ABSTRACT
Hepatocellular carcinoma (HCC) is the most common primary liver cancer. Sirtuins are NAD+-dependent histone deacetylases that regulate many cellular processes such as proliferation, apoptosis, and metabolism. SIRT (silent information regulator)-1, 5, 6 and 7, members of the mammalian Sirtuin family of proteins (SIRT1–SIRT7), are involved in carcinogenesis, prognosis, metastasis, and chemical resistant of HCC. These proteins act through the deacetylation of tumor suppressor or oncogenic factors. MicroRNAs (miRNAs) are a group of small non-coding RNAs that down regulate gene expression by targeting the 3'-untranslated region of miRNAs. MiRNAs can function as tumor suppressors or as oncogenes and are involved in progression, differentiation, apoptosis and drug resistance of tumor cells. The focus of this review is to delineate the relationship between some microRNAs and their target, Sirtuins, and to present an overview of their function in HCC as currently understood.
Introduction
Hepatocellular carcinoma
Cancer is the second leading cause of mortality throughout the world [Citation1], behind only ischemic heart disease and stroke (World Health Organization [WHO]). Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide [Citation2]. The incidence of HCC is highest in Asia and Africa and HCC occurs most often in conjunction with chronic liver disease, such as cirrhosis caused by hepatitis B or C infection. Hepatocellular carcinoma is more common in people who drink large amounts of alcohol and/or who have fatty livers [Citation3]. HCC is estimated to be responsible for 750,000 deaths per year, i.e. 9% of all cancer deaths [Citation4]. Inflammation, necrosis, fibrosis, and continuous regeneration characterize the cirrhotic liver and contribute to HCC. Other risk factors include food contaminated with aflatoxin B1, aging, and obesity. Treatment with a combination of chemotherapy and aggressive surgery has greatly improved the prognosis of HCC patients over the last few decades and can lead to increased survival [Citation5]. Thus, there remains investigation of novel diagnostic biomarkers and therapeutic targets for HCC.
Sirtuin
Silent information regulator 2 (SIR2) proteins- Sirtuins- are a family of histone deacetylases (HDACS) that catalyze the deacetylation of both histone and none histone lysine residues. In mammals, there are 7 homologs of SIR 2 termed Sirtuins (SIRT1-SIRT7). All seven require cellular nicotinamide adenosine dinucleotide (NAD+) as a cofactor for deacetylation reactivity [Citation6,Citation7]. They are characterized by a conserved 275-amino acid catalytic core and specifically added N-terminal and/or C-terminal sequences of diverse lengths [Citation8]. Sirtuins are implicated in the control of different biological processes including metabolism, cell cycle, stress responses, DNA repair, senescence and apoptosis. The significance of Sirtuins is showed by their roles in several major human pathologic conditions, such as diabetes, cancer, neurodegenerative and cardiovascular diseases [Citation9].
Sirtuins are widely expressed in the hepatoma cells and several mechanisms involved in dysregulation of them have been proposed in cancer. Sirtuins can serve as either a tumor promoter or tumor suppressor, depending on the carcinogenic pathway. Silencing of these proteins has been shown to suppress cell proliferation and lead to apoptosis and cycle arrest [Citation10]. In sum, Sirtuins may be a promising target in HCC therapy [Citation11].
MicroRNAs
MicroRNAs (miRNAs/miRs) are a group of endogenous RNAs of 21–25 nucleotides in length. They exert their functions in a variety of biological processes by integrating into the RNA-inducing silencing complex of their target mRNA where they attach to the 3'-untranslated regions of genes and inhibit mRNA translation or induce its degradation [Citation12]. MiRNA regulation of gene expression plays a role in progression, differentiation, apoptosis and drug resistance of tumor cells. Evidence indicates that miRNAs can function either as tumor suppressors or tumor promoters by down-regulating oncogenic targets, and negatively regulating oncosuppressor proteins [Citation1]. Abnormally expressed miRNAs are reported to be closely correlated with the incidence and development of HCC. Because of their small size and secondary structure, mature mRNAs are highly stable, enhancing their utility as biomarkers and indicators for diagnosis [Citation13]. It is important to clarify the function of microRNAs in tumor progression and pathogenesis. Furthermore, miRNAs-interference may be useful in the treatment of HCC.
MiR-34a
Cell migration and invasion are significant biological processes related to tumor metastasis. Some studies have illustrated that miR-34a acts as a tumor suppressor. MiR-34a was down-regulated in HCC cells which were involved in the venous metastasis of Hepatitis B virus-positive HCC [Citation14]. Additionally, the protein and mRNA levels of SIRT1 were overexpressed in HCC cells. On the other hand, the overexpression of SIRT1 promoted HCC metastasis by the epithelial-mesenchymal transition (EMT) (). EMT is a process by which epithelial cells gain invasive properties and metastasize [Citation13]. SIRT1 may function as an oncogene and is capable of reducing the ability of p53 to promote cell cycle arrest and/or attenuate cellular apoptosis. SIRT1 appears to bind directly to the p53 protein C-terminal Lys 382 residue and thus negatively regulates the acetylation level and transcriptional activity of p53 protein [Citation15].
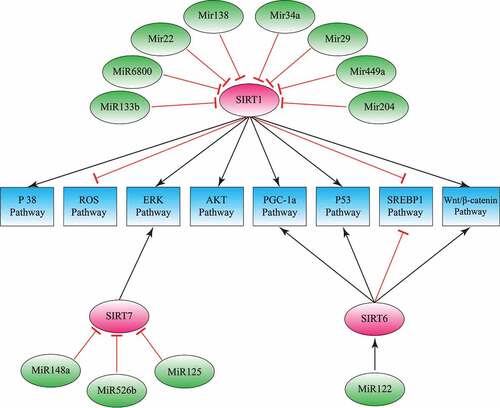
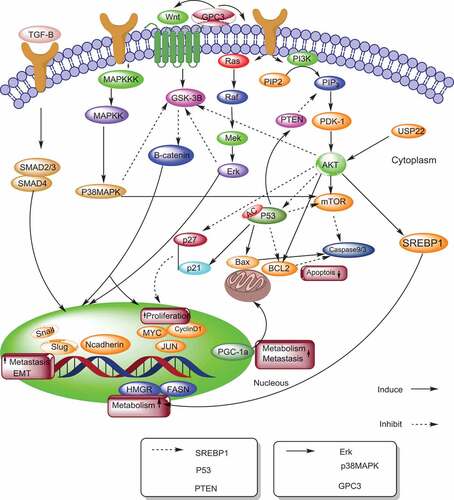
Figure 1. The mechanisms of sirtuins in hepatocellular carcinoma. This model illustrates sirtuins and their upstream and downstream pathways involved in hepatocellular carcinoma. SREBP (Sterol regulatory element binding protein), FASN (Fatty acid synthase), HMGCR (3-hydroxy-3-methylglutaryl CoA reductase), EMT (Epithelial-to mesenchymal transition), PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), CDK (cyclin-dependent kinase) PI3K (phosphatidylinositol 3 kinase), TGF-β (Transforming growth factor-beta),GPC3, (Glypican-3), ERK (Extracellular signal-regulated kinases), USP22 (upregulated ubiquitin-specific peptidase 22), PDK-1(phosphoinositide-dependent protein kinase-1)
SIRT1 expression is down-regulated by the overexpression of miR-34a, showing that miR-34a may inhibit HCC metastasis via the downregulation of SIRT1, suppressing cell migration and invasion [Citation13]. Therefore, the application of miR-34a inducers may have a potential role in HCC metastasis, diagnosis and treatment [Citation14].
MiR-449
It is now realized that cancer is no longer induced only through genetic and genomic alterations, but it is as well as related to lipid metabolism. In addition, modified lipid metabolism has been linked to HCC pathogenesis. Scholars believe that the de novo fatty acid synthesis plays a key role in tumor progression. Blocking abnormal cholesterogenesis and lipogenesis is a promising therapeutic approach for the prevention or treatment of malignancy [Citation16]. Sterol regulatory element-binding protein (SREBP)-1 c is an oncogene transcription factor.
Dysregulation of SREBPs and two downstream target Fatty acid synthase (FASN), genes and 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) are associated with cholesterogenesis and lipogenesis. It has been reported that these enzymes are involved in the progression of cancer [Citation17–19].
SIRT1 regulates mitochondrial biogenesis, modulates fat accumulation and activates fatty acid oxidation. SIRT1 can cause unlimited cell growth through repressing the transcription of cyclin-dependent kinase (CDK) inhibitors, allowing continued cellular proliferation. CDKs are a family of kinases which moves cell from a resting state to cell division. These findings support the suggestion of oncogenic SIRT1 involvement in hepatocellular malignant proliferation and transformation [Citation20]. SREBP-1 c deacetylation by SIRT1 prevents SREBP-1 c activity and decreases its stability [Citation21].
MiRNAs have been reported to regulate multiple biological processes like metabolism [Citation22]. The miR- 449 is a potent inducer of cell death, cell cycle arrest and cell differentiation. MiR-449 down regulates SIRT1 and functions as a tumor suppressor. In fact, miR-449 downregulates SREBP-1 c and their downstream modulated genes such as HMGCR and FASN. It also inhibited cell proliferation, mitotic entry and DNA synthesis, through reduced expression of SIRT1, the levels of cholesterol and fatty acid in hepatoma cells. Restoration of the newly identified miR-449 has been explored to be a promising target for HCC therapy [Citation20].
MiR-22
Overexpression of miR-22 has anti-proliferative and anticancer effects in various cancer cells. The decreased miR-22 level is considered to be responsible for elevated tumorigenicity, development, and metastasis. Cellular death may be mediated by ROS production [Citation23–26]. SIRT1 was reported to regulate the cellular antioxidants, such as superoxide dismutase (SOD), to maintain the reactive oxygen species (ROS) at physiological levels [Citation27]. SIRT1 expression is high in the tumor cells and it increases the expression of intracellular antioxidants such as SOD, thereby maintaining the pro-proliferative state and anti-apoptotic. SIRT1 was also shown to be responsible for cancer cell survival and drug resistance. Several studies demonstrated that SIRT1 decreases oxidative stress by directly deacetylating several transcription factors that regulate antioxidant genes expression. SIRT1 maintains mitochondrial potential by activating glutathione peroxidase (GPx1), catalase, and SOD. Decreased SIRT1 expression by several chemotherapeutic drugs was reported to induce the cell death and inhibit tumor growth inhibition [Citation24]. SIRT1 is a direct target of miR-22. MiR-22 down regulates the SIRT1 levels. B-cell lymphoma 2 (Bcl-2) is an apoptosis suppressor gene [Citation28]. Overexpression of this protein in cancer cells may delay or block apoptosis. It was proved that down-regulation of SIRT1 by miR-22 caused in prevention of bcl2 expression, while enhancement the expression of caspase 9, cytochrome c and caspase 3 leads to apoptosis in HCC [Citation24].
Both Glycogen synthase kinase-3 (Gsk-3) and phosphatase and tensin homolog (PTEN) are tumor suppressor genes. In HCC, the tumor suppressor PTEN, a phosphatase, is frequently inactivated to enable the function of phosphatidylinositol 3 kinase (PI3K). Then, PI3K phosphorylates and activates AKT with the assistance of phosphoinositide-dependent protein kinase-1(PDK-1). Overexpression of SIRT1 deacetylates PTEN and stop its activity, thereby triggers PTEN/PI3K/AKT-induced mitotic entry, growth, and proliferation of HCC cells [Citation29].
MiR-22-mediated SIRT1 downregulation results in decreased p-Akt and β-catenin expression, and increased gsk-3 and PTEN expression. In conclusion, these events may lead to reduced cell proliferation and raised apoptosis resulted in hepatic tumor suppression [Citation24].
Moreover, some surveys outlined that miR-22, as a tumor suppressor gene, could negatively regulate cluster of differentiation 147 (CD147), ezrin, galectin-9 and in hepatocellular carcinoma [Citation30–33].Study of Lou et al showed that overexpression of miR-22 could remarkably suppress the HCC cell proliferation, migration and invasion [Citation30]. Moreover, miRNA-22 decreased the expression of CD147 by binding specific target site within the CD147 3'UTR. CD147 is a transmembrane glycoprotein was shown highly expressed in various type of cancer which take parts in cancer cells growth and proliferation [Citation34]. Compounds which induce production of miRNA-22 such as Waltonitone or those mimic its function showed promising results for treatment of HCC [Citation35].
MiR-138
There have been miRNAs known as metasta-miRs that are involved in the metastatic processes such as miR-138. It has been shown that miR-138 expression was at a low level while the expression of SIRT1 mRNA was at a high level in hepatocellular carcinoma tissues and cell lines. Moreover, SIRT1 is highly expressed while miR-138 expression significantly reduced in cancer cell lines such as Bel7404, HepG2, HCCM3 and SMMC7721 compared to the normal hepatic cell line L02 [Citation5].
MiR-138 is confirmed to play an essential role as a tumor suppressor in hepatocellular carcinoma development and metastasis [Citation36]. SIRT1 has been identified as an independent prognostic indicator of metastasis formation and metastasis-free survival. It should be noted that the up-regulation of miR-138 expression in HCC cells effectively down-regulates SIRT1 expression. In conclusion, there appears to be a direct link between miR-138 and SIRT1. Using a luciferase reporter assay showed that miR-138 directly binds to 3' UTR region of the SIRT1 gene and represses it in HCC. Moreover, restoration of miR-138 expression is involved in the clinical management of hepatocellular carcinoma [Citation5].
MiR-6800
Long noncoding RNAs (lncRNAs) are a class of transcripts longer than 200 nucleotides without or limited protein-coding potential. HULC is the first identified lncRNA that is specifically overexpressed in human HCC tissues. Studies have revealed that HULC has a critical role in liver oncogenesis and acts as a carcinogenic lncRNA [Citation37].
Autophagy is thought to play a dual role in HCC. It is involved in tumorigenesis and tumor suppression. The protective autophagy has been proved to be a potential mechanism for chemoresistance in cancer cells [Citation38].
Upregulated ubiquitin-specific peptidase 22 (USP22), a member of deubiquitinases (DUBs), can catalyze the removal of ubiquitin from target proteins. USP22 participates in the deubiquitination of NAD+-dependent protein deacetylase SIRT1. A growing body of evidence has shown that USP22 is involved in oncogenesis associated with poor prognosis in a variety of cancers including HCC. LncRNA HULC has been shown to dramatically upregulate USP22. USP22 has a pivotal role in mediating the HULC-induced deubiquitination and stabilization of SIRT1 in HCC cells [Citation39].
Moreover, the mechanism by which HULC elevates SIRT1 protein is mediated by the ‘three miRNAs (miR-6825-5p, miR-6845-5p and miR-6886-3p)-USP22' pathway. In fact, HULC inhibited the expression and activity of these three novel miRNAs (miR-6825-5p, miR-6845-5p and miR-6886-3p) through targeting USP22 3'-UTR directly. USP22 may reduce levels of p53 through SIRT1 stabilization to suppress cell apoptosis. USP22 binds to SIRT1 and activates the AKT pathway, enhancement the chemo-resistance in HCC. In summary, HULC decreases the expression and activities of the three miRNAs (miR-6825-5p, miR-6845-5p and miR-6886-3p), which increases the level of USP22 protein, elevates the deubiquitination of SIRT1 which ultimately triggers the autophagy of HCC cells via enhancing SIRT1-mediated deacetylation of Atg5 and Atg7. This pathway attenuates the chemosensitivity of HCC cells. These findings provide a novel target for developing a sensitizing strategy for HCC chemotherapy [Citation40].
MiR-204
MiR-204-5p can promote or suppress cancer progression. Reports showing low levels of miR-204-5P expression in HCC suggest that MiR-20405p may act as a tumor suppressor in HCC. The expression level of miR-204 in HCC tissues is associated with large tumor size, the number of tumors and the advanced TNM stage [Citation41].
LncRNAs, contribute to miRNA degradation and may inhibit their activities [Citation42]. Metastatic-associated lung adenocarcinoma transcript1 (MALT1), a conserved lncRNA in mammals, is highly expressed in HCC tissues and promotes cell migration and invasion. MALT1 acts as a sponge RNA for miR-204 [Citation43].
Since SIRT1 is a putative target of miR-204-5P, SIRT1 could increase the proto-oncogene factor, YAP, and mitogen-activated protein kinase kinase 3 (MKK3) expression via p38 signal pathway in HCC and its overexpression is associated with cancer metastasis and EMT [Citation41]. It has been shown that overexpression of miR-204-5p causes significant down-regulation of both SIRT1 mRNA and protein in HCC cells. MicroRNA-204-5p by targeting the SIRT1, inhibits the invasion, suppresses survival, and stimulated apoptosis of HCC cells.
MALT1 and SIRT1 are bound to the same site on miR-204. Therefore, MALT1 may compete with SIRT1 for binding to miR-204, and thereby promote HCC migration/invasion by sponging miR-204. MALT1 prevents the binding of SIRT1 to microRNA [Citation44]. Furthermore, miR-204 induces apoptosis by down-regulation of Bcl-2 [Citation45]. Ultimately, miR-204 may represent a diagnostic marker and a therapeutic target in HCC treatment through the application of miR-204 inducers and SIRT1 inhibitors [Citation44].
MiR-133b
MiR-133b is notably reduced in HCC where it acts primarily as a suppressor [Citation46]. The overexpression of miR-133b suppresses SIRT1 expression. Furthermore, miR-133b overexpression results in the induction of apoptosis and inhibition of development and invasion in HCC cells. SIRT1 is a direct functional target of miR-133b in HCC and the overexpression of miR133b suppresses SIRT1 expression. SIRT1 overexpression is frequently observed in HCC [Citation47].
Glypican-3 (GPC3) is the most highly overexpressed membrane-bound protein in HCC. It plays a significant role in the signaling pathway involved in hepatocarcinogenesis. GPC3 is a diagnostic marker and predicts poor prognosis. SIRT1 downregulation could attenuate GPC3 mRNA expression and anti-apoptotic proteins [Myeloid cell leukemia 1 (Mcl-1), B-cell lymphoma-extra large (BCL-xL) and Bcl-2)] expression and at the same time enhance E-cadherin mRNA expression [Citation48]. GPC3 also promotes EMT by Extracellular signal-regulated kinases (ERK) pathway [Citation49]. GPC3 interacts with Wnt ligands acting on the canonical pathway to stimulate cell proliferation and migration in HCC. Downregulation of GPC3 decreased the cytosolic accumulation and nuclear translocation of the transcription factor β-catenin while increasing E-cadherin expression. MiR-133b up-regulation reduces cell progression and invasion. It induces apoptosis by suppressing SIRT1. The decline in SIRT1 results in the down-regulation of GPC3, which prevents the activation of Wnt/β-catenin pathway. In conclusion, MiR-133b induced suppresses cell growth, cell apoptosis and migration by prevention the SIRT1-GPC3-Wnt/beta-catenin pathway suggesting that MiR-133b inducers may have a potential therapeutic role in HCC treatment [Citation47].
MiR-29
MiR-29a is efficiently down-regulated in HCC. A lower miR-29a expression is correlated with increased vascular invasion and tumor size. So, a poor disease-free survival (DFS) and overall survival (OS) time for HCC patients have been related to lower miR29a. Several studies have shown that overexpression of miR-29a effectively suppresses cell proliferation, cell colony-forming ability, cell cycle progression, and exhibits tumor-suppressing effects for HCC. MiR-29a suppresses cell proliferation in hepatocellular carcinoma. Most HCC develop in cirrhotic livers, a microenvironment where fibrotic tissue replaces parenchymal cells [Citation50]. Transforming growth factor-beta (TGF-β) acts as a tumor suppressor in the initial stage of cancer and as a tumor oncogene in late phases. MiR- 29a has been reported to regulate TGF-β-induced EMT by affecting DNA methylation thus promoting HCC progression [Citation51].
Overexpression of SIRT1 promotes tumorigenesis of hepatocellular carcinoma by PI3K/PTEN/AKT signaling pathway [Citation52]. The energy levels of the tumorigenic metabolism are maintained through elevated mitochondrial biogenesis and are controlled via peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1 α). SIRT1 facilitates hepatocellular carcinoma metastasis by promoting peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α)-mediated mitochondrial biogenesis [Citation33]. Other studies suggested that overexpression of SIRT1 promotes metastasis through EMT in hepatocellular carcinoma. It is demonstrated that SIRT1 is a direct target of miR-29a. MiR-29a overexpression can reduce SIRT1 mRNA and protein expression levels. Overall, miR-29a overexpression suppressed cell proliferation by downregulating SIRT1 in HCC. Thus, these results may provide information for exploring novel therapeutic methods for HCC [Citation50].
P21, a potent inhibitor of CDKs, acts through prevention cell cycle progression and by blocking the transition from cell growth (G1) to the DNA synthesis or S phase. Indeed, increased p21 levels have been suggested to prevent cell growth by inducing cell cycle arrest at the G1 or growth phase. Aberrant SIRT1 overexpression is demonstrated in HCC [Citation53]. SIRT1 hyperactivity suppresses cell cycle checkpoints, including p15, p21 and p27, and modulates the activity of cyclins to foster G1/S transition of liver cancer cells. SIRT1 suppression leads to hypophosphorylation of pRb, which inactivates elevated eukaryotic elongation factor 2 (E2F)/DP1 target gene transcription, and thereby caused a significant increase of HCC cells to remain in the G1/S phase [Citation51]. Thus, it has been reported that SIRT1 inactivation suppresses in vitro cell growth of liver cancer cells. These results support the suggestion of oncogenic SIRT1 involvement in hepatocellular malignant proliferation and transformation. Notably, miR-29 c expression was significantly down-regulated in HCC cells, and the lower expression of miR-29 c was correlated with poor prognosis in HCC [Citation54]. It also has been shown that miR-29 c acts as tumor suppressor miRNAs by targeting Bcl-2 and Mcl-1 [Citation51]. Evidence is mounting to suggest that miR-29 c is an endogenous regulator of SIRT1 in HCC cells. Some findings indicated that miR29c functions as a tumor suppressor via directly suppressing oncogenic SIRT1 in both normal liver cells and HCC cells. On the other hand, ectopic expression of miR-29 c resulted in the recovery of the activity of negative cell cycle regulators while suppressing cyclins and CDKs, thereby inhibiting in vitro tumor proliferation in HCC cells. These data suggest a potential role for miR-29 c in HCC carcinogenesis. MiR-29 c may have potential therapeutic value for the treatment of liver cancer [Citation54].
MiR-299-3p
MiR-299-3p plays a critical role in cancer development through regulating tumor cell growth, migration, cell cycle, invasion, cell sensibility and apoptosis. SIRT5 is a downstream target of miR-299-3p in HCC cells. MiR-299-3p under-expression has been shown to occur both in HCC tissues and cell lines [Citation55]. The decreased level of miR-299-3p is closely associated with large tumor size, the presence of venous infiltration, advanced Edmondson-Steiner grading, high TNM stage and poorer prognosis.
Previous studies have reported that SIRT5 is downregulated in HCC and acts as an anti-oncogene in HCC. While some other studies showed that SIRT5 is increased in HCC and promotes HCC progression. SIRT5 has been reported to mediate the impact of miR-299-3p on migration, invasion, and proliferation of HCC cells. It appears that overexpressed miR-299-3p inhibits HCC cell migration, invasion, and proliferation by downregulating SIRT5. MiR-299-3P may be a potential tumor biomarker as well as a prognosis indicator. MiR-299-3P also may be a useful therapeutic target in HCC [Citation56].
MiR-122
MiR-122 is one of the first examples of a tissue-specific miRNA. It plays a major role in liver function, cholesterol and fatty acid synthesis, and β-oxidation. Inhibition of miR-122 results in a reduction of SREBP1 and SREBP2 [Citation57]. MiR-122 is a tumor suppressor that is downregulated in HCC cells. It also promotes apoptosis of HCC cells through repression of bcl2 levels HCC [Citation53].
SIRT6 has been shown to have a major role in metabolism and liver cells [Citation58]. SIRT6 is a tumor suppressor and expression of SIRT6 reduces the levels of SREBP1 and SREBP2. The Warburg effect or aerobic glycolysis is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen [Citation26,Citation59–62]. Hence, a high rate of glucose metabolism is required to meet increased energy needs to support rapid tumor progression. SREBP1 protects tumor cells via enhancing aerobic glycolytic activity [Citation57]. SIRT6 dampen cancer progression by inhibition of this glycolytic activity. SIRT6 also up-regulates bcl-2 and phosphorylates the ERK pathway which can activate a variety of target molecules including cyclin D1, thus promoting HCC [Citation63].
Interestingly, MiR-122 and SIRT6 take part in many similar pathways but act in opposite directions. Upregulation of MiR-122 and SIRT6 reduces the genes α fetoprotein (AFP), GPC3, insulin growth factor 2 (IGF2) and H19. All of these genes are involved in cancer initiation. MiR-122 and SIRT6 repress each other. SIRT6 downregulates miR-122 by deacetylating H3K56 in the promoter region. On the other hand, miR-122 binds to three sites on the SIRT6 3'UTR and reduces its level. The correlation between these two markers is associated with survival. Studies have shown that patients with low survival rates display a negative correlation [Citation57]. Therefore, these findings suggest that this regulatory mechanism may be involved in controlling various aspects of liver function and could serve as a prognostic biomarker.
MiR-526b
MiR-526b is a miRNAs associated with pregnancy and locates on chromosome 19q13.42 [Citation64]. Under-expression of miR-526b is related to venous infiltration, large tumor size and advanced tumor stage. More importantly, a low miR-526b level independently predicts a significantly shorter overall survival of HCC patients. SIRT7 exhibits an oncogenic role by promoting proliferation and cell cycle progression in vitro and facilitating in vivo growth of HCC [Citation65]. The ERK pathway plays an important role in promoting HCC [Citation40]. ERK can upregulate Cyclin D1, c-Myc, SNAIL-related zinc-finger transcription factor (SLUG), c-Jun and SNAIL expression, all proto-oncogenes which contribute to tumor recurrence, drug resistance, metastasis, and cancer progression [Citation66]. Several studies have suggested that miR526b can inactivate the ERK pathway and its downstream targets [Citation67]. Restoration of SIRT7 reversed miR-526b-induced downregulation of MEK and p-ERK. Mechanistically, it appears that miR-526b binds to 3'UTR of SIRT7 mRNA and represses its expression. MiR526b and SIRT7 have been reported to have a negative correlation in HCC tissue. There is growing evidence that miR-526b inhibits growth, migration, and invasion of HCC cells, and reduces tumor proliferation and metastasis via inactivation of the ERK pathway through suppressing SIRT7. Therefore, miR-526b may have the potential to act as a biomarker for the prognosis of HCC patients [Citation65].
MiR-125
MiR-125a-5p (miR-125a) is involved in the hepatocyte/hepatitis B virus interaction, with hepatitis B being an important risk factor for HCC development. MiR-125a has been shown to interfere with the expression of the hepatitis B virus surface antigen thus limiting viral replication. MiR-125a as a functional target is frequently down-regulated in HCC [Citation4]. SIRT7 is a direct target of miR-125a which and its up-regulation in HCC is attenuated by miR-125a [Citation68].
SIRT7 prevents transcriptional activation of p21 and activates cyclin D1 in liver cells. These events may exert a potent mitotic stimulation causing unlimited cell proliferation during HCC progression. Furthermore, SIRT7 inactivation suppresses ectopic protein expression. Decreased protein synthesis and increased autophagic cell death occur during HCC tumorigenesis [Citation69]. MiR-125a contributes to anti-proliferative pathways because it attenuates the downregulation of p21 expression by potentiating cell cycle arrest. MiR-125a acts through the downregulation of sirtuin-7 (SIRT7), which leads to the prevention of cell proliferation, angiogenesis, and cell migration. There are several reports that the expression of miR-125b is abnormal in a variety of tumors and a significant reduction of miR-125b is observed in HCC tissue and cell line [Citation68]. SIRT7 plays a vital role in the progression of human cancer. It is a direct target of miR-125b and is suppressed by this miRNA [Citation70].
MiR-125b functions through regulating the expression of SIRT7, which subsequently suppresses tumor cell development and invasion in HCC. MiR-125b acts as a tumor suppressor gene in HCC by regulating its target gene, SIRT7 [Citation69].
MiR-148a
MiR-148a has been shown to control cholesterol and triglyceride homeostasis and circulating lipoprotein levels, in addition to hepatocytic differentiation and the pathogenesis of HCC [Citation71]. MiR-148a is downregulated in HCC and is correlated with poor prognosis. Numerous studies have exhibited that miR-148a acts as a tumor suppressor function in human HCC and suppresses growth, EMT, metastasis and invasion by inhibition of various oncoprotein signaling pathways [Citation72]. Deletion of miR148a elevated expression of several important factors involved in hepatic lipid metabolic transcriptional regulation, like SIRT7. So, miR-148a may represent a promising candidate for miRNA replacement therapy in HCC through the down-regulation of SIRT7 [Citation14]. . summarized various miRNAs and SIRTs and their downstream signaling involved in the modulation of HCC development.
Table 1. MicroRNAs and SIRTs: The mechanisms involved in the modulation of HCC development
Conclusion
Sirtuins are involved in carcinogenesis and metastasis of HCC. They affect HCC cells proliferation, differentiation, and chemotherapy resistance. MiRNAs directly target sirtuins and inverse the effects of Sirtuins on HCC tumorigenesis by different downstream signaling pathways (). Although Sirtuin regulate diverse cellular processes, the net effect of their activity has been well investigated in HCC. For example, overexpression of SIRT1 has been shown in both cancer cell lines and patients with HCC. So, directly targeting SIRT1, combining conventional chemotherapy with SIRT1 inhibitors, or upregulating tumor-suppressive miRNAs may improve therapeutic efficacy and patient outcomes. Sirtuins and miRNAs may be helpful as a prognostic indicator and therapeutic targets for HCC. For instance, sorafenib, an antitumor medicine, exerts its function by induction of miR-125a-5p. Also, butyrate, a short-chain fatty acid, induced apoptosis by overexpression of miR-22 and followed by downregulation of SIRT1. Other studies also reported that inhibition of SIRT1 in HCC cells by knockdown with shRNA or with the selective inhibitor can impair tumor cell growth in vitro and in vivo. Consequently, as a new strategy for HCC treatment, we can consider designing the next-generation drug based on targeting miRNAs and SIRTs. As a matter of fact, identifying medicines inducing and inhibiting miRNAs and Sirtuins, respectively, may be applied to evaluate their efficacy in HCC. Undoubtedly, a better understanding of the mechanism(s) by which miRNAs affect Sirtuins may enable the development of more new medicines and compounds based on this understanding.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Karbasforooshan H, Roohbakhsh A, Karimi G. SIRT1 and microRNAs: the role in breast, lung and prostate cancers. Exp Cell Res. 2018;367(1):1–6.
- Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):448–458.
- Herold C, Reck T, Fischler P, et al. Prognosis of a large cohort of patients with hepatocellular carcinoma in a single European centre. Liver. 2002;22(1):23–28.
- Coppola N, de Stefano G, Panella M, et al. Lowered expression of microRNA-125a-5p in human hepatocellular carcinoma and up-regulation of its oncogenic targets sirtuin-7, matrix metalloproteinase-11, and c-Raf. Oncotarget. 2017;8(15):25289.
- Luo J, Chen P, Xie W, et al. MicroRNA-138 inhibits cell proliferation in hepatocellular carcinoma by targeting Sirt1. Oncol Rep. 2017;38(2):1067–1074.
- Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomed Pharmacother. 2017;90:386–392.
- Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic retinopathy. Biomed Pharmacother. 2018;97:190–194.
- Pan M, Yuan H, Brent M, et al. SIRT1 contains N- and C-terminal regions that potentiate deacetylase activity. J Biol Chem. 2012;287(4):2468–2476.
- Bosch-Presegué L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011;2(6):648–662.
- Chen J, Zhang B, Wong N, et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011;71(12):4138–4149.
- Elhanati S, Ben-Hamo R, Kanfi Y, et al. Reciprocal regulation between SIRT6 and miR-122 controls liver metabolism and predicts hepatocarcinoma prognosis. Cell Rep. 2016;14(2):234–242.
- Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3(3):83–92.
- Zhou J, Zhou W, Kong F, et al. microRNA-34a overexpression inhibits cell migration and invasion via regulating SIRT1 in hepatocellular carcinoma. Oncol Lett. 2017;14(6):6950–6954.
- Cheng L, Zhu Y, Han H, et al. MicroRNA-148a deficiency promotes hepatic lipid metabolism and hepatocarcinogenesis in mice. Cell Death Dis. 2017;8(7):e2916–e2916.
- Prives C, Manley JL. Why is p53 acetylated? Cell. 2001;107(7):815–818.
- Qin H, Ruan ZH. The role of monoacylglycerol lipase (MAGL) in the cancer progress. Cell Biochem Biophys. 2014 Sept;70(1):33–36.
- Cassim S, Raymond V-A, Dehbidi-Assadzadeh L, et al. Metabolic reprogramming enables hepatocarcinoma cells to efficiently adapt and survive to a nutrient-restricted microenvironment. Cell Cycle. 2018;17(7):903–916.
- Truman J-P, García-Barros M, Obeid LM, et al. Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochim Biophys Acta. 2014;1841(8):1174–1188.
- Shamir Cassim V-AR, Lacoste B, Lapierre P, et al. Metabolite profiling identifies a signature of tumorigenicity in hepatocellular carcinoma. Oncotarget. 2018;9(42):26868.
- Zhang H, Feng Z, Huang R, et al. MicroRNA-449 suppresses proliferation of hepatoma cell lines through blockade lipid metabolic pathway related to SIRT1. Int J Oncol. 2014;45(5):2143–2152.
- Huang W-C, Li X, Liu J, et al. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res. 2012;10(1):133–142.
- Chen B, Li H, Zeng X, et al. Roles of microRNA on cancer cell metabolism. J Transl Med. 2012;10(1):228.
- Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006 Sept 1;10(3):175–176.
- Pant K, Yadav AK, Gupta P, et al. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017 Aug 1;12:340–349.
- Galadari S, Rahman A, Pallichankandy S, et al. Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radic Biol Med. 2017;104:144–164.
- Cassim S, Vučetić M, Ždralević M, et al. Warburg and beyond: the power of mitochondrial metabolism to collaborate or replace fermentative glycolysis in cancer. Cancers (Basel). 2020;12(5):1119.
- Cheng Y, Takeuchi H, Sonobe Y, et al. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. J Neuroimmunol. 2014;269(1–2):38–43.
- Yang L, Sui W, Li Y, et al. Substance P inhibits hyperosmotic stress-induced apoptosis in corneal epithelial cells through the mechanism of akt activation and reactive oxygen species scavenging via the neurokinin-1 receptor. PLoS One. 2016;11(2):e0149865–e0149865.
- McCubrey JA, Steelman LS, Bertrand FE, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5(10):2881–2911.
- Luo L-J, Zhang L-P, Duan C-Y, et al. The inhibition role of miR-22 in hepatocellular carcinoma cell migration and invasion via targeting CD147. Cancer Cell Int. 2017 Feb 2;17(1):17.
- Zhou L, He J, Zhang Y. MicroRNA-22 expression in hepatocellular carcinoma and its correlation with ezrin protein. J Int Med Res. 2013;41(4):1009–1016.
- Chen S, Pu J, Bai J, et al. EZH2 promotes hepatocellular carcinoma progression through modulating miR-22/galectin-9 axis. J Exp Clin Cancer Res. 2018;37(1):1–12.
- Chen M, Hu W, Xiong C-L, et al. miR-22 targets YWHAZ to inhibit metastasis of hepatocellular carcinoma and its down-regulation predicts a poor survival. Oncotarget. 2016;7(49):80751.
- Weidle UH, Scheuer W, Eggle D, et al. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010 May-June;7(3):157–169.
- Yang F, Gong J, Wang G, et al. Waltonitone inhibits proliferation of hepatoma cells and tumorigenesis via FXR-miR-22-CCNA2 signaling pathway. Oncotarget. 2016;7(46):75165–75175.
- Wang W, Zhao L-J, Tan Y-X, et al. MiR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis. 2012;33(5):1113–1120.
- Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46.
- Shteingauz A, Boyango I, Naroditsky I, et al. Heparanase enhances tumor growth and chemoresistance by promoting autophagy. Cancer Res. 2015;75(18):3946–3957.
- Lin Z, Yang H, Kong Q, et al. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol Cell. 2012;46(4):484–494.
- Xiong H, Ni Z, He J, et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017 June 22;36(25):3528–3540.
- Jiang G, Wen L, Zheng H, et al. miR‐204‐5p targeting SIRT1 regulates hepatocellular carcinoma progression. Cell Biochem Funct. 2016;34(7):505–510.
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874.
- Yoshimoto R, Mayeda A, Yoshida M, et al. MALAT1 long non-coding RNA in cancer. Biochim Biophys Acta. 2016 Jan;1859(1):192–199.
- Hou Z, Xu X, Zhou L, et al. The long non-coding RNA MALAT1 promotes the migration and invasion of hepatocellular carcinoma by sponging miR-204 and releasing SIRT1. Tumour Biol. 2017;39(7):1010428317718135.
- Lima RT, Busacca S, Almeida GM, et al. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011;47(2):163–174.
- Guo L, Bai H, Zou D, et al. The role of microRNA-133b and its target gene FSCN1 in gastric cancer. J Exp Clin Cancer Res. 2014;33(1):99.
- Tian Z, Jiang H, Liu Y, et al. MicroRNA-133b inhibits hepatocellular carcinoma cell progression by targeting Sirt1. Exp Cell Res. 2016 May 1;343(2):135–147.
- Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle. 2008;7(18):2787–2790.
- Wu Y, Liu H, Weng H, et al. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int J Oncol. 2015 Mar;46(3):1275–1285.
- Zhang Y, Yang L, Wang S, et al. MiR-29a suppresses cell proliferation by targeting SIRT1 in hepatocellular carcinoma. Cancer Biomark. 2018;22(1):151–159.
- Xiong Y, Fang JH, Yun JP, et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010 Mar;51(3):836–845.
- Kogure T, Kondo Y, Kakazu E, et al. Involvement of mi RNA‐29a in epigenetic regulation of transforming growth factor‐β‐induced epithelial–mesenchymal transition in hepatocellular carcinoma. Hepatol Res. 2014;44(8):907–919.
- Rastogi N, Mishra DP. Therapeutic targeting of cancer cell cycle using proteasome inhibitors. Cell Div. 2012 Dec 26;7(1):26.
- Bae HJ, Noh JH, Kim JK, et al. MicroRNA-29c functions as a tumor suppressor by direct targeting oncogenic SIRT1 in hepatocellular carcinoma. Oncogene. 2014;33(20):2557–2567.
- Wang JY, Jiang JB, Li Y, et al. MicroRNA-299-3p suppresses proliferation and invasion by targeting VEGFA in human colon carcinoma. Biomed Pharmacother. 2017 Sept;93:1047–1054.
- Bringman-Rodenbarger LR, Guo AH, Lyssiotis CA, et al. Emerging roles for SIRT5 in metabolism and cancer. Antioxid Redox Signal. 2018;28(8):677–690.
- Dang S, Zhou J, Wang Z, et al. MiR-299-3p functions as a tumor suppressor via targeting Sirtuin 5 in hepatocellular carcinoma. Biomed Pharmacother. 2018;106:966–975.
- Marquardt JU, Fischer K, Baus K, et al. Sirtuin-6–dependent genetic and epigenetic alterations are associated with poor clinical outcome in hepatocellular carcinoma patients. Hepatology. 2013;58(3):1054–1064.
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324(5930):1029–1033.
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482.
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20.
- Cassim S, Pouyssegur J. Tumor microenvironment: a metabolic player that shapes the immune response. Int J Mol Sci. 2020;21(1):157.
- Lin CJ-F, Gong H-Y, Tseng H-C, et al. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375(3):315–320.
- Hromadnikova I, Kotlabova K, Ondrackova M, et al. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol. 2015;34(6):437–457.
- Liu X, Yang L, Tu J, et al. microRNA-526b servers as a prognostic factor and exhibits tumor suppressive property by targeting Sirtuin 7 in hepatocellular carcinoma. Oncotarget. 2017;8(50):87737–87749.
- Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013 Nov;58(5):1667–1680.
- Huang X-H, Jian W-H, Wu Z-F, et al. Small interfering RNA (siRNA)-mediated knockdown of macrophage migration inhibitory factor (MIF) suppressed cyclin D1 expression and hepatocellular carcinoma cell proliferation. Oncotarget. 2014;5(14):5570.
- Potenza N, Mosca N, Zappavigna S, et al. MicroRNA‐125a‐5p is a downstream effector of sorafenib in its antiproliferative activity toward human hepatocellular carcinoma cells. J Cell Physiol. 2017;232(7):1907–1913.
- Kim JK, Noh JH, Jung KH, et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR‐125a‐5p and MiR‐125b. Hepatology. 2013;57(3):1055–1067.
- Zhao L, Wang W. miR-125b suppresses the proliferation of hepatocellular carcinoma cells by targeting Sirtuin7. Int J Clin Exp Med. 2015;8(10):18469–18475.
- Goedeke L, Wagschal A, Fernández-Hernando C, et al. miRNA regulation of LDL-cholesterol metabolism. Biochim Biophys Acta. 2016;1861(12):2047–2052.
- Li L, Liu Y, Guo Y, et al. Regulatory MiR‐148a‐ACVR1/BMP circuit defines a cancer stem cell‐like aggressive subtype of hepatocellular carcinoma. Hepatology. 2015;61(2):574–584.