-regulation suppressed cell proliferation, whereas miR-34a knockdown promoted cell proliferation in HG-induced RVECs (). Compared with NG-induced RVECs, the apoptosis of HG-induced RVECs was increased. And the apoptosis of HG-induced RVECs was promoted by miR-34a up-regulation and repressed by miR-34a knockdown in HG-induced RVECs (). Therefore, these data show that miR-34a overexpression inhibits cell proliferation and promotes apoptosis of RVECs.
Figure 5. MiR-34a overexpression inhibits cell proliferation and promotes apoptosis of RVECs
MiR-34a overexpression inhibits cell proliferation and promotes apoptosis of RVECs by inhibiting SIRT1 expression
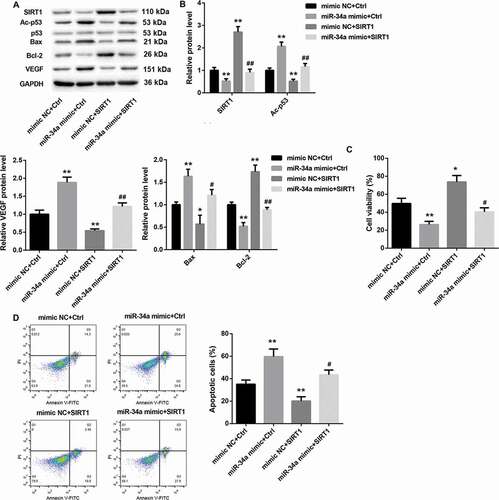
To further explore the role of miR-34a/SIRT1 in RVECs, RVECs were transfected with miR-34a mimic or mimic NC and pcDNA3.1-SIRT1 or pcDNA3.1-NC. Then, the modified RVECs were treated with HG. As shown in and , SIRT1 expression is severely down-regulated in HG-induced RVECs after transfected with miR-34a mimic. SIRT1 overexpression facilitated SIRT1 expression in HG-induced RVECs. And the inhibiting effect of miR-34a overexpression on the expression of SIRT1 was rescued by SIRT1 up-regulation. Furthermore, miR-34a overexpression caused a boost of Ac-p53 and VEGF expression, whereas SIRT1 overexpression led to a decrease of Ac-p53 and VEGF expression in HG-induced RVECs. And the inhibiting effect of miR-34a overexpression on Ac-p53 and VEGF expression was rescued by SIRT1 up-regulation. MiR-34a overexpression promoted the expression of Bax, whereas SIRT1 overexpression repressed the expression of Bax in HG-induced RVECs. And the influence conferred by miR-34a overexpression was abolished by SIRT1 overexpression. However, Bcl-2 expression exhibited the opposite results ( and ). In addition, the effect of miR-34a/SIRT1 on cell proliferation and apoptosis of RVECs was assessed by MTT assay and flow cytometry. MiR-34a overexpression led to a decrease in cell proliferation and caused a boost in apoptosis. On the contrary, SIRT1 overexpression led to an increase in cell proliferation and caused a decrease in apoptosis. And the influence conferred by miR-34a overexpression was abolished SIRT1 overexpression ( and ). Therefore, these data indicate that miR-34a overexpression inhibits cell proliferation and promotes apoptosis of RVECs by inhibiting SIRT1 expression.
Figure 6. MiR-34a overexpression inhibits cell proliferation and promotes apoptosis of RVECs by inhibiting SIRT1 expression
Discussion
MiRNAs have a pivotal role in various biological processes such as cell evolution, cell proliferation, apoptosis, tumors, and immune responses [Citation12]. Existing researches have recognized that miR-34a has a vital role in the occurrence and development of various diseases. Surveys such as that conducted by Kukreti have confirmed that miR-34a is highly expressed in the muscles of aging mice and in the myoblasts of insulin-resistant humans. And miR-34a leads to ceramide accumulation and affects the insulin signaling pathway via targeting ceramide kinase in aging skeletal muscle [Citation13]. Ovarian cancers exhibit a significant lower level of miR-34 family as compared with normal ovarian tissues. Low miR-34 level is associated with a worse overall survival and progression free survival [Citation14]. In DR, HG promotes the expression of miR-34a and induces cellular senescence in human retinal microvascular endothelial cells. And miR-34a plays a critical role in HG-induced stress-associated premature senescence in retinal endothelial cells via mitochondrial dysfunction and loss of antioxidant activities [Citation15]. In our study, we found that miR-34a was significantly increased and SIRT1 was notably decreased in the retinal tissues of DR rats. The study of Gok shows that SIRT1 is highly expressed in the serum of type 2 diabetes patients, whereas there is no difference for miR-34a expression between type 2 diabetes patients and normal people [Citation16]. This is different from our results. It may be that the source of the cells is different, causing different results. And miR-34a may be closely associated with DR, but not related to DM. Thus, these findings indicate that miR-34a and SIRT1 are closely associated with DR.
Furthermore, DR rats exhibited evident retinal damage and a high level of EB permeability in the retinal tissues, which could be effectively improved by miR-34a silencing. Thus, these data suggest that miR-34a knockdown effectively attenuates the progression of DR in rats. In addition, miR-34a had an inhibiting effect on SIRT1 expression in retinal tissues. And Ac-p53 and Bax expression were up-regulated and Bcl-2 expression was down-regulated in retinal tissues of DR rats, which could be effectively improved by knockdown of miR-34a. Previous evidence suggests that miR-34 family are direct transcriptional targets of p53, and loss of miR-34 impairs p53-mediated apoptosis [Citation17]. Bax and Bcl-2 are apoptosis-related genes, and the ratio between Bax, Bcl-2 determines whether the fate of cells is survival or apoptosis [Citation18]. Therefore, it indicates that miR-34a silencing inhibits apoptosis of the retinal tissues in DR rats via p53 and Bcl-2 pathways. VEGF was highly expressed in the retinal tissues in DR rats, which was inhibited by knockdown of miR-34a. VEGF is a major vascular factor that promotes the development of DR. MiR-93 improves DR progression by down-regulating VEGF secretion via VEGF pathway [Citation19]. Our data have shown that miR-34a silencing alleviates DR by inhibiting VEGF expression. Taken together, these data suggest that miR-34a silencing inhibits the progression of DR in rats via regulating apoptosis and VEGF expression.
There have been a number of longitudinal studies involving SIRT1 that have reported that SIRT1 has a pivotal role in DR. The mRNA and protein level of SIRT1 is enhanced in the peripheral blood mononuclear cells of DR patients [Citation20]. Fenofibrate inhibits the NF-κB activity by promoting SIRT1 expression in the retinal tissues, thereby treating DR in rats [Citation21]. SNHG7 attenuates HG-induced angiogenesis in the DR by regulating miR-543-mediated SIRT1/VEGF pathway [Citation22]. Our study showed that miR-34a interacted with the 3'UTR of SIRT1 and suppressed SIRT1 expression in HG-induced RVECs. MiR-34a overexpression promoted the expression of Ac-p53, Bax, VEGF, and inhibited the expression of SIRT1 and Bcl-2. And miR-34a up-regulation led to a boost in apoptosis of RVECs. It indicates that miR-34a up-regulation promotes apoptosis of RVECs via p53 and Bcl-2 pathways. Moreover, miR-34a overexpression caused a decrease in cell proliferation of RVECs. Ultimately, up-regulation of SIRT1 abrogated the effect of miR-34a overexpression on cell proliferation and apoptosis of RVECs. Thus, these data indicate that miR-34a overexpression inhibits cell proliferation and promotes apoptosis of RVECs by inhibiting SIRT1 expression.
In summary, our study has demonstrated that miR-34a promotes apoptosis of RVECs by targeting SIRT1 in rats with DR. Our findings suggest that the miR-34a/SIRT1 axis could be a valuable target for DR therapies.
Supplemental Material
Download Zip (87.4 KB)Disclosure statement
The authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7(2):140–149.
- Martinez B, Peplow PV. MicroRNAs as biomarkers of diabetic retinopathy and disease progression. Neural Regen Res. 2019;14(11):1858–1869.
- Liu TT, Hao, Q, Zhang, Y, et al. Effects of microRNA-133b on retinal vascular endothelial cell proliferation and apoptosis through angiotensinogen-mediated angiotensin II- extracellular signal-regulated kinase 1/2 signalling pathway in rats with diabetic retinopathy. Acta Ophthalmol. 2018;96(5):e626–e635.
- Xia F, Sun J-J, Jiang Y-Q, et al. MicroRNA-384-3p inhibits retinal neovascularization through targeting hexokinase 2 in mice with diabetic retinopathy. J Cell Physiol. 2018;234(1):721–730.
- Gong Q, Li F, Xie J, et al. Upregulated VEGF and Robo4 correlate with the reduction of miR-15a in the development of diabetic retinopathy. Endocrine. 2019;65(1):35–45.
- Shao Y, Dong L-J, Takahashi Y, et al. miRNA-451a regulates RPE function through promoting mitochondrial function in proliferative diabetic retinopathy. Am J Physiol Endocrinol Metab. 2019;316(3):E443–E452.
- Chen P, Miao Y, Yan P, et al. MiR-455-5p ameliorates HG-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells. J Cell Physiol. 2019;234(12):21915–21924.
- Tong P, Peng Q-H, Gu L-M, et al. LncRNA-MEG3 alleviates high glucose induced inflammation and apoptosis of retina epithelial cells via regulating miR-34a/SIRT1 axis. Exp Mol Pathol. 2019;107:102–109.
- Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic retinopathy. Biomed Pharmacothe. 2018;97:190–194.
- Zhao S, Li T, Li J, et al. miR-23b-3p induces the cellular metabolic memory of high glucose in diabetic retinopathy through a SIRT1-dependent signalling pathway. Diabetologia. 2016;59(3):644–654.
- Liu Y, Li R, Xie J, et al. Protective effect of hydrogen on sodium iodate-induced age-related macular degeneration in mice. Front Aging Neurosci. 2018;10:389.
- Shukla GC, Barik SJ. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3(3):83–92.
- Kukreti H, Amuthavalli K. MicroRNA-34a causes ceramide accumulation and effects insulin signaling pathway by targeting ceramide kinase (CERK) in aging skeletal muscle. J Cell Biochem. 2020;121(5–6):3070–3089.
- Welponer H, Tsibulak I, Wieser V, et al. The miR-34 family and its clinical significance in ovarian cancer. J Cancer. 2020;11(6):1446–1456.
- Thounaojam MC, Jadeja, RN, Warren, M, et al. MicroRNA-34a (miR-34a) mediates retinal endothelial cell premature senescence through mitochondrial dysfunction and loss of antioxidant activities. Antioxidants. 2019;8(9):328.
- Gok O, Karaali Z, Ergen A, et al. Serum sirtuin 1 protein as a potential biomarker for type 2 diabetes: increased expression of sirtuin 1 and the correlation with microRNAs. J Res Med Sci. 2019;24(1):56.
- He L, He X, Lowe SW, et al. microRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007;7(11):819–822.
- Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619.
- Long J, Wang Y, Wang W, et al. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. 2010;285(30):23457–23465.
- Maghbooli Z, Emamgholipour S, Aliakbar S, et al. Differential expressions of SIRT1, SIRT3, and SIRT4 in peripheral blood mononuclear cells from patients with type 2 diabetic retinopathy. Arch Physiol Biochem. 2018:1–6.
- Chen N, Jiang K, Yan GG. Effect of fenofibrate on diabetic retinopathy in rats via SIRT1/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(19):8630–8636.
- Ke N, Pi L-H, Liu Q, et al. Long noncoding RNA SNHG7 inhibits high glucose-induced human retinal endothelial cells angiogenesis by regulating miR-543/SIRT1 axis. Biochem Biophys Res Commun. 2019;514(2):503–509.
