ABSTRACT
Ischemic heart disease (IHD) is one of the most deadly diseases worldwide. To detect the regulatory mechanism, the circular RNA (circRNA)-differentially expressed in normal cells and neoplasia domain containing 4 C (DENND4C) was explored in the H9c2 cells.
The circRNA-DENND4C overexpressing plasmid, si-circRNA-DENND4C and miR-320 mimic were transfected into the H9c2 cells and treated with OGD/R stimulation. We took CCK-8 method, Annexin V-FITC/PI-flow cytometer to search for viability and apoptotic ability. With the help of qRT-PCR and western blot, the expression of circRNA-DENND4C and miR-320, as well as the Bax, Cleaved PARP/caspase 3 and signal proteins were separately determined. Regulation of circRNA-DENND4C and miR-320 was confirmed by dual-luciferase reporter assay.
OGD/R induced suppression of cell viability, but enhancement of apoptosis and block of ERK and mTOR pathways. Moreover, circRNA-DENND4C was up-regulated after OGD/R stimulation and augmented OGD/R-stimulated damage while circRNA-DENND4C silencing displayed opposite influences. miR-320 was negatively controlled and targeted by the circRNA-DENND4C.The overexpressed miR-320 impeded the effects of circRNA-DENND4C. Besides, circRNA-DENND4C relieved the suppression of ERK and mTOR pathways caused by OGD/R stimulation, and all promoting impacts of circRNA-DENND4C were reversed by the miR-320 mimic.
Overexpressed circRNA-DENND4C in H9c2 cells attenuated OGD/R-induced injuries by the down-regulation of miR-320 through the ERK and mTOR activation.
Introduction
Ischemic heart disease (IHD), which is also called coronary artery disease (CAD), is one of the most deadly diseases worldwide [Citation1]. Death caused by IHD accounts for more than 20% of total deaths [Citation2]. Many patients suffer from stable and long-term morbidity angina or ischemic heart failure as well [Citation3]. There are many risk factors, including exercise deficiency, cigarettes, alcoholism, and hypertension that are responsible for the occurrence of IHD [Citation4]. What is more, IHD results in serious financial burdens especially in low-income countries [Citation5,Citation6]. There are no definitive answers for the pathogenesis or treatment of the disease. Hence, understanding the molecular mechanisms that are associated with the disease will provide an important theoretical basis for the clinical treatment of IHD.
Circular RNAs (CircRNAs) are novel RNA molecules that are widely present in eukaryotes in the form of covalently closed loops [Citation7]. Current researches have indicated that circRNA is conserved among different species, and they are currently receiving increasing attentions due to the specificity of expression and the complexity of regulation as well as its important role in diseases [Citation8,Citation9]. A novel result demonstrated that hundreds of circRNAs were controlled and dysregulated after cerebral ischemia injury, which was mechanically dependent on the protein kinase (PKα) pathway [Citation10]. Circular RNA-differentially expressed in normal cells and neoplasia domain containing 4 C (hsa_circ_0005684, chr9:19,286,766–19,305,525) is derived from DENND4C gene. According to Chen’s report, the cZNF292 and circRNA-DENND4C were promoted during hypoxia stimulation in endothelial cells [Citation11].
microRNA (miRNAs) could regularly and post-transcriptionally act toward translation, messenger RNAs (mRNA) degradation, signaling pathway activation, or diseased regulation [Citation12]. After ischemia and reperfusion treatment, several miRNAs such as miR-146a and miR-320 exhibited shifted expression levels [Citation13]. Abundant miR-424 could lessen ischemic injury via suppressing apoptosis and microglia activation in brain tissues [Citation14]. It has been verified that miR-320 was closely related to myocardial ischemia, where it could be up-regulated by hypoxia induction. Suppressing miR-320 displayed protective effects on myocardial ischemic damages [Citation15,Citation16].
Based on these previous investigations, we wonder how the circRNA-DENND4C and miR-320 serve in the progress of myocardial ischemia. Thus, we detected those changes in H9c2 cell line after oxygen-glucose deprivation/reperfusion (OGD/R) simulation.
Materials and methods
H9c2 cells cultivation and treatment
H9c2 cells derived from rat embryonic heart (ventricular) were bought from ATCC and grew at 37°C in an atmosphere filled with 5% CO2. The penicillin, streptomycin (100 µg/ml) and the sterile serum (10%)-constituted the DMEM complete medium (all from Gibco, Grand Island, NY, USA), and the DMEM was used as a source of nutrients for those H9c2 cells. What is more, H9c2 cells were treated for 4 h by an oxygen-glucose deprivation (OGD) stimuli. Meanwhile, the glucose (Solarbio, Beijing, China) in the medium was removed and the 95% air was replaced by 94% nitrogen and 1% O2. Then, the H9c2 cell line was returned into the original environment (95% room air-5% CO2, 37°C) and maintained for 24 h for an OGD/R injury induction.
Apoptosis detection
Flow cytometry analysis was conducted to identify the amount of apoptotic H9c2 cells with an Annexin V-FITC/PI kit (Sigma-Aldrich, St. Louis, MO, USA). The H9c2 cells at a seeding concentration of 100,000 cells/well were seeded in the cultivation plate. After wash and suspension, those H9c2 cells were measured by a flow cytometer (Thermo Fisher Scientific, Waltham, MA, USA) and the final data was collected.
Viability assay
H9c2 cells (5000 cells/well) were initially maintained in a multi-well plate, cell’s viability was assessed using the CCK-8 (Dojindo Molecular Technologies, Kumamoto Ken, Kyushu, Japan). After the specific stimulation, those cultures mixed with the CCK-8 reagent and they were maintained at 37°C in humidified air (95%) containing CO2 (5%). The absorbance under 450 nm was detected using a microplate reader (Bio-Rad, Hercules, CA).
Cell transfection
The circRNA-DENND4C knocking down recombinant (si-circRNA-DENND4C, 5’-AAGUAGCACUGCUCUUCAAAA-3’) and its NC (5’-UCUCCGAA CGUGUCACGUTT-3’) were synthesized using a small interference RNA. The circRNA-DENND4C overexpressing plasmid was synthesized based on a pcDNA vector. All of them were bought from GenePharma (Shanghai, China). With the assistance of INTERFERin (Polyplus transfection, Strasbourg, Bas-Rhin, France), 30 nM oligonucleotides were transfected into the H9c2 cells, when reaching 60% confluence. We stopped this assay 24 h post transfection for next analysis.
Dual-luciferase reporter assay
The fragment containing miR-320 binding site or mutant binding site from circRNA-DENND4C was amplified by PCR. They were used for forming DENND4C-wt or DENND4C-mut reporter vector with the pmirGlO Vector (Promega, Madison, WI, USA). Subsequently, those vectors (5’-CAGUACUUUUGUGUAGUACAA-3’) and miR-320 mimic (5’-GCUUCGCUCCCCUCCGCCUUCUCUUCCCGGUUCUUCCC GGAGUCGGGAAAAGCUGGGUUGAGAGGGCGAAAAAGGAUGAGGU-3’) were co-transfected into target cells, and the Dual-Luciferase Reporter Assay System (Promega) was utilized for determining the luciferase activity. Firefly luciferase activity was normalized to the corresponding renilla luciferase activity.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The concentration and integrity of RNA were valued using the Qubit® RNA Kit (Promega, Madison, WI, USA) and RNA Nano 6000 kit (Agilent Technologies, CA, USA). After nuclease treatment (Sigma-Aldrich), we quantified those RNA using the MultiscribeRT kit and miRNA RT Kit, as well as SYBR Mix and random hexamers (Applied Biosystems). The samples untreated with RNase were used to detect β-actin and U6. Relative expressions of mRNA and miRNA were calculated via the 2−ΔΔCt method. Primers were listed:
F-circDENND4C, 5’-GGGGCAGCAGTATTGTGAAA-3’, R-circDENND4C, 5’-AAGACTGTGTGCTCCCCATT-3’; F-miR-320, 5’-ACACTCCAGCTGGGAAAAG CTGGGTTGAGA-3’, R-miR-320, 5’-TGG TGT CGT GGA GTC G-3’; F-β-actin, 5’-TCATGAAGTGTGACGTGGACATC-3’; R-β-actin 5’-CAGGAGGAGCAATGAT CTTGATCT-3’.
Western blot
Tissues were lysed in Tissue Protein Extraction Reagent (Thermo Fisher). Gels for SDS-polyacrylamide gel electrophoresis (PAGE) were prepared with the Bis-Tris Gel System (Bio-Rad). Protein sample was added to the concentrated gels in the presence of loading buffer. After electrophoresis, those proteins on the separation gel were transferred to the polyvinylidene fluoride (PVDF) membrane (Life Technologies, Cergy Pontoise, France) via currents and electroacoustic buffer. Primary antibodies and the secondary antibody were sequentially co-incubated with this membrane at 4°C for a whole night or at room temperature for 1 h. Image LabTM software (Bio-Rad) was used to detect optical signals. Antibodies included Bax (#2772, Cell Signaling Technology, Danvers, MA, USA), cleaved PARP (#94,885), cleaved Caspase-3 (#9661), phosphorylated extracellular regulated protein kinases (p-ERK) (#9101), total-ERK (#4695, t-ERK), p70S6K1 (ab32359), p-p70S6K1 (ab59208), p-mammalian target of rapamycin (mTOR) (ab63552, Abcam, Cambridge, MA), mTOR (ab2732) and β-actin (#4967), as well as anti-rabbit, HRP-linked antibody (#7074).
Statistical analysis
After three parallel experiments, data were presented like mean + standard deviation (SD). In further, SPSS 19.0 was used for statistical analysis by t-test or one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05 was a significant boundary indicating a statistical significance.
Results
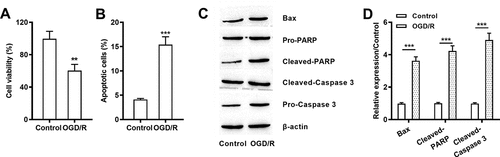
OGD/R stimulation-induced damages in H9c2 cells
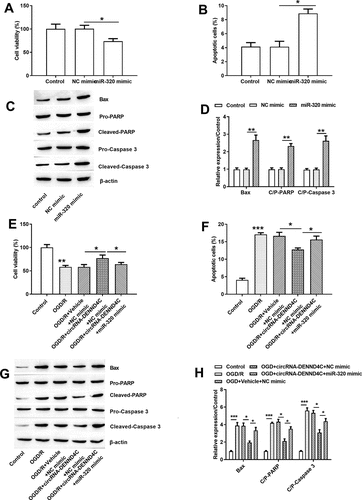
After OGD/R processing, cell viability (P < 0.01, )) was severely declined. Moreover, increased apoptotic H9c2 cells (P < 0.001, )) and enhanced Bax, Cleaved PARP/caspase 3 and C/P-PARP/caspase 3 were noticed after OGD/R stimulation (all P < 0.001, -)). Those data represented that OGD/R treatment induced hypoxic damage in H9c2 cell line by declining viability but promoting apoptosis.
Figure 1. OGD/R stimulation-induced damages in H9c2 cells
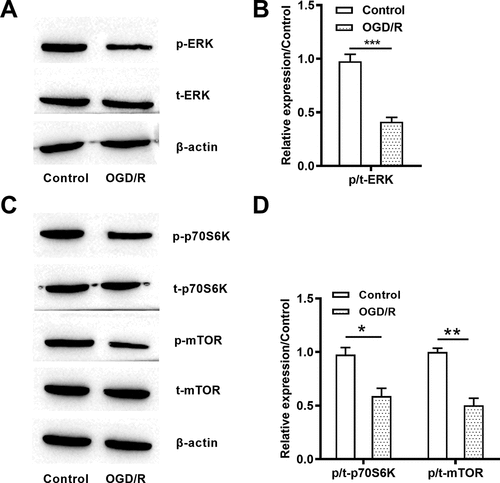
ERK and mTOR signaling pathways were blocked due to OGD/R stimulation
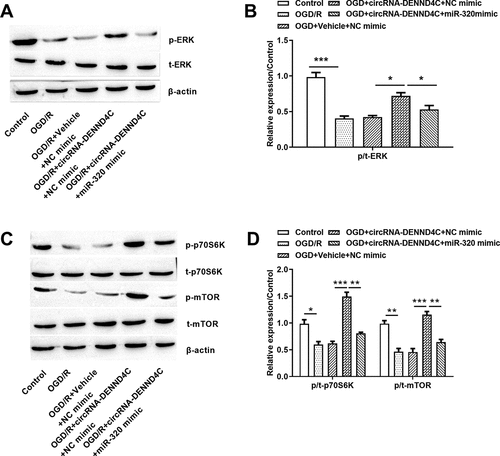
At the mechanism level, the ERK and mTOR pathways were controlled by OGD/R stimuli. As shown in , the p-ERK ()) and p/t-ERK (P < 0.001, )) were declined after OGD/R treatment. In addition, the reduced p-p70S6K, p-mTOR ()) as well as p/t-p70S6K (P < 0.05) and p/t-mTOR (P < 0.01) ()) were observed in OGD/R-treated cells. Those data showed that ERK and mTOR pathways were blocked after OGD/R induction.
Figure 2. OGD/R deactivated ERK and mTOR pathways
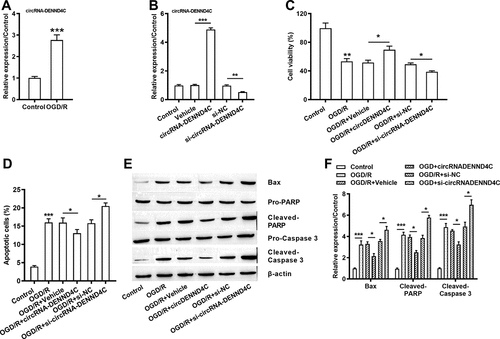
Overexpressed circRNA-DENND4C and under-expressed circRNA-DENND4C in OGD/R-induced H9c2 cells
Interestingly, OGD/R induction stimulated the high expression of circRNA-DENND4C in H9c2 cell line (P < 0.001, )). Besides, si-circRNA-DENND4C transfection inhibited the expression of circRNA-DENND4C (P < 0.01) and the circRNA-DENND4C overexpressing vector enhanced the generation of circRNA-DENND4C (P < 0.001) ()). Apart from, comparing with the single OGD/R treatment group, cell viability was enhanced due to circRNA-DENND4C overexpression (P < 0.05), but was decreased owing to silencing si-circRNA-DENND4C (P < 0.05) ()). Moreover, the amount of apoptotic H9c2 cells were reduced after circRNA-DENND4C overexpressing (P < 0.05), but were increased after circRNA-DENND4C knocking down (P < 0.05), representing a negative regulation between the circRNA-DENND4C and OGD/R injury ()). Being consistent with this, overexpressing circRNA-DENND4C and/or silencing circRNA-DENND4C extremely abated and/or facilitated the generation of Bax, Cleaved PARP/caspase 3 (all P < 0.05, -)). We could hypothesize that in H9c2 cells, circRNA-DENND4C was up-regulated after OGD/R treatment and circRNA-DENND4C might play protective roles in H9c2 cell’s growth.
Figure 3. Overexpressed circRNA-DENND4C and under-expressed circRNA-DENND4C in OGD/R-induced H9c2 cells
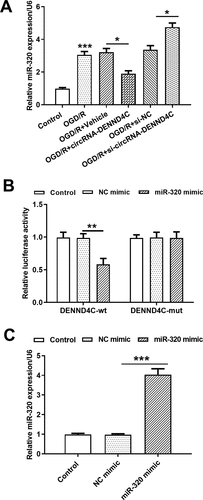
CircRNA-DENND4C directly targeted miR-320 in H9c2 cells
The mRNA level of miR-320 was elevated after OGD/R stimulation (P < 0.001), and qRT-PCR confirmed a significant down-regulation (P < 0.05) and/or overexpression (P < 0.05) of miR-320 in H9c2 cells when transfected with circRNA-DENND4C vector and/or si-circRNA-DENND4C ()). To experimentally determine whether circRNA-DENND4C could directly target the miR-320, a dual-luciferase reporter assay was conducted. As shown in ), the co-transfection of miR-320 mimic with circRNA-DENND4C-wt, but not with circRNA-DENND4C-mut, led to visible down-regulation of luciferase activity than that in control group (P < 0.01). Next, the expression of miR-320 in the H9c2 cells was significantly increased after miR-320 mimic transfection (P < 0.001, )). These results suggested that circRNA-DENND4C could directly sponge miR-320, leading to miR-320 suppression. It seemed like that overexpression of circRNA-DENND4C attenuated OGD/R-induced H9c2 cell’s damage by down-regulating miR-320.
Figure 4. CircRNA-DENND4C directly targeted miR-320 in H9c2 cells
Overexpression of circRNA-DENND4C attenuated OGD-induced injury by down-regulating miR-320
In an attempt to investigate the functions of miR-320 in the growth of H9c2 cells, we noticed that the anti-hypoxia impact of circRNA-DENND4C was eliminated due to miR-320 overexpression. In detail, the decreased cell viability (P < 0.05, ,e)), increased apoptotic cells (P < 0.05, ,f)) as well as the higher Bax, Cleaved-PARP and caspase 3 expression levels (P < 0.05 or P < 0.01, -)) were caused by miR-320 mimic in H9c2 cells. Based on these findings, we concluded miR-320 overexpression was adverse to H9c2 cell’s growth and circRNA-DENND4C might protect H9c2 cells against OGD/R damage by suppressing miR-320.
Figure 5. miR-320 mimic impeded H9c2 cell’s growth by facilitating apoptosis and hindering viability
The overexpressed circRNA-DENND4C promoted activation of ERK and mTOR signaling pathways by down-regulating miR-320
Based on those above results, we studied the signaling mechanisms of circRNA-DENND4C and miR-320 by focusing on the ERK and mTOR cascades using western blot. Surprisingly, the circRNA-DENND4C was overexpressed to increase p-ERK ()) and p/t-ERK (P < 0.05, )), p-p70S6K and p-mTOR ()), p/t-p70S6K and p/t-mTOR (both P < 0.001, )) in cells. Nonetheless, those influences were all disturbed by the miR-320 mimic (P < 0.05, P < 0.01 or P < 0.001). Those assays revealed that circRNA-DENND4C overexpression contributed to activate ERK and mTOR signaling pathways by down-regulating miR-320.
Figure 6. Overexpressed circRNA-DENND4C promoted activation of ERK and mTOR pathway by down-regulating miR-320
Discussion
Our data confirmed that OGD/R treatment induced apparent hypoxic damage in H9c2 cells, leading to inactivation of ERK and mTOR pathways. Overexpression of circRNA-DENND4C contributed to relieve OGD/R-induced viability inhibition and apoptosis enhancement, while circRNA-DENND4C knocking down exhibited an opposite outcome. miR-320 was identified as an inhibitory target of circRNA-DENND4C and there was a negative correlation between them. Increased miR-320 could restrain cell’s growth and disturb the protective effects of circRNA-DENND4C. We found the ERK and mTOR cascades that triggered by the circRNA-DENND4C was blocked by miR-320 mimic.
CircRNAs have a quite stable cyclic structure that gives them broad and conservative functions [Citation17,Citation18]. CircRNAs are capable of regulating gene expression and being intimately connected with many diseases, encompassing cancers, fibrosis, and heart disorders in a tissue/time-specific way [Citation19]. For instance, circRNA-ANRIL affected the progression of CAD [Citation20]. What is more, hsa_circ_0124644 also had some connections with CAD development and thus the hsa_circ_0124644 might be a precise biomarker for CAD diagnosing [Citation21]. Hsa_circ_0001879 and hsa_circ_0004104 were irregularly expressed in CAD patients. Wang et al. implied that hsa_circ_0004104 overproduction resulted in the dysregulation of genes which were related to the atherosclerosis and CAD [Citation22]. CircRNA-DENND4C was a critical participator in breast cancer, and researchers identified that overexpression of circRNA-DENND4C helped increase the breast tumor size, while knocking-down circDENND4C inhibited the proliferation of tumor cells [Citation23]. A parallel outcome was achieved in our research. Lacking of oxygen and sugars contributed to augment apoptosis and proliferation suppression of H9c2 cells, and thus the ERK and mTOR pathways were mechanically blocked. Furthermore, circRNA-DENND4C was up-regulated due to the OGD/R stimuli. The gain/loss-of function experiments revealed that circRNA-DENND4C acted as an advantageous factor in H9c2 cell line against OGD/R, and circRNA-DENND4C knocking-down was adverse to the survival of the cells. Those phenomena suggested that circRNA-DENND4C facilitated by the exogenous OGD/R stimulation helped protect H9c2 cells from hypoxic disorders.
miRNAs are small regulator RNAs in cells. Given the wide distribution of those small molecules in animals, plants, viruses, and single-cell eukaryotes, its function had been widely researched [Citation24]. They have a crucial role in the pathogenesis and movement of myocardial ischemia or ischemia-reperfusion (I/R) injury [Citation25–27–Citation27]. Liu et al. reported that miR-124 expression was promoted after OGD/R treatment, and overexpressed miR-124 could augment OGD/R-induced cell death and apoptosis by suppressing the sphingosine kinase 1 (SphK1) in a myocardial infarction (MI) model [Citation28]. It had been reported in the literature that miR-320 was significantly elevated in ischemic myocardium [Citation16]. The exact behavior of miR-320 was proved by Song and the colleagues that miR-320 inhibition mitigated myocardial I/R injury while miR-320 overexpression played opposite roles [Citation29]. We observed in our experiments that overexpression of circRNA-DENND4C protected H9c2 cells against OGD/R-induced damages; nevertheless, this kind of protection was eliminated owing to the up-regulation of miR-320. What is more, increased miR-320 restrained cell growth by inhibiting viability and promoting apoptosis. Obviously, we could hypothesize that miR-320 might be beneficial to the deterioration of ischemic accident. miR-320 was required for the circRNA-DENND4C to play protective roles in OGD/R-treated H9c2 cell line. Despite little has been known regarding how circRNA-DENND4C controlled the expression of miR-320 formerly, we confirmed a direct combining relationship between them by conducting the dual-luciferase assay. Similar conclusion was also observed in a previous report that silencing circRNA-DENND4C helped restrain hypoxia-induced breast cancer cells metastasis by sponging miR-200b and miR-200 c [Citation30].
The ERK cascade could take part in the transcriptional adjustment of target genes that is correlated with inflammation and apoptosis [Citation31,Citation32]. Recently, the ERK pathway has been shown to have a profitable role in defending cells against I/R-induced damage [Citation33]. For example, trimetazidine (TMZ) displayed cardio-protection functions against hypoxic injury by triggering ERK signaling pathway [Citation34]. In the endoplasmic reticulum I/R model, hypoxia stimulation restrained the expression of miR-423-3p and activated the ERK pathway [Citation35]. Acting as a potential influencing factor during cardiac energy privation and ischemia, mTOR kinase is required for regular cardiac structure and metabolic homeostasis [Citation36]. Besides, in myocardial I/R injury assays, mTOR cascade also showed excellent functional potentials. For instance, basic fibroblast growth factor (bFGF) was beneficial to the recovery of heart function and cardiomyocytes survival via activating mTOR pathway [Citation37,Citation38]. Those conclusions demonstrated that the ERK and mTOR cascades were positively related to ischemic injury in cardiomyocytes. In the immediate experiment, circRNA-DENND4C overproduction relieved OGD/R-resulted ERK and mTOR deactivation via down-regulating miR-320. In another word, the promoting effect of circRNA-DENND4C toward ERK and mTOR was ameliorated by the overexpressed miR-320. What we found here was consistent with the previous data. Circ_0006528 modulated the ERK pathway to regulate the proliferation and apoptosis of breast cancer cells by acting as a sponge of miR-7-5p [Citation39]. In addition, Huang et al. implied that circRNA-100,338 regulated the activity of mTOR signaling pathway through modulating miR-141-3p in HCC tissues [Citation40]. However, there also are some limitations in our research, because little is known with respect to the roles of circRNA-DENND4C and miR-320 in in vivo experiments. For example, more evidence that how circRNA-DENND4C silencing or overexpressing affects the myocardial function in rats is needed for our conclusion.
Conclusion
All in all, overexpression of circDENND4C in H9c2 cells attenuated OGD/R-induced damages, which was probably by the down-regulation of miR-320 through the ERK and mTOR activation.
Disclosure statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- El Bakry SA, Fayez D, Morad CS, et al. Ischemic heart disease and rheumatoid arthritis: do inflammatory cytokines have a role? Cytokine. 2017 Aug;96:228–233. PubMed PMID: 28477538; eng.
- Lagedal R, Elfwen L, Jonsson M, et al. Coronary angiographic findings after cardiac arrest in relation to ECG and comorbidity. Resuscitation. 2019 Sep 24. DOI:10.1016/j.resuscitation.2019.09.021. PubMed PMID: 31560991; eng.
- Imai T, Miyamoto K, Sezaki A, et al. Traditional Japanese diet score - association with obesity, incidence of ischemic heart disease, and healthy life expectancy in a global comparative study. J Nutr Health Aging. 2019;23(8):717–724. PubMed PMID: 31560029; eng
- Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the global burden of disease 2010 study. Circulation. 2014 Apr 8;129(14):1493–1501. PubMed PMID: 24573351; PubMed Central PMCID: PMCPmc4181601. eng.
- Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011 Jul 19;124(3):314–323. PubMed PMID: 21730306; eng.
- Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the global burden of disease study 2013. bmj. 2016;354:i3857.
- Sheng M, Wei N, Yang HY, et al. CircRNA UBAP2 promotes the progression of ovarian cancer by sponging microRNA-144. Eur Rev Med Pharmacol Sci. 2019 Sep;23(17):7283–7294. PubMed PMID: 31539115; eng.
- Rong D, Sun H, Li Z, et al. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017 Sep 22;8(42):73271–73281. PubMed PMID: 29069868; PubMed Central PMCID: PMCPmc5641211. eng.
- Yu T, Wang Y, Fan Y, et al. CircRNAs in cancer metabolism: a review. J Hematol Oncol. 2019 Sep 4;12(1):90. PubMed PMID: 31484561.
- Liu W, Jia C, Luo L, et al. Novel circular RNAs expressed in brain microvascular endothelial cells after oxygen-glucose deprivation/recovery. Neural Regen Res. 2019 Dec;14(12):2104–2111. PubMed PMID: 31397348; eng.
- Boeckel JN, Jae N, Heumuller AW, et al. Identification and characterization of hypoxia-regulated endothelial circular RNA. Circ Res. 2015 Oct 23;117(10):884–890. PubMed PMID: 26377962; eng.
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Nat Acad Sci. 2010;107(14):6328–6333.
- Hendgen-Cotta UB, Messiha D, Esfeld S, et al. Inorganic nitrite modulates miRNA signatures in acute myocardial in vivo ischemia/reperfusion. Free Radic Res. 2017 Jan;51(1):91–102. PubMed PMID: 28090786; eng.
- Zhao H, Wang J, Gao L, et al. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke. 2013;44(6):1706–1713.
- Zhu XA, Gao LF, Zhang ZG, et al. Down-regulation of miR-320 exerts protective effects on myocardial I-R injury via facilitating Nrf2 expression. Eur Rev Med Pharmacol Sci. 2019 Feb;23(4):1730–1741. PubMed PMID: 30840298; eng.
- Tian ZQ, Jiang H, Lu ZB. MiR-320 regulates cardiomyocyte apoptosis induced by ischemia-reperfusion injury by targeting AKIP1. Cell Mol Biol Lett. 2018;23:41. PubMed PMID: 30181740; PubMed Central PMCID: PMCPmc6114048. eng
- You X, Vlatkovic I, Babic A, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015 Apr;18(4):603–610. PubMed PMID: 25714049; PubMed Central PMCID: PMCPmc4376664. eng.
- Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013 Mar 21;495(7441):384–388. PubMed PMID: 23446346; eng.
- Salzman J, Chen RE, Olsen MN, et al. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9(9):e1003777. PubMed PMID: 24039610; PubMed Central PMCID: PMCPmc3764148. eng
- Burd CE, Jeck WR, Liu Y, et al. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010 Dec 2;6(12):e1001233. PubMed PMID: 21151960; PubMed Central PMCID: PMCPmc2996334. eng.
- Zhao Z, Li X, Gao C, et al. Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci Rep. 2017 Jan;3(7):39918. PubMed PMID: 28045102; PubMed Central PMCID: PMCPmc5206672. eng.
- Wang L, Shen C, Wang Y, et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis. 2019 Jul;286:88–96. PubMed PMID: 31103880; eng.
- Liang G, Liu Z, Tan L, et al. HIF1alpha-associated circDENND4C promotes proliferation of breast cancer cells in hypoxic environment. Anticancer Res. 2017 Aug;37(8):4337–4343. PubMed PMID: 28739726; eng.
- Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014 Jan;15(1):1–19. PubMed PMID: 23175680; PubMed Central PMCID: PMCPmc3896928. eng
- Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013 Jan;123(1):11–18. PubMed PMID: 23281405; PubMed Central PMCID: PMCPmc3533276. eng
- Guo H, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010 Aug 12;466(7308):835–840. PubMed PMID: 20703300; PubMed Central PMCID: PMCPmc2990499. eng.
- Alberto Arias-Pérez DR-T 1, Rodríguez ME 1, Portela-Bens S 1, et al. In silico detection and FISH analysis to determine location of miRNAs in solea senegalensis chromosomes using BACs. OBM Gene. 2018;2(4). DOI:10.21926/obm.genet.1804044.
- Liu BF, Chen Q, Zhang M, et al. MiR-124 promotes ischemia-reperfusion induced cardiomyocyte apoptosis by targeting sphingosine kinase 1. Eur Rev Med Pharmacol Sci. 2019 Aug;23(16):7049–7058. PubMed PMID: 31486506; eng.
- Song CL, Liu B, Diao HY, et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget. 2016 Jun 28;7(26):39740–39757. PubMed PMID: 27175593; PubMed Central PMCID: PMCPmc5129967. eng.
- Ren S, Liu J, Feng Y, et al. Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J Exp Clin Cancer Res. 2019;38(1):388. PubMed PMID: 31488193; eng
- Ottani A, Galantucci M, Ardimento E, et al. Modulation of the JAK/ERK/STAT signaling in melanocortin-induced inhibition of local and systemic responses to myocardial ischemia/reperfusion. Pharmacol Res. 2013 Jun;72:1–8. PubMed PMID: 23535516; eng.
- Sun Y, Liu W-Z, Liu T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct. 2015;35(6):600–604.
- Lamsal Y, Stuber GD, Snider WD. ERK/MAPK Signaling Is Required for Pathway-Specific Striatal Motor Functions. J Neurosci. 2017;37(34):8102–8115. doi:10.1523/jneurosci.0473-17.2017
- Liu Z, Chen JM, Huang H, et al. The protective effect of trimetazidine on myocardial ischemia/reperfusion injury through activating AMPK and ERK signaling pathway. Metabolism. 2016 Mar;65(3):122–130. PubMed PMID: 26892523; PubMed Central PMCID: PMCPmc4967934. eng.
- Yang TR, Zhang T, Mu NH, et al. Resina draconis inhibits the endoplasmic-reticulum-induced apoptosis of myocardial cells via regulating miR-423-3p/ERK signaling pathway in a tree shrew myocardial ischemia- reperfusion model. J Biosci. 2019 Jun;44(2). PubMed PMID: 31180066; eng. DOI:10.1007/s12038-019-9872-8
- Parra V, Verdejo HE, Iglewski M, et al. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFkappaB-Opa-1 signaling pathway. Diabetes. 2014 Jan;63(1):75–88. PubMed PMID: 24009260; PubMed Central PMCID: PMCPmc3868041. eng.
- Wang ZG, Wang Y, Huang Y, et al. bFGF regulates autophagy and ubiquitinated protein accumulation induced by myocardial ischemia/reperfusion via the activation of the PI3K/Akt/mTOR pathway. Sci Rep. 2015 Mar;19(5):9287. PubMed PMID: 25787015; PubMed Central PMCID: PMCPmc4365411. eng.
- Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J clin oncol. 2010;28(2):245.
- Gao D, Qi X, Zhang X, et al. hsa_circRNA_0006528 as a competing endogenous RNA promotes human breast cancer progression by sponging miR-7-5p and activating the MAPK/ERK signaling pathway. Mol Carcinog. 2019 Apr;58(4):554–564. PubMed PMID: 30520151.
- Huang XY, Huang ZL, Zhang PB, et al. CircRNA-100338 is associated with mTOR signaling pathway and poor prognosis in hepatocellular carcinoma. Front Oncol. 2019;9:392. PubMed PMID: 31157168; PubMed Central PMCID: PMCPmc6528706. eng
