ABSTRACT
Radiotherapy is an essential treatment for endometrial cancer (EC), especially in advanced, metastatic, and recurrent cases. Combining radiotherapy, which mainly causes DNA double-strand breaks (DSBs), with small molecules targeting aberrantly activated homologous recombination (HR) repair pathways holds great potential for treating ECs in advanced stages. Here, we demonstrate that diosmetin (DIO), a natural flavonoid, suppresses HR, therefore inhibiting cell proliferation and enhancing the sensitivity of EC to radiotherapy. Clonogenic experiments revealed that combining DIO and X-ray significantly inhibited the viability of EC cells compared to cells treated with diosmetin or X-ray alone. The survival fraction of EC cells decreased to 40% when combining 0.4 Gy X-ray and 4 μM DIO; however, each treatment alone only caused death in approximately 15% and 22% of cancer cells, respectively. Further mechanistic studies showed that diosmetin inhibited the recruitment of RPA2 and RAD51, two critical factors involved in the HR repair pathway, upon the occurrence of DSBs. Thus, we propose that a combination of diosmetin and irradiation is a promising therapeutic strategy for treating endometrial cancer.
Introduction
Endometrial cancer is the fourth most common gynecologic malignancy and the sixth leading cause of cancer death in women, as estimated by the American Cancer Society, in the United States [Citation1], with 76,000 deaths among women every year worldwide [Citation2]. The incidence and death rates of endometrial cancer are still rapidly rising. Contemporary treatment of endometrial cancer mainly includes surgery, which is often followed by radiotherapy, chemotherapy, or both, depending on risk factors including grade, lymph node metastasis, and muscle layer and vascular invasion [Citation3]. Radiotherapy, which involves the production of excessive DNA damage to induce cell death, has been widely used for decades to treat more than 50% of cancer patients, and postoperative radiotherapy significantly reduces the risk of local recurrence in EC patients [Citation4]. DNA double-strand breaks (DSBs), the most lethal type of DNA damage to eukaryotic cells, are mainly repaired by homologous recombination (HR) and non-homologous end joining (NHEJ) in higher eukaryotes. NHEJ can be activated throughout the cell cycle, and HR is an accurate repair pathway that occurs during S and G2 phases, when sister chromatids are present [Citation5]. As malignant endometrial cells rapidly divide [Citation6], they may experience high replication stress. HR is the major pathway for relieving such stress [Citation7], thereby promoting cell survival. Indeed, several studies have indicated that HR is upregulated in a number of tumor types in comparison to normal adjacent tissues Citation8–10]. On the other hand, upregulated DSB repair by HR in cancer cells might confer radioresistance. Therefore, suppressing DNA repair pathways, especially HR, might constitute an efficacious strategy to increase the sensitivity of EC cells to DNA damage inducers such as irradiation.
Diosmetin (DIO) is an O-methylated flavone (3′,5,7-trihydroxy-4′-methoxyflavone) and the aglycone component of the flavonoid glycoside diosmin. Diosmetin can be extracted from citrus fruit and olive leaves [Citation11,Citation12]. It is also the active compound of Galium verum L, a traditional Chinese herb that has been used for decades to promote blood circulation and expel miasma [Citation13]. Previous studies have shown that diosmetin possesses anti-inflammatory [Citation14], antioxidant [Citation15] and antitumor properties in different biological contexts. As reported, diosmetin induces cell cycle arrest as well as apoptosis in liver cancer [Citation16], prostate cancer [Citation17], breast cancer [Citation18] and chronic myeloid leukemia [Citation19] by targeting different pathways. Diosmetin has also been shown to downregulate expression of MMP-2 and MMP-9 to inhibit metastasis of hepatocellular carcinoma cells [Citation20]. In addition, diosmetin has been demonstrated to act as a natural dietary agonist of AhR that is capable of inhibiting CYP1A1 enzyme activity to suppress carcinogen activation in breast cancer [Citation21,Citation22]. Nevertheless, its effect on endometrial cancer is not yet clear. Here, we corroborate that diosmetin destabilizes genomes by suppressing DSB repair, especially HR, in a cell cycle-independent manner. Diosmetin inhibits the recruitment of both RPA2 and RAD51 upon the occurrence of DSBs induced by X-ray. Further clonogenic studies indicate that combining diosmetin with X-ray synergistically suppresses the survival of endometrial cancer cells, suggesting that it has potential in future clinical applications.
Materials and methods
Cell culture
Hec-1B, ISK and AN3CA cells were cultured in DME/F-12 (SH30023.01, HyClone, Jiangsu, China) supplemented with 10% FBS (10270–106, Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (15140–122, Gibco, Grand Island, NY, USA). HCA2-hTERT cells were cultured in Minimum Essential Medium Eagle (M4655, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% FBS (10270–106, Gibco, Grand Island, NY, USA), 1% MEM NEAA (11140–050, Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (15140–122, Gibco, Grand Island, NY, USA).
All cell lines were incubated in a 5% CO2 humidified incubator (Thermo Fisher Heracell 240i, Thermo Fisher, Waltham, MA, USA) at 37°C. Mycoplasma testing was conducted regularly.
Cell viability assay
Hec-1B, ISK and AN3CA cells were seeded in 6-well plates at a density of 20,000 cells per well, followed by treatment with DMSO or diosmetin at the indicated concentrations at 24 hours after passaging. The cells were then collected, and cell numbers were calculated after 24, 48, and 72 hours of incubation.
Clonogenic assay
Hec-1B, ISK and AN3CA cells were seeded in 6-well plates at a density of 200 cells per well. At 24 hours postseeding, the cells were treated with DMSO or diosmetin at the indicated concentrations, followed by a 72-hour incubation. Afterward, the medium was replaced with fresh medium with no supplemented drugs.
Hec-1B cells were seeded in 6-well plates at a density of 200 cells per well. On day 1 postseeding, the cells were incubated with DMSO or diosmetin at the indicated concentrations for 24 hours. Then, the cells were X-ray irradiated at increasing dosages. The irradiated cells were cultured in the presence of diosmetin for 2 days, after which the medium was replaced with fresh medium.
The cells were incubated for 14 days before staining with Coomassie reagent (methanol: acetic acid: Coomassie: H2O = 50: 10: 0.25: 40) for a time period of 4 hours. Afterward, the cells were washed with distilled water three times. Colonies with at least 50 cells were counted.
Comet assay
Hec-1B cells were seeded in 6-well plates at a density of 20,000 cells per well. After 24 hours, the cells were incubated with DMSO or the indicated concentration of diosmetin for 72 hours. The cells were collected, washed with PBS once and diluted to 300,000 cells per milliliter. Genomic stability was analyzed with a Trevigen alkaline comet assay kit (Cat. number: 4250–050-K, Trevigen, Gaithersburg, MD, USA) according to the instructions. Pictures were taken and analyzed with Cometscore software (Sumerduck, VA, USA). At least 50 cells were quantified for each sample.
EdU incorporation assay
Hec-1B cells were seeded in 6-well plates at a density of 20,000 cells per well. At 24 hours postseeding, the cells were treated with DMSO or diosmetin at the indicated concentrations, followed by a 72-hour incubation. The cells were then harvested, incubated with 10 μM EdU at 37°C for 2 hours, collected and treated with a Click-iT EdU Assay Kit (Invitrogen, Waltham, MA, USA, C10634) according to the manufacturer’s instructions. EdU-positive cells were quantified using a FACSVerse (BD Biosciences, San Jose, CA, USA). At least 10,000 cells for each sample were analyzed.
Analysis of HR/NHEJ repair efficiency
Hec-1B cells were seeded in 10-cm dishes at a density of 1,000,000 cells per dish. At 24 hours postseeding, the cells were pretreated with DMSO or diosmetin at the indicated concentration for 24 hours, followed by transfection using a Lonza 4D machine. The transfected cells were immediately incubated with the above reagents for 72 hours before analysis using a FACSVerse (BD Biosciences, San Jose, CA, USA). At least 20,000 cells for each sample were analyzed.
Immunofluorescence assay
HCA2-hTERT cells were seeded on coverslips in 12-well plates at a density of 10,000 cells per well. After 24 hours, the cells were pretreated with DMSO or diosmetin (10 μM) for 24 hours and then irradiated with X-ray (2 Gy) in the presence of diosmetin. At the indicated time points post-IR, the medium was removed, and the cells were washed twice with PBS, followed by fixation with 4% paraformaldehyde for 15 min and permeabilization with 0.25% Triton X-100 (Sigma, Cat. #X100) for 10 min. The fixed and permeabilized cells were blocked with 2% bovine serum albumin for 1 hour and subsequently incubated with the primary antibody at 4°C overnight, followed by incubation with the specific fluorescent secondary antibody for 1 hour in the dark. Pictures were taken with a laser scanning confocal microscope (TCS SP8; Leica, Wetzlar, Germany), and for each sample, the number of foci for at least 50 cells was counted.
Results
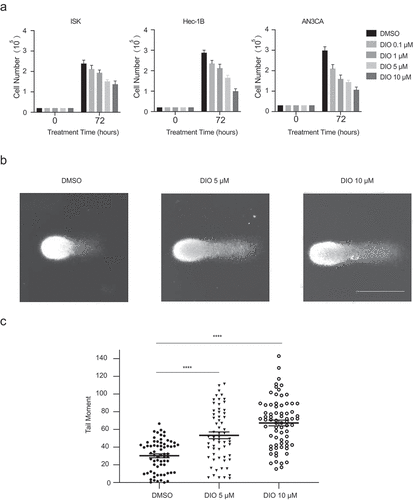
Diosmetin impairs the genomic stability of EC cells and inhibits cell proliferation
The structure of diosmetin used in this study is shown in Figure S1A. We first examined whether diosmetin affects EC cell proliferation. Three different EC cell lines, ISK, Hec-1B and AN3CA, were treated with diosmetin at increasing concentrations. We observed a dose-dependent decline in cell proliferation rates in all three cell lines (), indicating a toxic effect of diosmetin on EC cells. Next, we examined whether diosmetin is able to impact genome integrity, therefore causing toxicity in EC cells. By using an alkaline comet assay, which is a sensitive method to measure genomic stability at the single-cell level, we observed that diosmetin destabilized the genomes of Hec-1B cells. Indeed, the tail moments of 5 μM diosmetin-treated Hec-1B cells were 2-fold higher than those of DMSO-treated cells (), demonstrating that diosmetin impaired the genomic stability of Hec-1B cells.
Figure 1. Diosmetin impairs the genomic stability of EC cells and inhibits the cell proliferation
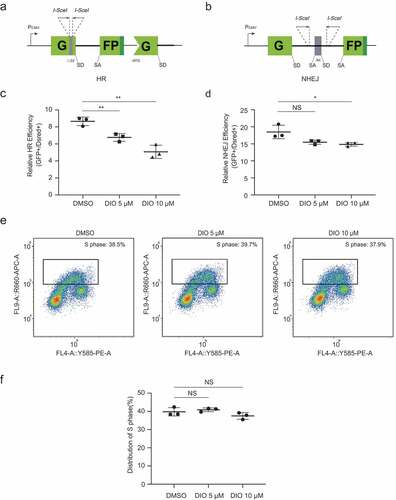
Diosmetin negatively regulates HR repair in EC cells
Given that diosmetin influences genomic stability and that DNA repair plays an indispensable role in the maintenance of genomic stability [Citation23], we hypothesized that diosmetin might have an impact on DNA repair. We compared the efficiency of HR and NHEJ, two major DSB repair pathways, between diosmetin-treated and DMSO-treated Hec-1B cells. A previously described [Citation24–26] well-established DSB repair reporter system was used to analyze the HR and NHEJ efficiency of Hec-1B cells in a quantitative manner (,b). For this assay, functional GFP genes were reconstructed and expressed only as an outcome of successful HR or NHEJ repair events. We transfected HR or NHEJ reporters digested by I-SceI enzymes in vitro, along with DsRed plasmids for normalization of transfection efficiency, into Hec-1B cells in the presence of diosmetin or DMSO, and the numbers of GFP+ and DsRed+ cells were quantified by flow cytometry at 72 hours post transfection. We observed a remarkable reduction in the HR efficiency of diosmetin-treated Hec-1B cells in a dose-dependent fashion () and a slight decrease in NHEJ efficiency (). The HR efficiency of Hec-1B cells decreased by 50% after treatment with diosmetin at a concentration of 10 μM, which might explain the genomic instability caused by diosmetin. Moreover, we did not observe a significant change in the number of cells in S phase after diosmetin treatment for a 24-hour period (,), indicating that the observed decrease in HR was not a secondary effect of cell cycle arrest.
Figure 2. Diosmetin negatively regulates HR repair in EC cells
Diosmetin reduces recruitment of RPA2
To reveal the underlying mechanisms involved, we first examined expression of major proteins involved in HR and NHEJ [Citation27], though we did not observe any significant difference in DSB repair-associated proteins in Hec-1B cells after 72 hours of diosmetin treatment (Figure S2A-B). ATM, a member of the phosphoinositide 3-kinase (PI3K)-related kinase (PIKK) family, is the major factor sensing DNA damage and initiating signal transduction, especially in response to IR-induced DSBs [Citation28]. In response to irradiation, ATM autophosphorylates serine residue S1981, which is crucial for its kinase activity [Citation29]. However, we did not observe any change in the level of ATM protein or ATM S1981 phosphorylation with or without diosmetin incubation after IR treatment (Figure S2C). Consequently, we did not detect any significant difference between the control and experimental groups with regard to the level of CHK2, the target of ATM, or CHK2-pT68, the ATM phosphorylated product [Citation30] (Figure S2C). Moreover, the level of H2AX phosphorylation at S139, which is also activated by ATM, did not decrease in the presence of diosmetin at 30 min post-IR (Figure S2C). All these data indicate that diosmetin suppresses DNA repair by HR in an ATM-independent manner, and the regulation of HR by diosmetin probably occurs at steps after activation of ATM and phosphorylation of H2AX.
Next, we examined the recruitment kinetics of phosphorylated H2AX (γH2AX), a well-recognized DSB damage marker, in response to the occurrence of DSBs upon X-ray treatment at different time points. We found that diosmetin treatment did not affect early foci formation of γH2AX, consistent with our above findings. Nonetheless, diosmetin treatment significantly delayed the clearance of γH2AX foci (). For instance, at 24 hours after X-ray treatment, the average number of γH2AX foci remained over 30% of the peak number in the presence of diosmetin; in control cells, the average number of γH2AX foci dropped to the basal level (). These data are in accordance with the abovementioned results that diosmetin impairs HR repair, destabilizing the genomes of EC cells.
Figure 3. Diosmetin reduces the recruitment of RPA2
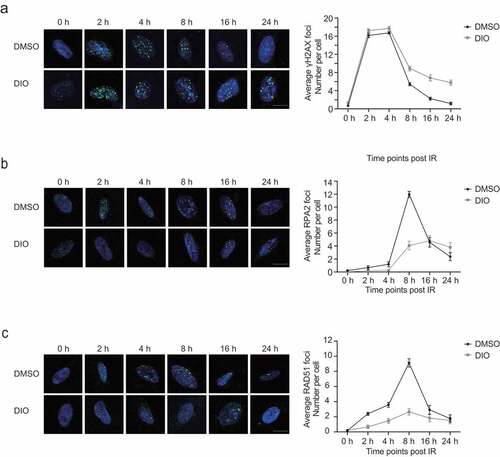
Then, we set out to identify the step at which diosmetin affects HR repair. We examined the kinetics of the recruitment of RPA2, a single-strand-binding protein involved in the step of end resection in the process of HR. We found that diosmetin strongly inhibited the recruitment of RPA2 to sites of DNA damage (), indicating that diosmetin disrupted the end resection step of HR repair. As a consequence, subsequent recruitment of RAD51, the major recombinase involved in HR repair [Citation23], was also abrogated in EC cells by diosmetin supplementation (). In contrast, we did not observe any significant change in the recruitment of the critical NHEJ factor 53BP1, which is in agreement with the result that the presence of diosmetin did not affect NHEJ efficiency (Figure S3A).
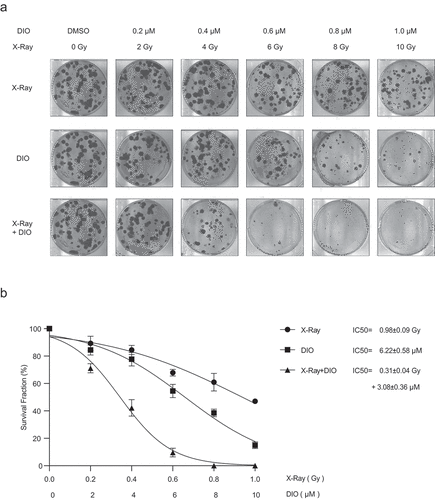
Diosmetin sensitizes EC cells to ionizing radiation
As radiotherapy is one of the most critical therapeutic methods in curing EC, we then examined whether diosmetin sensitizes EC cells to X-ray using a clonogenic assay. We found that in Hec-1B cells, the IC50 of X-ray or diosmetin alone was 0.98 Gy and 6.22 μM, respectively (,b). Intriguingly, combining the two treatments greatly reduced the dosages of X-ray and diosmetin required, with IC50s of 0.31 Gy for X-ray and 3.08 μM for diosmetin (,b). These data strongly indicate that the combination of X-ray and diosmetin synergistically suppresses the survival of EC cells, hinting that diosmetin has potential for treating EC as a sensitizer of radiotherapy.
Figure 4. Diosmetin sensitizes EC cells to ionizing radiation
Discussion
Diosmetin is a natural flavonoid abundant in citrus fruit, olive leaves, and some other kind of teas and dry herbs [Citation12], and it has been featured as an antitumor agent in various kinds of cancers. For instance, diosmetin induces apoptosis in prostate cancer by inhibiting X-linked inhibitor of apoptosis (XIAP) [Citation17]. In acute myeloid leukemia, diosmetin is reported to induce apoptosis through estrogen receptor β [Citation31]. In addition, diosmetin has been shown to inhibit cell proliferation and induce apoptosis by regulating autophagy [Citation32] and by upregulating p53 via the TGF-beta signaling pathway [Citation33] in hepatocellular carcinoma HepG2 cells. Diosmetin also induces ROS accumulation by downregulating the PI3K/Akt/GSK-3β/Nrf2 pathway, resulting in apoptosis in NSCLC cells [Citation34]. In the present study, we found that diosmetin enhanced the effect of radiotherapy in endometrial cancer by suppressing DSB repair by negatively regulating DNA end resection (Figure S4A). This finding extends the antitumor spectrum of diosmetin and identifies a novel role of this drug.
Notably, in some previous studies, the antitumor function of diosmetin has been largely attributed to its effect on the cell cycle. For example, diosmetin caused S-phase arrest at concentrations of 5 μM, 10 μM, 20 μM, and 40 μM in PC-3 cells [Citation17]. However, we did not observe any significant discrepancy in S-phase distribution between the diosmetin-treated group and DMSO-treated group of Hec-1B cells (,), which suggested that diosmetin at concentrations of 5 μM and 10 μM might not have an S-phase arrest effect in these cells. In addition, Ma [Citation16] and colleagues demonstrated that diosmetin induces Chk2-dependent G2/M cell cycle arrest at concentrations of 10 μg/ml (33.3 μM) and 15 μg/ml (50 μM); in our experiments, we did not observe obvious alterations in the protein levels of Chk2 and p-Chk2 after diosmetin treatment at a concentration of 10 μM, which is nearly as low as one-fifth of the dosage used by Ma et al. Therefore, we assume that diosmetin exerts its cell cycle arrest function only at high dosages, while at low concentrations, it can sensitize cancer cells to radiotherapy by targeting HR repair.
Previous studies have indicated that diosmetin might play distinct roles in different types of cancers, and the underlying mechanisms could be tissue type dependent. In skin cancer, diosmetin has been reported to impair tumor angiogenesis [Citation35]. Moreover, it blocks tumor metastasis by inhibiting the epithelial-mesenchymal transition in human bronchial epithelial cells [Citation36], by activating E-cadherin expression and inhibiting the TGF-β signaling pathway in glioma cells [Citation37] and by downregulating expression of MMP-2 and MMP-9 in liver cancer cells [Citation20]. To date, no research has focused on diosmetin’s effect on endometrial cancer, and our study showed that diosmetin is able to inhibit cell proliferation and enhance sensitivity to radiotherapy by suppressing homologous recombination in endometrial cancer, providing additional mechanisms of diosmetin’s antitumor effect. Although we demonstrated that diosmetin modulates HR by suppressing recruitment of RPA2 as well as RAD51, how diosmetin affects RPA2 recruitment remains to be elucidated. Chen and colleagues [Citation38] showed that diosmetin can reduce the stability of Nrf2, which is reported to be capable of regulating many factors in HR repair and that inhibiting Nrf2 using all-trans retinoic acid (ATRA) or Nrf2 knockdown caused significant downregulation of HR repair. Whether diosmetin impairs the recruitment of RPA2 and RAD51 through Nrf2 needs further investigation. Xu and colleagues [Citation39] observed that diosmetin elevates levels of γH2AX at 1 hour post-IR in lung cancer cells, which is in agreement with our results in EC cells. They also found that the Akt signaling pathway was downregulated and speculated that Akt signaling may be involved in the diosmetin-mediated DNA damage response. Activated Akt is reported to suppress HR and activate NHEJ via different mechanisms [Citation40], which may partially explain why NHEJ efficiency declines slightly in diosmetin-treated Hec-1B cells.
Radiotherapy has been widely used for decades either alone or in combination with surgery, chemotherapy and targeted medicines to treat more than 50% of cancer patients; however, the response to irradiation treatment differs due to the complexity of a variety of factors [Citation41] and is limited by acquired resistance, which can result in relapse and metastasis [Citation42]. Additionally, a sufficient dose of pelvis radiotherapy is often associated with serious toxicities, such as urinary incontinence and fecal leakage, which will have a long-lasting influence on the quality of life of patients [Citation43]. Attempts have been made to improve radiotherapy efficacy and to reduce the effective dose. It is believed that DNA repair inhibitors targeting several crucial molecules in DNA repair pathways can effectively sensitize cancer cells to irradiation treatment and, more importantly, reduce the effective dose of irradiation [Citation23]. For example, AZD1390, an ATM inhibitor, is proven to be an effective radiosensitizer in central nervous system malignancies in early clinical development [Citation44]. The PARP inhibitor talazoparib is reported to enhance the efficacy of radiotherapy in small-cell lung cancer both in vivo and in vitro [Citation45], and RI-1 and B02, small molecules targeting RAD51, cause significant radiosensitization in glioblastoma [Citation46].
Whether patients can benefit from such DNA repair inhibitors as radiosensitizers is partially dependent on the correlation of DNA repair and cancer type or subtype [Citation41]. Previous research has demonstrated that the development of endometrial cancer is tightly associated with DSB repair; for example, 27% of high-risk ECs are associated with loss of nuclear RAD51 [Citation47]. Decreased expression of Ku70 is also found in endometrioid endometrial cancer [Citation48]. Our study showed that diosmetin suppresses DSB repair by negatively regulating the DNA end resection step. As a result, diosmetin not only inhibits proliferation but also sensitizes endometrial cancers to radiotherapy. Altogether, we propose that the combination of diosmetin and irradiation is a promising therapeutic strategy for endometrial cancer.
Supplemental Material
Download Zip (7.6 MB)Disclosure statement
All authors declare no competing interests.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020;70(1):7–30. Epub 2020/ 01/09. PubMed PMID: 31912902.
- Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19(9):510–521. Epub 2019/ 08/08. PubMed PMID: 31388127.
- Wright JD, Barrena Medel NI, Sehouli J, et al. Contemporary management of endometrial cancer. Lancet. 2012;379(9823):1352–1360. Epub 2012/ 03/27. PubMed PMID: 22444602.
- Brooks RA, Fleming GF, Lastra RR, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69(4):258–279. Epub 2019/ 05/11. PubMed PMID: 31074865.
- Mao Z, Bozzella M, Seluanov A, et al. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle (Georgetown, Tex). 2008;7(18):2902–2906. Epub 2008/ 09/05. PubMed PMID: 18769152; PubMed Central PMCID: PMCPmc2754209.
- Rosenberg P, Wingren S, Simonsen E, et al. Flow cytometric measurements of DNA index and S-phase on paraffin-embedded early stage endometrial cancer: an important prognostic indicator. Gynecol Oncol. 1989;35(1):50–54. Epub 1989/ 10/01. PubMed PMID: 2792902.
- Scully R, Panday A, Elango R, et al. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol. 2019. Epub 2019/ 07/03. PubMed PMID: 31263220. DOI:10.1038/s41580-019-0152-0
- Raderschall E, Stout K, Freier S, et al. Elevated levels of Rad51 recombination protein in tumor cells. Cancer Res. 2002;62(1):219–225. Epub 2002/ 01/10. PubMed PMID: 11782381.
- Maacke H, Jost K, Opitz S, et al. DNA repair and recombination factor Rad51 is over-expressed in human pancreatic adenocarcinoma. Oncogene. 2000;19(23):2791–2795. PubMed PMID: WOS:000087318500009.
- Mao Z, Jiang Y, Liu X, et al. DNA repair by homologous recombination, but not by nonhomologous end joining, is elevated in breast cancer cells. Neoplasia (New York, NY). 2009;11(7):683–691. Epub 2009/ 07/02. PubMed PMID: 19568413; PubMed Central PMCID: PMCPmc2697354.
- Meirinhos J, Silva BM, Valentao P, et al. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat Prod Res. 2005;19(2):189–195. Epub 2005/ 02/18. PubMed PMID: 15715265.
- Patel K, Gadewar M, Tahilyani V, et al. A review on pharmacological and analytical aspects of diosmetin: a concise report. Chin J Integr Med. 2013;19(10):792–800. Epub 2013/ 10/05. PubMed PMID: 24092244.
- Kahn SR. The clinical diagnosis of deep venous thrombosis: integrating incidence, risk factors, and symptoms and signs. Arch Intern Med. 1998;158(21):2315–2323. Epub 1998/ 11/25. PubMed PMID: 9827782.
- Chen Y, Wang Y, Liu M, et al. Diosmetin exhibits anti-proliferative and anti-inflammatory effects on TNF-alpha-stimulated human rheumatoid arthritis fibroblast-like synoviocytes through regulating the Akt and NF-kappaB signaling pathways. Phytother Res. 2019. Epub 2019/ 12/14. PubMed PMID: 31833613. DOI:10.1002/ptr.6596
- Liao WZ, Ning ZX, Chen LY, et al. Intracellular antioxidant detoxifying effects of diosmetin on 2,2-Azobis(2-amidinopropane) Dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J Agr Food Chem. 2014;62(34):8648–8654. PubMed PMID: WOS:000340992100014. .
- Ma A, Zhang R. Diosmetin inhibits cell proliferation, induces cell apoptosis and cell cycle arrest in liver cancer. Cancer Manag Res. 2020;12:3537–3546. Epub 2020/ 06/18. PubMed PMID: 32547191; PubMed Central PMCID: PMCPMC7244522.
- Oak C, Khalifa AO, Isali I, et al. Diosmetin suppresses human prostate cancer cell proliferation through the induction of apoptosis and cell cycle arrest. Int J Oncol. 2018;53(2):835–843. PubMed PMID: WOS:000440581500033.
- Wang C, Li S, Ren H, et al. Anti-proliferation and pro-apoptotic effects of diosmetin via modulating cell cycle arrest and mitochondria-mediated intrinsic apoptotic pathway in MDA-MB-231 Cells. Med Sci Monit. 2019;25:4639–4647. Epub 2019/ 06/23. PubMed PMID: 31228347; PubMed Central PMCID: PMCPMC6601365.
- Liu Y, Shao Z, Liao Y, et al. Targeting SKP2/Bcr-Abl pathway with Diosmetin suppresses chronic myeloid leukemia proliferation. Eur J Pharmacol. 2020;173366. Epub 2020/ 07/18. doi:10.1016/j.ejphar.2020.173366
- Liu J, Wen X, Liu B, et al. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol Med Rep. 2016;13(3):2401–2408. Epub 2016/ 02/06. PubMed PMID: 26847170; PubMed Central PMCID: PMCPMC4768952.
- Ciolino HP, Wang TTY, Yeh GC. Diosmin and diosmetin are agonists of the aryl hydrocarbon receptor that differentially affect cytochrome P450 1A1 Activity. Cancer Res. 1998 JULY 1;58:2754–2760.
- Androutsopoulos VP, Mahale S, Arroo RR, et al. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21(6):1525–1528. Epub 2009/ 05/09. PubMed PMID: 19424633.
- Srivastava M, Raghavan SC. DNA double-strand break repair inhibitors as cancer therapeutics. Chem Biol. 2015;22(1):17–29. Epub 2015/ 01/13. PubMed PMID: 25579208.
- Seluanov A, Mao Z, Gorbunova V. Analysis of DNA double-strand break (DSB) repair in mammalian cells. J Vis Exp. 2010:43. Epub 2010/ 09/25. PubMed PMID: 20864925; PubMed Central PMCID: PMCPmc3157866. DOI:10.3791/2002.
- Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science (New York, NY). 2011;332(6036):1443–1446. Epub 2011/ 06/18. PubMed PMID: 21680843.
- Zhang L, Zhang F, Zhang W, et al. Harmine suppresses homologous recombination repair and inhibits proliferation of hepatoma cells. Cancer Biol Ther. 2015;16(11):1585–1592. Epub 2015/ 09/19. PubMed PMID: 26382920; PubMed Central PMCID: PMCPMC4846143.
- Scully R, Panday A, Elango R, et al. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Bio. 2019;20(11):698–714. PubMed PMID: WOS:000492310900009.
- Goldstein M, Kastan MB. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–143. Epub 2014/ 11/26. PubMed PMID: 25423595. .
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. PubMed PMID: WOS:000180670600036.
- Ahn JY, Schwarz JK, Piwnica-Worms H, et al. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60(21):5934–5936. PubMed PMID: WOS:000165230300006.
- Roma A, Rota SG, Spagnuolo PA. Diosmetin induces apoptosis of acute myeloid leukemia cells. Mol Pharm. 2018;15(3):1353–1360. Epub 2018/ 02/08. PubMed PMID: 29412683.
- Liu J, Ren H, Liu B, et al. Diosmetin inhibits cell proliferation and induces apoptosis by regulating autophagy via the mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Oncol Lett. 2016;12(6):4385–4392. Epub 2017/ 01/20. PubMed PMID: 28101201; PubMed Central PMCID: PMCPMC5228182.
- Liu B, Shi Y, Peng W, et al. Diosmetin induces apoptosis by upregulating p53 via the TGF-beta signal pathway in HepG2 hepatoma cells. Mol Med Rep. 2016;14(1):159–164. Epub 2016/ 05/14. PubMed PMID: 27176768; PubMed Central PMCID: PMCPMC4918616.
- Chen X, Wu Q, Chen Y, et al. Diosmetin induces apoptosis and enhances the chemotherapeutic efficacy of paclitaxel in non-small cell lung cancer cells via Nrf2 inhibition. Br J Pharmacol. 2019;176(12):2079–2094. Epub 2019/ 03/03. PubMed PMID: 30825187; PubMed Central PMCID: PMCPMC6534779.
- Choi J, Lee DH, Park SY, et al. Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer. Biomed Pharmacother. 2019;117:109091. Epub 2019/ 06/23. PubMed PMID: 31228803.
- Ge A, Ma Y, Liu YN, et al. Diosmetin prevents TGF-beta1-induced epithelial-mesenchymal transition via ROS/MAPK signaling pathways. Life Sci. 2016;153:1–8. Epub 2016/ 04/23. PubMed PMID: 27101925.
- Yan Y, Liu X, Gao J, et al.Inhibition of TGF-beta Signaling in Gliomas by the Flavonoid Diosmetin Isolated from Dracocephalum peregrinum L. Molecules. 2020; 25(1). Epub 2020/ 01/08. PubMed PMID: 31906574; PubMed Central PMCID: PMCPMC6982745. DOI:10.3390/molecules25010192.
- Chen X, Wu Q, Chen Y, et al. Diosmetin induces apoptosis and enhances the chemotherapeutic efficacy of paclitaxel in non‐small cell lung cancer cells via Nrf2 inhibition. Br J Pharmacol. 2019;176(12):2079–2094.
- Xu Z, Yan Y, Xiao L, et al. Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PloS One. 2017;12(4):e0175977. Epub 2017/ 04/18. PubMed PMID: 28414793; PubMed Central PMCID: PMCPMC5393875.
- Liu Q, Turner KM, Alfred Yung WK, et al. Role of AKT signaling in DNA repair and clinical response to cancer therapy. Neuro Oncol. 2014;16(10):1313–1323. Epub 2014/ 05/09. PubMed PMID: 24811392; PubMed Central PMCID: PMCPMC4165418.
- Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–253. Epub 2011/ 03/25. PubMed PMID: 21430696.
- Ozpiskin OM, Zhang L, Li JJ. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics. 2019;9(5):1215–1231. Epub 2019/ 03/15. PubMed PMID: 30867826; PubMed Central PMCID: PMCPMC6401500.
- Nout RA, van de Poll-franse LV, Lybeert ML, et al. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J Clin Oncol. 2011;29(13):1692–1700. Epub 2011/ 03/30. PubMed PMID: 21444867.
- Durant ST, Zheng L, Wang Y, et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci Adv. 2018;4(6):eaat1719. Epub 2018/ 06/26. PubMed PMID: 29938225; PubMed Central PMCID: PMCPMC6010333.
- Laird JH, Lok BH, Ma J, et al. Talazoparib is a potent radiosensitizer in small cell lung cancer cell lines and xenografts. Clin Cancer Res. 2018;24(20):5143–5152. Epub 2018/ 06/28. PubMed PMID: 29945991; PubMed Central PMCID: PMCPMC6742772.
- King HO, Brend T, Payne HL, et al. RAD51 is a selective dna repair target to radiosensitize glioma stem cells. Stem Cell Reports. 2017;8(1):125–139. Epub 2017/ 01/12. PubMed PMID: 28076755; PubMed Central PMCID: PMCPMC5233453.
- Auguste A, Genestie C, De Bruyn M, et al. Refinement of high-risk endometrial cancer classification using DNA damage response biomarkers: a TransPORTEC initiative. Modern Pathol. 2018;31(12):1851–1861. PubMed PMID: WOS:000451623400008.
- Lomnytska MI, Becker S, Gemoll T, et al. Impact of genomic stability on protein expression in endometrioid endometrial cancer. Br J Cancer. 2012;106(7):1297–1305. Epub 2012/ 03/15. PubMed PMID: 22415234; PubMed Central PMCID: PMCPmc3314786.
