ABSTRACT
Tuberculosis is one of the most important infectious diseases worldwide and macrophage apoptosis is the major host defense mechanism against TB. We attempted to characterize the role of miRNA (miR)-125b-5p on mycobacterium tuberculosis (Mtb) infection and macrophages behaviors in vitro. According to fluorescence-activated cell separation (FACS), primary monocytes (CD14+) in TB patients were accumulated, and apoptotic monocytes were decreased. Peripheral blood mononuclear cells (PBMCs)-derived macrophages (MDMs) and monocytic cells THP-1-derived macrophage-like cells (TDMs) in vitro were used to be infected with H37Rv. After infection, colony-forming units assay revealed the increase of bacterial activity, FACS demonstrated the decrease of apoptosis rate of MDMs and TDMs, as well as promoted levels of IL-6, TNF-α, Bax, and Bim and suppressed levels of IL-10 and Bcl-2, examined by enzyme-linked immunosorbent assay (ELISA) and western blot assay. Expression of miR-125b-5p and DNA damage-regulated autophagy modulator 2 (DRAM2) was examined, and real-time PCR and western blot assay showed that miR-125b-5p was upregulated, whereas DRAM2 was downregulated in primary monocytes and H37Rv-infected macrophages (MDMs and TDMs). Moreover, blocking miR-125b-5p could attenuated H37Rv-induced bacterial activity and inflammatory response of MDMs and TDMs, accompanied with apoptosis inhibition. Whereas these effects of miR-125b-5p knockdown were abolished by downregulating DRAM2. In mechanism, DRAM2 was a downstream target of miR-125b-5p, as evidenced by dual-luciferase reporter assay. Collectively, silencing miR-125b-5p could protect human macrophages against Mtb infection through promoting apoptosis and inhibiting inflammatory response via targeting DRAM2, suggesting a novel target for Mtb eliminating.
Abbreviations: TB: tuberculosis; PBMCs: peripheral blood mononuclear cells; Mtb: mycobacterium tuberculosis; AFB: acid fast bacilli; FITC: fluorescein isothiocyanate; MDMs: monocytes-derived macrophages; TDMs: THP-1-derived macrophage-like cells; ERFP: Mtb-enhanced red fluorescent protein; CFU: colony-forming units; ELISA: enzyme-linked immunosorbent assay; FACS: fluorescence-activated cell separation; PI: propidium iodide; DRAM2: DNA damage-regulated autophagy modulator 2; Real-time PCR: real-time polymerase chain reaction; in-miR-125b-5p: miR-125b-5p inhibitor; si-DRAM2: siRNA against DRAM2
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) infection is one of the top 10 causes of death in developing countries, and owes the second-highest mortality rate of infectious diseases [Citation1,Citation2]. Mtb is an intracellular pathogen, which is capable of undergoing various genomic reprogramming events to subsequently prevent the immune system from completely eliminating latent infectious agents [Citation3]. The cellular immunity response of host cells determines whether the infection becomes a latent TB infection or progresses into active TB [Citation4,Citation5]. In the condition that infected individuals fail to control the initial pulmonary infection, or the immune system is weakened, Mtb may cause pulmonary TB or extrapulmonary TB. However, there are only 10% of the infected individuals exhibiting active TB [Citation6], indicating the vital role of host immune defense and genetic factors in the regulation of Mtb infection. The major types of innate immune cells after Mtb infection include macrophages, dendritic cells, neutrophils, and natural killer cells [Citation7]; classically, activated macrophages play a central role in eliminating Mtb [Citation8]. It has been proved that Mtb infection-induced apoptosis and necrosis of macrophages that contribute to the pathogenesis of TB [Citation9]. Therefore, we attempted to figure out the mechanism of macrophage apoptosis after Mtb infection, as well as an inflammatory response.
MicroRNAs (miRNAs) are small noncoding RNAs that play important roles in cell immunity by regulating target gene expression at the levels of transcription, RNA processing, and translation. Recently, increasing numbers of studies have demonstrated that some miRNAs are implicated in regulating the immune response of macrophages against bacterial pathogens [Citation5], as well as Bacillus Calmette-Guerin (BCG) treatment [Citation10,Citation11], the only effective approach to prevent active TB. For example, upregulation of miRNA (miR)-196b-5p could attenuate BCG uptake in macrophages via activating STAT3 from patients with active pulmonary TB [Citation10]; miR-124 expression is provoked by BCG infection in murine macrophages and lungs, and negatively regulated TLR signaling in alveolar macrophages in response to Mtb infection [Citation11]. In regard to miR-125b-5p, its expression has been disclosed to be markedly increased in Vγ2Vδ2 T cells derived from TB patients [Citation12], as well as the serum of TB patients [Citation13]. Besides, miR-125b-5p is one tumor-suppressive miRNA and apoptosis-related miRNA in cancers [Citation14–16]. However, the role of miR-125b-5p in macrophage apoptosis after Mtb infection remains to be characterized.
DNA damage-regulated autophagy modulator 2 (DRAM2) is thought to participate in the initiation of autophagy [Citation17]. Essentially, DRAM2 encodes a transmembrane lysosomal protein localized in the lysosome [Citation18]. The silencing of endogenous DRAM2 could attenuate cell death in tumor cells [Citation17]. It is also reported that DRAM2 is responsible for autophagy through serving as a target of miR-125b-1 [Citation19]. However, there is a little investigation focused on illuminating the role of DRAM2 during Mtb infection.
In this study, we analyzed the expression of miR-125b-5p in patients with TB, and its effect on apoptosis and inflammatory response of human macrophages in vitro after Mtb infection. Notably, we identified a novel miR-125b-5p/DRAM2 axis in macrophages in TB.
Materials and methods
Study population
A total of 68 samples of peripheral blood were collected: 34 healthy control (median age 40.17 ± 15.36 years; male 47.1%) and 34 patients with pulmonary TB (median age 45.62 ± 15.17 years; male 50.0%). Detailed information regarding the patients and controls is shown in . All TB patients enrolled in this study were based on clinical symptoms, chest X radiography, acid-fast bacilli (AFB) staining of sputum smears. The peripheral blood mononuclear cells (PBMCs) were purified with density gradient centrifugation at 1,500 × g at 4oC depending on Ficoll-Paque (GE Healthcare Biosciences, Pittsburgh, PA, USA). The study protocols were approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University, and informed written consent was obtained from all participants prior to commencement.
Table 1. Demographic and clinical characteristics in patients with active tuberculosis (TB) and healthy control individuals
Fluorescence-activated cell separation (FACS) method
Peripheral blood samples (5 ml) were obtained from the antecubital vein of HC and TB patients on an empty stomach, and the surface antibody was stained (within 6 h) with fluorescein isothiocyanate (FITC)-labeled mouse anti-human CD14+ monoclonal antibody (abcam, Cambridge, UK, #28,061) for 1 h on ice in the dark. The cells were subsequently analyzed using Beckman CXP (version 2.0) software on a FC-500 Flow Cytometer (Beckman Counter, Brea, CA, USA). The appropriate homeotypic antibody was used to determine the background level of staining.
Additionally, in vitro cell apoptosis rate was measured using Annexin V-FITC Apoptosis Detection Kit (Beyotime, Shanghai, China) according to the manufacturer’s instruction. In brief, 1 × 105 cells (MDMs and TDMs) with H37Rv infection or not were harvested, washed, and successively stained with Annexin V-FITC and propidium iodide (PI). Then, stained cells were set in Annexin V-FITC/PI quadrant, and the percentage of apoptotic cells was in Annexin V-FITC+/PI+ and Annexin V-FITC+/PI- quadrants was calculated.
Total RNA and protein isolation
PBMCs were lysed in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. After adding chloroform, the solution came to stratification: the colorless water phase in the upper layer (for RNA), the white membrane in the interlayer (for protein), and the organic phase in the sublayer (for DNA). And the interlayer suffered from a series of treatment of precipitation with isopropanol, rinse with 0.3 M guanidine hydrochloride (in 95% ethanol) and vacuum drying. Total protein in PBMCs was redissolved in 1% sodium dodecyl sulfate (SDS) containing protease inhibitor phenylmethanesulfonyl fluoride. In MDMs and TDMs, total protein was isolated with RIPA lysis buffer (Beyotime).
Real-time polymerase chain reaction (real-time PCR)
The cDNA was acquired with reverse transcription using a Reverse transcription kit (Abcam). The amplification of cDNA was performed by SYBR Premix Ex Taq Master Mix (Invitrogen) on Applied Biosystem 7500 real-time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). Fold changes in expressions of miR-125b-5p and DRAM2 were calculated according to the comparative threshold cycle value (2−ΔΔCt) method, compared with U6 and β-actin. All operations were carried out at least 3 times. The primer sequence was listed as follows: miR-125b-5p, 5ʹ-TCCCTGAGA CCCTAACTTGTGA-3ʹ forward and 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ reverse; DRAM2, 5ʹ-CCTTTCCTACCAAATGCAGCCC-3ʹ forward and 5ʹ-GCCACTGTGCAAAACTGATGAGC-3ʹ reverse; β-actin, 5ʹ-CACAGAGCCTCGCCTTTGCC-3ʹ forward and 5ʹ-ACCCATGCCCACCATCACG-3ʹ reverse; U6, 5ʹ-GCAGGAGGTCTTCACAGAGT-3ʹ forward and 5ʹ-TCTAGAGGAGAAGCTGGGGT-3ʹ reverse.
Cell cultures
Human peripheral blood monocytes-derived macrophages (MDMs) were obtained from PBMCs. In brief, PBMCs were seeded onto a 24-well plate (Corning, NY, USA) with differentiation for 5 days in the presence of 4 ng/ml human CSF/macrophage colony-stimulating factor (M6518; Sigma-Aldrich, St. Louis, MO, USA); then, nonadherent cells were removed, and the adherent cells were regarded as differentiated MDMs.
THP-1 human monocytic cells were originally from BeNa Culture Collection (Beijing, China), and then were treated with 20 nM phorbol-12-myristate-13-acetate (P8139; Sigma-Aldrich) for 24 h to induce differentiation into macrophage-like cells (TDMs).
The human MDMs and TDMs were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Gibco), 50 U/ml penicillin, 50 μg/ml streptomycin, and 2 mM L-glutamine at 37oC in 5% CO2 condition.
Cell transfection
The siRNA against DRAM2 (si-DRAM2; GUGCUUCACAUGAUCACUACUG), miR-125b-5p mimic (miR-125b-5p; UCCCUGAGACCCUAACUUGUGA), and miR-125b-5p inhibitor (in-miR-125b-5p; TCACAAGTTAGGGTCTCAGGGA) and their respective controls (si-NC, UUCUCCGAACGUGUCACGU; miR-NC, UUCUCCGAACGUGUCACGUTT; in-miR-NC, CAGUACUUUUGUGUAGUACAA) were provided by GenePharma (Shanghai, China), and transfected into MDM and TDM cells individually or in combination. All transfection reactions were performed using Lipofectamine 2000 (Invitrogen) following the standard instruction of the manufacturer. Transfected cells were cultured for an additional 48 h prior to Mtb infection.
Mtb culture and infection
Mtb H37Rv was provided by American Type Culture Collection (25618TM; ATCC, Manassas, VA, USA). Mycobacteria was grown in Middlebrook 7H9 broth with ADC enrichment (Medium 1395, ATCC). Bacterial cultures were harvested by centrifugation at 500 × g for 20 min, and the pellets were re-suspended in a bacterial culture medium. The H37Rv was tagged with an enhanced red fluorescent protein (ERFP), and ERFP-H37Rv was cultivated in 7H9-OADC plus kanamycin (60,615; Sigma-Aldrich). We used 50 μg/ml kanamycin to culture the Mtb-ERFP strain. Bacterial strains were divided into 1 ml aliquots and stored at -70 oC.
The mid-log phase bacteria (absorbance 0.4) were used, and bacterial clumps were removed by passing the washed suspension through a 22-gauge syringe. MDMs and TDMs were infected with a multiplicity of infection (MOI) of 5 for 4 h, washed with phosphate buffer saline (PBS) for 3 times, and further cultivated for another 2 days. About 5 MOI of H37Rv infection for 48 h prior to further analysis.
Colony-forming units (CFU) assay
The bacterial viability in MDMs and TDMs was determined with CFU assay. Briefly, after 5 MOI of H37Rv infection for 48 h, MDMs and TDMs were washed with PBS for 3 times prior to be lysed in sterile normal saline. Then, 10-fold serial dilutions were transferred on Middlebrook 7H10 agar (Medium 173, ATCC) for another 3-week cultivation. The CFUs were counted and transformed into a log10 value.
Enzyme-linked immunosorbent assay (ELISA)
ELISA kits (ExCell, Shanghai, China) employing the conventional “sandwich” formats were used to quantify the cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10. After H37Rv infection for 2 days, cell extract of MDMs and TDMs was obtained and performed according to corresponding ELISA kits. Eventually, the optical density at 450 nm was spectrophotometrically measured by a microplate reader (BioTek, Burlington, VT, USA).
Western blot assay
The protein extracts were quantified with the BCA™ Protein Assay kit (Pierce; Thermo Fisher Scientific) and 20 μg of samples was utilized for western blotting according to standard procedures, including SDS-polyacrylamide gel electrophoresis, polyvinylidene difluoride membrane transferring, antibody incubation, and chemiluminescence detection. The primary antibodies were purchased from abcam and as follows: Bax (#18,283); Bcl-2 (#196,495); Bim (#7888); DRAM2 (#230,191); β-actin (#9484). The proteins were visualized using enhanced chemiluminescence (Millipore, Bedford, MA, USA). The experiment was performed in triplicate, and β-actin on the same membrane was used as a loading control.
Dual-luciferase reporter assay
The putative target prediction of hsa-miR-125b-5p on the DRAM2 gene was performed through Targetscan software. The potential binding sites on DRAM2 in its 3ʹuntranslated region were mutated and cloned by the PCR method into the plasmid pmirGLO-luciferase report vector (Promega, Madison, WI, USA). MDM and TDM cells were plated in a 24-well plate (Corning) at 1 × 104 cells/well, followed by co-transfection with 20 nM of miR-125b-5p/NC mimic and 20 ng of wild type/mutant of DRAM2 3ʹ UTR (DRAM2-WT/MUT) for 48 h. Cells were collected to measure the relative luciferase activities using the dual-luciferase reporter assay system (Promega) according to the manufacturer’s information. All experiments were carried out in triplicate.
Statistical analysis
The results were presented as mean ± standard deviation (SD). Statistical analyses were performed using Graphpad 6.0 (GraphPad Software, La Jolla, CA, USA). The P values were calculated using the Student’s t test and one-way analysis of variance (ANOVA). Tukey’s post hoc test was performed following ANOVA. P < 0.05 was considered to indicate a statistically significant difference.
Results
Monocytes were accumulated in PBMCs of TB patients with apoptosis inhibition
In order to evaluate the dysregulation of miR-125b-5p in PTB patients, we isolated the PBMCs from healthy control (HC; n = 34) and PTB patients (TB; n = 34). Firstly, the comparative analysis of PBMCs number was determined with the FACS method, and the data showed that the percentage of CD14+ monocytes was significantly higher in the TB group ()). Additionally, apoptosis-positive cells were lower in the blood of TB patients, compared with that in HC ()). These observations indicated monocytes were highly induced after Mtb infection in patients.
Figure 1. Effect of Mycobacterium tuberculosis (Mtb) infection on human monocytes in pulmonary tuberculosis (TB) patient. Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy control (HC; n = 34) and pulmonary TB patients (TB; n = 34). (a) CD14-positive (CD14+) moncytes were sorted and determined by fluorescence-activated cell sorting (FACS). (b) Apoptosis positive cells in above primary moncytes was measured on FACS. All operations were performed in triplicate and * P < 0.05
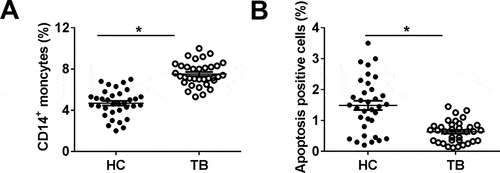
MiR-125b-5p was highly induced in monocytes of TB patients and Mtb-infected human macrophages
The expression of miR-125b-5p after Mtb infection was examined with real-time PCR. As a result, level of miR-125b-5p was upregulated (2.8-fold, and P < 0.05) in monocytes from TB patients ()), and H37Rv-infected MDMs (3.8-fold) and TDMs (3.2-fold) (,)). H37Rv infection in MDMs and TDMs highly induced bacterial activity, as indicated by elevated CFU ()). Besides, FACS data showed that the apoptosis rate was lowered in MDMs and TDMs ()), accompanied by increased Bcl-2 and decreased Bax and Bim ()). In terms of inflammation, ELISA kits demonstrated that H37Rv infection promoted secretions of pro-inflammatory cytokines IL-6 and TNF-α, but inhibited anti-inflammatory cytokine IL-10 production ()). These findings showed that miR-125b-5p was upregulated in human monocytes in response to Mtb infection both in vitro and in vivo, suggesting miR-125b-5p as a potential contributor in TB pathogenesis.
Figure 2. Expression of miR-125b-5p in human primary moncytes and macrophages infected by H37Rv in vitro. Relative expression level of miR-125b-5p was measured with real-time PCR in (a) PBMCs from HC and TB patients, and (b, c) H37Rv-infected human peripheral blood monocytes-derived macrophages (MDMs) and THP-1 cells-derived macrophages (TDMs) for 48 h. (d) The mycobacterial viability in transfected MDMs and TDMs after H37Rv infection was determined by colony-forming units (CFU) assay. (e) FACS examined apoptosis rate of H37Rv-infected MDMs and TDMs. (f-h) Enzyme-linked immunosorbent assay (ELISA) measured levels of inflammatory factors, interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α in cell culture supernatant of H37Rv-infected MDMs and TDMs. (i) Western blot measured levels of apoptosis-related proteins, B cell lymphoma (Bcl)-2, Bcl-2-associated X Protein (Bax), and Bcl-2-interacting mediator (Bim) in H37Rv-infected MDMs and TDMs. All experiments were performed in triplicate and * P < 0.05
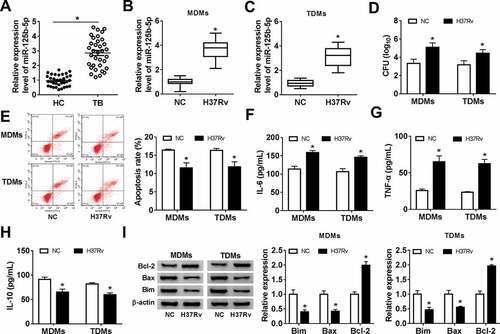
Knockdown of miR-125b-5p attenuated Mtb-induced apoptosis inhibition and inflammatory response in human macrophages in vitro
Subsequently, the role of miR-125b-5p in apoptosis of macrophages was further figured out under H37Rv infection. Firstly, in-miR-125b-5p was pretransfected in MDMs and TDMs, and the levels of miR-125b-5p were markedly decreased ()). Next, with H37Rv infection, CFU was diminished and the apoptosis rate was upregulated in MDMs and TDMs with miR-125b-5p depletion (,)). Levels of IL-6, TNF-α, Bax, and Bim were declined in in-miR-125b-5p-transfected MDMs and TDMs with following H37Rv infection ()), along with promoted IL-10 and Bcl-2 levels (,)). These results demonstrated that silencing miR-125b-5p could prevent macrophages in vitro from H37Rv-induced inflammation and apoptosis inhibition.
Figure 3. Effect of miR-125b-5p blockage on human macrophage apoptosis in vitro with H37Rv infection. (a) Real-time PCR detected miR-125b-5p expression level in MDMs and TDMs transfected with miR-125b-5p inhibitor (in-miR-125b-5p) or its negative control (in-miR-NC). (bh) Above transfected MDMs and TDMs at 48 h were then subjected with H37Rv infection for 48 h. (b) Real-time PCR detected miR-125b-5p expression level. (c) CFU assay determined mycobacterial viability. (d) Apoptosis rate was recorded by FACS. (e–g) ELISA measured IL-6, IL-10 and TNF-α levels in cell culture supernatant. (h) Western blot assay examined Bcl-2, Bax and Bim levels with normalization to β-actin. All operations were carried out at least three times and * P < 0.05
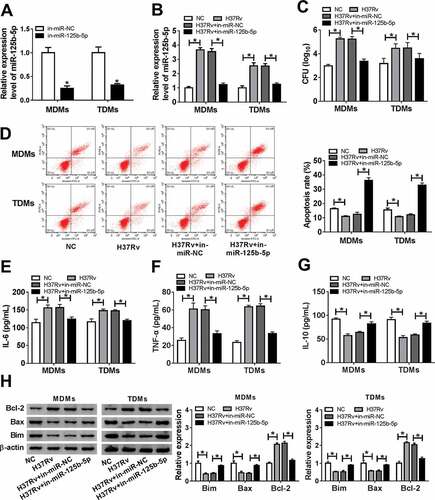
DRAM2 was downregulated with Mtb infection and was a direct target of miR-125b-5p
To identify the mechanism underlying the role of miR-125b-5p in regulating host defenses in Mtb infection, we preliminarily selected 10 genes according to the Targetscan database, and DRAM2 was the most sensitive gene in MDMs with miR-125b-5p downregulation (Supplementary Figure 1). Thus, we further testified DRAM2 as the target of miR-125b-5p, and the bioinformatic analysis showed that complementary sites between DRAM2-WT and miR-125b-5p ()). Luciferase assays showed that the luciferase activity of the DRAM2-WT vector instead of DRAM2-MUT was significantly reduced in MDMs and TDMs with co-transfection of miR-125-5p (,)); contrarily, it was elevated with co-transfection of in-miR-125-5p (,)). Next, the expression of DRAM2 in response to Mtb infection was evaluated. In PBMCs from TB patients, DRAM2 mRNA expression was downregulated (0.3-fold and P < 0.05) ()); besides, we observed downregulation of DRAM2 mRNA in H37Rv-infected MDMs and TDMs (,)). Furthermore, DRAM2 protein expression was upregulated within miR-125-5p transfection, and was downregulated with miR-125-5p mimic transfection in MDMs and TDMs ()), as indicated by western blot assay. There was an inverse correlation between the expression of DRAM2 mRNA and miR-125b-5p in TB patients ()) according to the Spearman rank correlation test. These results revealed that DRAM2 was directly and negatively regulated by miR-125b-5p in human macrophages.
Figure 4. DNA damage-regulated autophagy modulator 2 (DRAM2) was a target gene of miR-125b-5p in human macrophages. (a) The potential binding sequences of hsa-miR-125b-5p in wild type of DRAM2 3ʹ UTR (DRAM2-WT) was presented and mutated to construct the mutation of DRAM2 3ʹ UTR (DRAM2-MUT). (b-e) Dual-luciferase reporter assays assessed luciferase activity of plasmid carrying DRAM2-WT/MUT in MDMs and TDMs co-transfected with (b, c) miR-125-5p/NC mimic (miR-125-5p/NC) or (d, e) in-miR-125-5p/NC inhibitor. (f-h) Real-time PCR measured DRAM2 expression in (f) human primary moncytes from TB and HC, and (g, h) macrophages (MDMs and TDMs) with H37Rv infection or not. (i) Western blot assay examined expression level of DRAM2 in MDMs and TDMs transfected with miR-NC, miR-125-5p, in-miR-NC, or in-miR-125-5p. (j) The correlation analysis was conducted between miR-125b-5p and DRAM2 expression with Spearman rank correlation test. All operations were carried out in triplicate. * P < 0.05
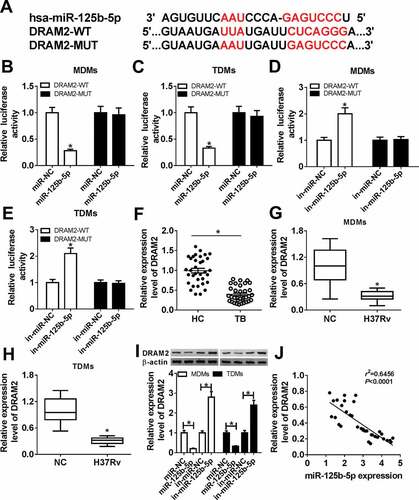
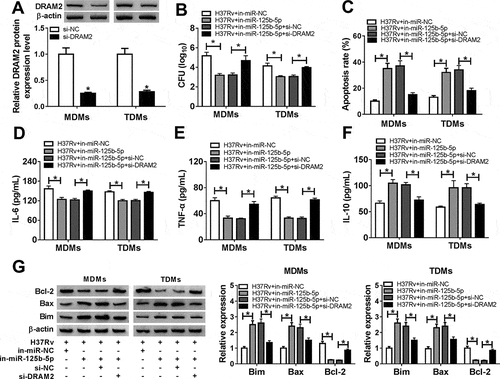
Restoration of DRAM2 abrogated the anti-TB role of miR-125b-5p knockdown in Mtb-infected macrophages in vitro
To further investigate the cross-talk between miR-125b-5p and DRAM2 in macrophages after Mtb infection, we conducted function-recovery experiments. The si-DRAM2 was used to knock down DRAM2 in MDMs and TDMs ()), and this transfection also caused diminishing effects on miR-125b-5p dysregulation role in H37Rv-infected cells. First of all, the inhibited bacterial activity mediated by silencing miR-125b-5p was attenuated by simultaneously downregulating DRAM2 ()). Then, high apoptosis of miR-125b-5p-silenced MDMs and TDMs under H37Rv infection was distinctly reduced due to si-DRAM2 introduction, as evidenced by reduced apoptosis rate, decreased Bax and Bim levels, as well as augmented Bcl-2 level (,)). In addition, DRAM2 deficiency partially reversed miR-125b-5p deletion-mediated promotion on IL-10 secretion, as well as an inhibition on IL-6 and TNF-α secretions in H37Rv-infected MDMs (-)). These outcomes indicated that miR-125b-5p knockdown exhibited an anti-TB role in human macrophages in vitro depending on DRAM2 upregulation, suggesting a miR-125b-5p/DRAM2 axis in the pathogenesis and treatment of TB.
Figure 5. The promotion of miR-125b-5p knockdown on human macrophages apoptosis in vitro with H37Rv infection was abolished by silencing DRAM2. (a) Western blot assay examined expression level of DRAM2 in MDMs and TDMs transfected with siRNA against DRAM2 (si-DRAM2) or its negative control (si-NC). (bg) MDMs and TDMs were transfected with in-miR-125b-5p alone or combined with si-DRAM2 for 48 h, followed with H37Rv infection for 48 h. (b) CFU assay determined mycobacterial viability. (c) Apoptosis rate was recorded by FACS. (d–f) ELISA measured IL-6, IL-10, and TNF-α levels in cell culture supernatant. (g) Western blot assay examined Bcl-2, Bax, and Bim levels. All operations were launched in triplicate. * P < 0.05
Discussion
TB was one of the world’s leading infectious diseases with an estimated rise of about 10 million new cases and 1.7 million deaths per year [Citation20]. To date, the BCG vaccine remained the only effective vaccine to provide protective effects against Mtb infection; however, the widespread application of BCG had also become a major contributor to the incidence of TB increasing over years [Citation21]. The pathogen had emerged with numerous drug-resistant strains ranging from multi-drug resistant to extensive drug resistant [Citation22], making it even more challenging to treat with the conventional therapy regimen. Furthermore, macrophages played a central role in Mtb pathogenesis and eliminating. Thus, we investigated the expression and role of miR-125b-5p in apoptosis of macrophages ex vivo and in vitro. And, it was demonstrated that inhibition of miR-125b-5p could elevate apoptosis rate and expressions of apoptosis-related genes Bax and Bim, as well as mitigated proinflammatory cytokines IL-6 and TNF-α secretion, thus protecting human macrophages from Mtb-induced injury.
In this work, we observed an upregulation of miR-125b-5p in monocytes from TB patients, which was consistent with previous data that serum miR-125b-5p in TB patients was 11.4-fold higher than healthy control [Citation13]. Next, the level of miR-125b-5p was revealed to be increased in H37Rv-infected MDMs and TDMs in vitro, and this finding was similar to that of Shen et al. [Citation12]. In that research, miR-125b-5p together with miR-337-3p were uncovered to be markedly upregulated in Vγ2Vδ2 T cells from TB patients [Citation12]. Here, apoptotic macrophages were restrained by Mtb infection, suggesting a deficiency of Mtb eliminating. It was reported by Keane et al. [Citation23] that apoptosis served as an autonomous response of primary human alveolar macrophages infected with live but not heat-killed Mtb. However, Mtb-induced inhibition on macrophage apoptosis could be regulated by multiple miRNAs. For example, miR-223 and miR-155 downregulation promoted the apoptosis rate of macrophages by targeting FOXO3 [Citation24,Citation25], which was involved in regulating cell cycle and innate immune responses, and resisting oxidation and cell apoptosis. Besides, inhibition of miR-20a-5p also resulted in more efficient mycobacterial clearance from infected THP-1 macrophages through modulating Bim and JNK2, which were closely related to apoptosis [Citation26]. In this present study, the apoptosis rate of MDMs and TDMs with H37Rv infection was promoted by miR-125b-5p inhibitor, as well as expression of Bax and Bim via regulating DRAM2. Furthermore, Mtb-induced inflammatory response was mitigated by silencing miR-125b-5p. This outcome hinted that miR-125b-5p might suppress antimicrobial activity against Mtb.
By the way, miR-125b-5p was one anti-apoptotic gene in human tumors. For example, overexpression of miR-125b-5p suppressed the proliferation and induced apoptosis of laryngeal squamous cell carcinoma cells through suppressed glucose consumption and lactate production by targeting hexokinase-2 [Citation27]. miR-125b-5p upregulation enhanced chemotherapy sensitivity to cisplatin through promoted cell apoptosis in gallbladder cancer cells in the presence of cisplatin by targeting Bcl-2 [Citation28]. In human circulating γδ T cells which rapidly responded to infections and tumorigenesis, either miR-125b-5p or miR-99a-5p could inhibit γδ T cell activation and promote γδ T cell apoptosis, thus suppressing the cytotoxicity of γδ T cells toward tumor cells [Citation29]. Since eliminating key host defense cells, apoptosis could facilitate the spreading and proliferation of the pathogen [Citation9]. Moreover, mycobacterial proteins such as SecA2 and NuoG were also involved in suppressing apoptosis, and cytosolically localized Mtb was no exception to being susceptible to autophagy. However, the effect of miR-125b-5p on macrophage autophagy was not further identified; however, this could be a novel direction to further study miR-125b-5p role in TB.
Initially, DRAM2 was thought to play a role only in the initiation of autophagy owing to the encoding of a transmembrane lysosomal protein [Citation17,Citation18]. For instance, DRAM2 together with targets ULK1, E2F1 was involved in HOTAIRM1/miRNAs (such as miR-20a, miR-106b, and miR-125b-5p) networks to regulate the degradation of PML-RARA oncoprotein and myeloid cell differentiation by promotion autophagy pathway [Citation30]. miR144* inhibited antimicrobial responses against Mtb in human monocytes and macrophages by targeting DRAM2, as well [Citation31]. In this study, DRAM2 was downregulated in monocytes of TB patients and H37Rv-infected MDMs and TDMs; its upregulation was hidden in a miR-125b-5p inhibitor-mediated promoting role in apoptosis and suppressive role in inflammation in human macrophages in vitro. Meanwhile, DRAM2 downregulation via siRNA transfection could suppress apoptosis and induce an inflammatory response in MDMs and TDMs in spite of miR-125b-5p knockdown. Notably, DRAM2 was a downstream target of miR-125b-5p in regulating Mtb-induced immune response in macrophages. Coincidently, Bai et al. [Citation32] declared miR-125b-5p decreased the luciferase intensity of wild-type DRAM2 3ʹ UTR by approximately 85% in human retinoblastoma HXO-Rb44 cells compared with the normal cells. These results together indicated that DRAM2 was directly regulated by miR-12b-5p to promote apoptosis across different cells.
In conclusion, we clarified that miR-125b-5p was upregulated in TB patients and macrophages with Mtb infection. Moreover, knockdown of miR-125b-5p could mitigate Mtb infection in human macrophages in vitro by promoting apoptosis and suppressing inflammation through targeting DRAM2. This study might provide a novel biomarker and target for the diagnosis and treatment of mycobacterium infection.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. The methods used in this study were performed in accordance with relevant guidelines and regulations.
Supplemental Material
Download Zip (102.2 KB)Disclosure statement
The authors declare that they have no conflict of interests.
Availability of data and materials
All original data and materials are available from the corresponding author upon request.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- P K D, Jeemon P, N K A, et al. Status of epidemiology in the WHO South-East Asia region: burden of disease, determinants of health and epidemiological research, workforce and training capacity. Int J Epidemiol. 2012;41(3):847–860.
- P J M, Lehrer DS, Vitamin D. cod liver oil, sunshine, and phototherapy: safe, effective and forgotten tools for treating and curing tuberculosis infections - a comprehensive review. J Steroid Biochem Mol Biol. 2018;177:21–29.
- Sgaragli G, Frosini M. Human tuberculosis I. epidemiology, diagnosis and pathogenetic mechanisms. Curr Med Chem. 2016;23(25):2836–2873.
- Subbian S, Bandyopadhyay N, Tsenova L, et al. Early innate immunity determines outcome of Mycobacterium tuberculosis pulmonary infection in rabbits. Cell Commun Signal. 2013;11:60.
- Sabir N, Hussain T, S Z A S, et al. miRNAs in tuberculosis: new avenues for diagnosis and host-directed therapy. Front Microbiol. 2018;9:602.
- Nonghanphithak D, Reechaipichitkul W, Namwat W, et al. Genetic polymorphisms of CCL2 associated with susceptibility to latent tuberculous infection in Thailand. Int J Tuberc Lung Dis. 2016;20(9):1242–1248.
- C H L, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14(12):963–975.
- Shimizu T, Tomioka H. Novel type of antimicrobial mechanism in host macrophages against mycobacterial infections. Nihon Hansenbyo Gakkai Zasshi. 2009;78(3):283–291.
- A H M, Kornfeld H. Cell death and autophagy in tuberculosis. Semin Immunol. 2014;26(6):497–511.
- Yuan Y, Lin D, Feng L, et al. Upregulation of miR-196b-5p attenuates BCG uptake via targeting SOCS3 and activating STAT3 in macrophages from patients with long-term cigarette smoking-related active pulmonary tuberculosis. J Transl Med. 2018;16(1):284.
- Ma C, Li Y, Li M, et al. microRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol Immunol. 2014;62(1):150–158.
- Shen H, Gu J, Xiao H, et al. Selective destruction of interleukin 23-induced expansion of a major antigen-specific gammadelta T-cell subset in patients with tuberculosis. J Infect Dis. 2017;215(3):420–430.
- Wagh V, Urhekar A, Modi D. Levels of microRNA miR-16 and miR-155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberculosis (Edinb). 2017;102:24–30.
- Zhang C, C D Z, M H M, et al. Three-microRNA signature identified by bioinformatics analysis predicts prognosis of gastric cancer patients. World J Gastroenterol. 2018;24(11):1206–1215.
- L L M, W J W, Y T Q, et al. miR-125b-5p functions as a tumor suppressor gene partially by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS One. 2017;12(10):e0185636.
- Venkatadri R, Muni T, A K I, et al. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016;7:e2104.
- J H Y, Her S, Kim M, et al. The expression of damage-regulated autophagy modulator 2 (DRAM2) contributes to autophagy induction. Mol Biol Rep. 2012;39(2):1087–1093.
- O’Prey J, Skommer J, Wilkinson S, et al. Analysis of DRAM-related proteins reveals evolutionarily conserved and divergent roles in the control of autophagy. Cell Cycle. 2009;8(14):2260–2265.
- C W Z, Z H C, X J Z, et al. MIR125B1 represses the degradation of the PML-RARA oncoprotein by an autophagy-lysosomal pathway in acute promyelocytic leukemia. Autophagy. 2014;10(10):1726–1737.
- Watts G. WHO annual report finds world at a crossroad on tuberculosis. BMJ. 2012;345:e7051.
- D G R, C E B 3rd, Flynn JL. Tuberculosis: what we don’t know can, and does, hurt us. Science. 2010;328(5980):852–856.
- Sotgiu G, Centis R, D’Ambrosio L, et al. Do we need a new Fleming epoque: the nightmare of drug-resistant tuberculosis. Int J Mycobacteriol. 2013;2(3):123–125.
- Keane J, M K B-S, H G R, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65(1):298–304.
- Xi X, Zhang C, Han W, et al. MicroRNA-223 is upregulated in active tuberculosis patients and inhibits apoptosis of macrophages by targeting FOXO3. Genet Test Mol Biomarkers. 2015;19(12):650–656.
- Huang J, Jiao J, Xu W, et al. MiR-155 is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO3. Mol Med Rep. 2015;12(5):7102–7108.
- Zhang G, Liu X, Wang W, et al. Down-regulation of miR-20a-5p triggers cell apoptosis to facilitate mycobacterial clearance through targeting JNK2 in human macrophages. Cell Cycle. 2016;15(18):2527–2538.
- Hui L, Zhang J, Guo X. MiR-125b-5p suppressed the glycolysis of laryngeal squamous cell carcinoma by down-regulating hexokinase-2. Biomed Pharmacother. 2018;103:1194–1201.
- Yang D, Zhan M, Chen T, et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci Rep. 2017;7:43109.
- Zhu Y, Zhang S, Li Z, et al. miR-125b-5p and miR-99a-5p downregulate human gammadelta T-cell activation and cytotoxicity. Cell Mol Immunol. 2018;16:112–125.
- Z H C, W T W, Huang W, et al. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 2017;24(2):212–224.
- J K K, H M L, K S P, et al. MIR144* inhibits antimicrobial responses against Mycobacterium tuberculosis in human monocytes and macrophages by targeting the autophagy protein DRAM2. Autophagy. 2017;13(2):423–441.
- Bai S, Tian B, Li A, et al. MicroRNA-125b promotes tumor growth and suppresses apoptosis by targeting DRAM2 in retinoblastoma. Eye (Lond). 2016;30(12):1630–1638.
