ABSTRACT
Microwave ablation (MWA) has been widely used in the treatment of solid tumors. Studies have been less conducted on the efficacy of MWA used with cell immunotherapy in treating hepatocellular carcinoma (HCC). The current study aimed at exploring the efficacy of MWA in combination with cell immunotherapy in treating HCC. Hepa1-6 HCC mice were treated by MWA, blockade, or the combined therapy (MWA used with blockade), or left untreated. Survival rates of the mice were plotted by Kaplan-Meier Curve, followed by log-rank test. 25 days after the operation, surviving mice were monitored for tumor recurrence, and tumor volumes were calculated every 5 days. Immunohistochemistry and flow cytometry were performed to detect the numbers of CD4+ and CD8+ cells in the tumors and spleens of mice. The expressions of related cytokines were detected and measured by ELISPOT and ELISA. The results showed that MWA combined with anti-PD-1/anti-CTLA-4 not only increased the survival time, protected the mice against tumor recurrence, but also enhanced the intratumoral infiltration of cytotoxic T lymphocyte and systemic T-cell immune responses induced by MWA through activation of synergistically specific antitumor immunity. In addition, the combined therapy increased T-helper 1 cell (Th1-type) cytokines, but reduced Th2-type cytokines, resulting in the polarization of Th1 cells. T-cell immune responses of HCC cells were activated by MWA. In addition, the combined therapy of MWA and anti-PD-1/anti-CTLA-4 induced Th1-type immune response, and showed specific antitumor immunity.
Introduction
Hepatocellular carcinoma (HCC) is a frequent primary liver cancer and a leading cause of cancer-related death around the world [Citation1], with increasing incidence [Citation2]. Evidence indicated that multiple risk factors, including viral hepatitis, toxin exposure, nonalcoholic fatty liver diseases, and alcoholic abuse, will lead to the occurrence of HCC [Citation3]. At present, surgical resection, liver implantation, and thermal ablation have been widely used in HCC treatment, and the use of thermal ablation has the features of causing minor trauma with high repeatability [Citation4]. However, the prognosis of HCC patients is still unsatisfactory, even after thermal ablation treatment [Citation5].
As a relatively novel strategy for HCC treatment, microwave ablation (MWA) was first introduced in 2008 [Citation6] for destroying a tumor through focal hyper-thermic injury [Citation7]. MWA method is a less invasive but convenient local treatment method with high safety [Citation8]. Compared with the frequently used thermal ablation technique radiofrequency ablation (RFA), MWA is a more recent technique with high performance [Citation9,Citation10], as it is an unresectable and non-metastatic pancreatic cancer treatment [Citation11]. Wei et al. showed that MWA is a safe and effective method for treating patients with oligometastatic non-small-cell lung cancer (NSCLC) [Citation8]. However, the specific mechanism and potential effects of MWA on HCC treatment is less discussed, thus, were determined in the current study.
In the fragments of tumor, pro-inflammatory signals produced by thermal ablation act as antigens to tumors and induce the generation of adaptive antitumor immunity [Citation12]. In a rat model, MWA induced T-cell immune response to osteosarcoma [Citation13]. Also, MWA-induced T-cell immune response has been discovered in HCC patients [Citation14]. However, tumor recurrence rate after MWA still high, and the prognosis is not satisfactory, suggesting that MWA alone could not meet the demands of cancer management, therefore, novel strategies on immunomodulation for enhancing antitumor immunity should be developed.
Programmed cell protein 1 (PD-1) is an immune check point that guards against autoimmunity and is a negative regulator of immune responses [Citation15]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a protein receptor functioning as an immune check point and reduces immune response [Citation16]. Studies demonstrated that inhibition of the expressions of PD-1 and CTLA-4 using anti-PD-1 or anti-CTLA-4 antibodies is one of the most promising therapeutic treatments for cancer patients [Citation17], and that anti-PD-1/anti-CTLA-4 antibody could cause durable antitumor activity in patients with melanoma [Citation18]. However, its efficacy in immune response of HCC patients is less reported.
MWA-induced immune responses in HCC remain poorly illustrated, therefore, the current research aimed to investigate whether MWA used with cell immunotherapy (anti-PD-1/anti-CTLA-4) had collaborative effects on HCC model in mice.
Materials and methods
Ethnical statement
All animal experiments were performed following the Guidelines of the China Council on Animal Care and Use. This study was approved by the Committee of Experimental Animals of the First Affiliated Hospital, Zhengzhou University (approval serial number: NK20190521). All possible efforts have been made to minimize pain and discomfort caused to the animals. The animal experiments were performed in the First Affiliated Hospital, Zhengzhou University.
Cell line
Murine hepatocellular carcinoma Hepa1-6 cells (catalog number: CRL-1830) and CT26 colon cancer cells (catalog number: CRL-2638) were purchased from American Type Culture Collection (Rockville, MD, USA). The cells were washed by phosphate-buffered saline (PBS), then cultured in RPMI-1640 medium (Sigma-Aldrich, Shanghai, China) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) and 50 IU/mL penicillin/streptomycin (Invitrogen, USA).
Animal models and microwave ablation
As previously described [Citation19,Citation20], the BALB/C mice (Guangdong Medical Laboratory Animal Center, Foshan, China) were used to establish the model in this study. For the tumor recurrence study, the Hepa1-6 cells (5 × 106) were suspended in 0.2 ml PBS and then injected via unilateral lumbar cord and rib into the BALB/C mice to for HCC model construction. The mice were sacrificed when the diameter of tumor was longer than 20 mm or when they became moribund during observation. The time of euthanization time was seen as the time of death.
To further assess the potential efficacy of MWA on the mice, the mice were assigned randomly to Untreated group (Untreated), Blockade group (Blockade), MWA group (MWA) and Combination group (Combination) (n = 10 for each group in survival analysis; n = 5 for each group in subsequent studies) after the treatment. The mice in the untreated group did not receive any treatment. For blockade group, the mice were injected with 2 mg/kg anti-PD-1 antibody (BE0033-2, mouse, Bio X Cell; West Lebanon, NH, USA) and 10 mg/kg anti-CTLA-4 antibody (BE0164, mouse, Bio X Cell, USA). For MWA group, when the tumor size reached a diameter of 8–10 mm on day 35 after tumor implantation, the mice with tumors were treated by MWA with a microwave generator (ECO-100A1, Yigao Microwave Electric Institute, Nanjing, China). Under anesthetization of inhalational isoflurane gas (Baxter Healthcare Corporation, Deerfield, IL, USA), a 17-gauge MWA antenna (Yigao Microwave Electric Institute, China) was inserted to the tumor center after the mice and the tumor area was disinfected using 75% alcohol. Following the previous experimental results, MWA was conducted at 5 W for 3 minutes (min) at the microwave irradiation frequency of 2450 MHz to ensure the complete ablation of tumors. For combination group, the mice were treated by 2 mg/kg anti-PD-1 antibody and 10 mg/kg anti-CTLA-4 antibody via intraperitoneal (i.p.) injection 30 min after MWA, and the antibody injection was repeated twice on day 3 and 6. Furthermore, the mice treated by MWA alone were intraperitoneally injected with an equivalent volume of PBS.
For tumor recurrence, 2 × 106 Hepa1-6 cells were injected into the mice (n = 4 for MWA group; n = 8 for Combination) that have survived for 25 days after MWA treatment. The volume of the recurrent tumor was measured and calculated every 5 days. Normal healthy mice (n = 5) were treated as Control group.
Survival analysis
After the treatment, the mortality of mice in the experimental groups was monitored and recorded every 10 days. The survived mice were recorded for 50 days. Then, the survival analysis was plotted using Kaplan-Meier Curve and log-rank test.
Immunohistochemical (IHC) analysis
After 7 days, the abdomen of the mice injected with cancer cells began to change. The tumor tissues of 5 mice were resected 7 days after MWA treatment and fixed by 4% formalin. The tissues were sectioned into 5-μm thick from the samples, paraffin-embedded. Then, used xylene to pre-clear the tissue section, and used a concentration gradient alcohol to hydrate it, and then put the tissue section into a water bath containing 0.01 M sodium citrate buffer (pH 6.0) for 10 min to repair the antigen. Then, block with goat serum for 20 min at room temperature. Next, stained by anti-mouse CD4 antibody (rat, MA1-81,588, 1:100, eBioscience, USA) and anti-mouse CD8 antibody (rat, catalog number: 14–0808-80, 1:800, eBioscience, USA) and then by horseradish peroxidase (HRP)-conjugated goat anti-rat IgG H&L secondary antibody (goat, ab150160, 1:1000, Abcam, UK). The sections were finally visualized by diaminobenzidine (DAB kit; P0202, Beyotime, Shanghai, China), and the nuclei were counterstained by hematoxylin. The positive cells were observed and counted from five random fields at 100 × and 200 × magnification. The results were further averaged for statistical analysis. IHC scores were assigned to each case according to the following criteria: 3+ (CD4 and CD8 expression was strongly positive, the tissue was dark brown, and single cells chromogenic in the positive part of the high power field were ≥20); 2+ (CD4 and CD8 expression was weakly positive, the tissues were pale brownish-yellow and brown, and the number of single cells in the positive part of the high power field was <20); 1+ (CD4 and CD8 expression was negative, and the tissue coloration was not obvious).
Flow cytometry
Seven days after the MWA treatment, flow cytometry was performed to measure the numbers of CD4+ and CD8+ cells in the spleens of the mice. The harvested spleens were gently minced using a plunger with a 10 mL syringe and passed through 70-μm nylon mesh cell strainers (Alkali Scientific Inc., Fort Lauderdale, FL, USA) to obtain suspension of a single cell. Red blood cells were removed by cell lysis buffer (#9803; Cell Signaling Technology, Danvers, MA, USA). Then, in order to stain intracellular cytokines, the harvested cells were stimulated by eBioscienceTM Cell Stimulation Cocktail (catalog number: 00–4975-03; Invitrogen, Carlsbad, CA, USA) for 5 hours (h). FITC-anti-CD4 antibody (catalog number: 561,842), APC/Cy7-anti-CD8a antibody (catalog number: 557,834) and control antibody were purchased from BD Biosciences (San Jose, CA, USA). Cy5 anti-mouse CD4 antibody (catalog number: 100,409), FITC anti-mouse Interferon-γ (IFN-γ) antibody (catalog number: 505,805) and matched control antibody were purchased from BioLegend (San Diego, CA, USA). Flow cytometric analysis was conducted in AQUIOS CL flow cytometry system (B39101; Beckman Coulter Life Sciences, Indianapolis, IN, USA), and the results were analyzed by Kaluza Analysis software (A82959; Beckman Coulter Life Sciences, USA).
Enzyme-linked immunospot (ELISPOT) assay
ELISPOT assay was conducted with Mouse IFN-γ Single-Color ELISPOT set (Cellular Technology Limited, Cleveland, OH, USA) following the instructions of the manufacturer. The spleen cells (5 × 104 cells/well) were separately mixed with mitomycin C-pretreated Hepa1-6 cells and irrelevant colorectal cancer CT26 cell, and then added into the PVDF plates (catalog number: 3654-WP-10, MABTECH Inc., Cincinnati, OH, USA) that had been pre-coated with 0.5 mg/mL anti-mouse IFN-γ antibody (catalog number: 505,801, BioLegend, USA). After incubating the plates at 37°C with 5% CO2 for 15 h, the plates were washed and then the cells were further cultured with IFN-γ biotinylated detection antibody (catalog number: 500-P119BT; PeproTech, Inc., Suzhou, China). Finally, the cells producing IFN-γ were visualized by AEC substrate set (BD Biosciences, USA). Spots number was counted using an AID ELISPOT reader (Autoimmun Diagnostika GmbH, Strasburg, Germany).
Enzyme-linked Immunosorbent Assay (ELISA)
The blood was collected into the tubes containing EDTA. Plasma was collected by centrifuging at 1000 × g for 10 min and stored at −70°C for subsequent experiments. Concentrations of IFN-γ, Interlukin-18 (IL-18), IL-2 secreted by Th1 cells and concentrations of IL-4, and IL-10 secreted by Th2 cells in the plasma were detected and quantified using ELISA kit (catalog numbers: KMC4022 for IFN-γ; BMS618-3 for IL-18; BMS601 for IL-2; BMS613 for IL-4 and BMS629 for IL-10, Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
All the experiments were independently conducted more than three times. The data were shown as mean ± standard deviation (SD). Statistical analysis was conducted using SPSS 21.0 software (IBM Corporation, Armonk, NY, USA). Statistics significances were determined by one-way ANOVA followed by Dunnett’s post hoc test and student’s t test. The survival rate was plotted by Kaplan-Meier method and compared by a log-rank test. P < 0.05 was considered to be statistically significant.
Results
MWA with anti-PD-1/anti-CTLA-4 increased the survival time and promoted immunity against tumor recurrence of mice
In order to detect the anti-tumor effects of MWA, cell immunotherapy and their combined use on HCC, the mice implanted with Hepa1-6 cells were divided into four groups as follows: Untreated group, MWA group, Blockade group, and Combination (MWA plus Blockade) group, with 10 in each group. Survival of the mice were monitored and compared every 10 days after the operation. As ) shown, the survival of the mice of MWA group was increased compared with those without any treatment (*P < 0.05). Also, the survival of the mice in Combination group was evidently higher than MWA group and Blockade group (^^P < 0.01, vs. MWA; ###P < 0.001, vs. Blockade). Moreover, the Cox regression proportional hazards model calculated the survival of mice (), P < 0.01), compared with the blockade group and the untreated group, the survival of mice in the MWA group was prolonged, but there was no significant difference in the survival rate of the untreated group and the Blockade group. The survival time of mice in Combination group was significantly longer than that in MWA group, Blockade group and untreated group, suggesting that MWA used with anti-PD-1/anti-CTLA-4 treatment increased the survival time of the mice.
Figure 1. Microwave ablation (MWA) combined with anti-PD-1/anti-CTLA-4 increased the survival time and enhanced immunity against tumor recurrence of mice. A: Kaplan-Meier Curves showed the survival rates of untreated mice and mice treated with Blockade, MWA, and Combination (MWA plus Blockade) (n = 10 for each group). *P < 0.05, vs. Untreated; ^^P < 0.01, vs. MWA; ###P < 0.001, vs. Blockade. B: The Cox regression proportional hazards model calculated the survival of mice. C: Tumor volumes of mice survived longer than 10 days after tumor recurrence, and the tumors were monitored every 5 days. D: Tumors were shown in the control group and the experimental group (n = 6 for Experimental group; n = 5 for Control group. Repeat three times for each independent experiment). &&P < 0.01, vs. Control. PD-1: Programmed cell protein 1; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4
Then, the mice survived 25 days after the MWA treatment (MWA group: n = 4; Combination group: n = 8) were re-implanted with 2 × 104 Hepa1-6 cells to simulate HCC recurrence. 10 days after the re-implantation, 6 in Combination group still survived, which allowed the second tumor evaluation, however, no mice in the MWA group survived. Consequently, the survived mice from Combination group (n = 6) were reallocated to the experimental groups, while the age-matched mice (n = 5) were treated as Control group. MWA used with anti-PD-1/anti-CTLA-4 allowed a second tumor complete rejection into 66.7% (4/6) of the mice, and tumor was developed in all the mice in Control group. Furthermore, as ) shown, tumor volumes of the mice of experimental group were evidently reduced compared with Control group (&&P < 0.01). In addition, we measured the tumor volume, and the results showed that compared with the control group, the tumor volume of the experimental group was significantly reduced ()).
MWA with anti-PD-1/anti-CTLA-4 promoted the intratumoral infiltration of cytotoxic T lymphocyte, but not T helper cells into the tumors treated by MWA
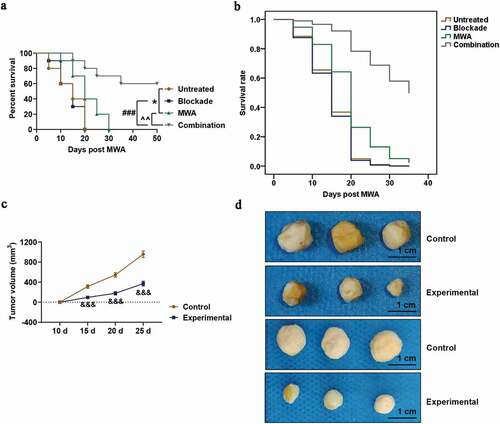
Anti-PD-1 and anti-CTLA4 could activate T-cell responses. Intratumoral infiltration of CD4+ T helper cells (Th cells) and CD8+ cytotoxic T lymphocyte (CTL) was detected and measured by immunohistochemistry in early stage of the HCC model mice. We found that the numbers of intratumorally infiltrated CD4+ Th cells in MWA group and Combination (MWA plus Blockade) group was higher than those in Untreated group ()). Furthermore, infiltration of CD4+ Th cells was not affected in blockade-treated or untreated tumors ()), while MWA and Blockade treatment used alone increased the number of intratumorally infiltrated CD8+ CTL in the tumors in comparison with untreated tumors ()). In addition, the combined treatment of Blockade and MWA further enhanced the intratumoral infiltration of CD8+ CTL into the tumors compared with MWA and Blockade treatment used alone ()).
Figure 2. Combined treatment of MWA with anti-PD-1/anti-CTLA-4 increased the intratumoral infiltration of cytotoxic CD8+ T lymphocyte cell into tumors, but not CD4+ T helper cells. A: Representative microphotographs of CD4+ in Untreated group, Blockade group, MWA group and Combination group, under 100 × and 200 × magnification. Staining of immunohistochemistry was conducted on the tumor specimens harvested 7 days after the treatment. n = 5. B: Representative microphotographs of CD8+ in Untreated group, Blockade group, MWA group and Combination group, under 100 × and 200 × magnification. Staining of immunohistochemistry was performed on the tumor specimens harvested 7 days after the treatment. n = 5. Each experiment was independently conducted in triplicate
MWA with anti-PD-1/anti-CTLA-4 promoted systemic T-cell immune responses induced by MWA treatment
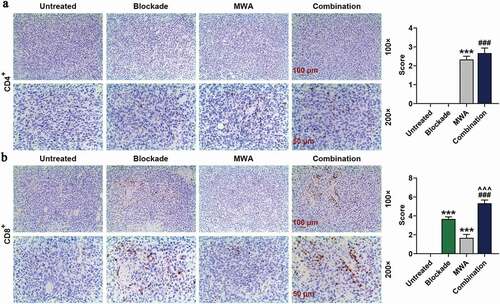
To explore the efficacy of MWA with anti-PD-1/anti-CTLA-4 on the peripheral lymphocytes, numbers of CD4+ and CD8+ T-cells in the spleens of mice of Untreated group, Blockade group, MWA group, and Combination group were analyzed by flow cytometry. 7 days after MWA treatment, at the early stage of HCC, CD4+ and CD8+ T cells in the spleens of the mice of MWA group were increased compared with Untreated group (, **P < 0.01). Furthermore, the combined treatment of MWA with anti-PD-1/anti-CTLA-4 increased the CD4+ and CD8+ T cells in the spleens of mice compared with those in the mice treated only by MWA or Blockade (, ^^P < 0.01, vs. MWA; ###P < 0.001, vs. Blockade). The CD4+ and CD8+ T cells in the spleens of the mice in Blockade group was similar to Untreated group (). The experimental data showed that MWA or combined used of anti-PD-1/anti-CTLA-4 could enhance the systemic immune responses of T cells, while anti-PD-1/anti-CTLA-4 treatment alone had almost no effects on T cells systemic immune responses.
Figure 3. MWA with anti-PD-1/anti-CTLA-4 enhanced systemic T-cell immune responses. Mononuclear cells were available from the spleens of mice on day 7 after the treatment and used for flow cytometry analysis. n = 5. CD4+ T cell and CD8+ T cell in Untreated group, Blockade group, MWA group and Combination group were analyzed using flow cytometry. **P < 0.01, vs. Untreated; ^^P < 0.01, vs. MWA; ###P< 0.001, vs. Blockade. Each experiment was independently conducted in triplicate
MWA with anti-PD-1/anti-CTLA-4 induced the secretion of IFN-γ of spleen cells
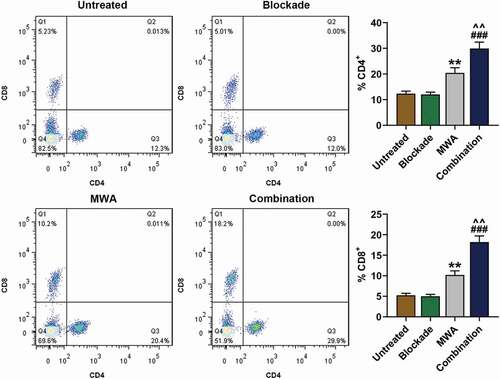
In order to further investigate the specificity of immune response induced by the treatments, ELISPOT assay was performed to detect the expression of IFN-γ in the splenocytes of mice 7 days after the treatment. As ) shown, the number of IFN-γ secreted by the Hepa1-6-specific cells in the Combination group was significantly increased than that in Blockade and MWA group (###P< 0.001, vs. Blockade; ^^^P < 0.001, vs. MWA), while the efficacy of MWA or anti-PD-1/anti-CTLA-4 treatment used alone did not result in a significant difference in comparison with Untreated group ()). In addition, no significant differences were found between the groups in which the solenocytes were stimulated with irrelevant CT26 colon cancer cells ()). Thus, the results suggested that MWA used with anti-PD-1/anti-CTLA-4 treatment could induce the secretion of IFN-γ of spleen cells.
Figure 4. MWA with anti-PD-1/anti-CTLA-4 induced specific antitumor immunity synergistically. A: Representative enzyme-linked immunospot pictures from Untreated group, Blockade group, MWA group and Combination group. Cells secreting IFN-(IFN-γ) in the spleens after Hepa1-6 cell implantation were analyzed by ELISPOT assay. ^^^P < 0.001, vs. MWA; ###P < 0.001, vs. Blockade. B: Representative ELISPOT pictures from Untreated group, Blockade group, MWA group and Combination group. Cells secreting IFN-γin the spleens after CT26 cell implantation were analyzed by enzyme-linked immunospot (ELISPOT) assay. Each experiment was independently conducted in triplicate
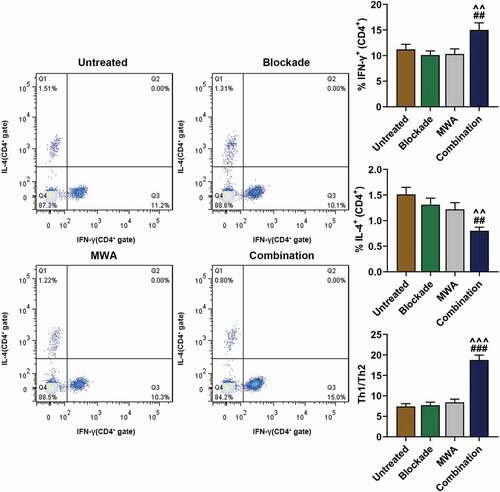
MWA with anti-PD-1/anti-CTLA-4 caused the polarization of Th1 cells
Anti-PD-1/anti-CTLA-4 and MWA have been found to induce the polarization or expression Th2 but not Th1 cells [Citation19,Citation21,Citation22]. We speculated that MWA used with anti-PD-1/anti-CTLA-4 treatment could cause the polarization of Th1 cells. In order to prove the speculation, both Th1 and Th2-type response were detected after the cytokine production by CD4+ T cells 7 days after MWA treatment. We found that the mice treated by MWA with anti-PD-1/anti-CTLA-4 had more Th1 cells producing IFN-γ in the spleens compared with those in Blockade and MWA group (, ##P < 0.01, vs. Blockade; ^^P < 0.01, vs. MWA). In contrast, Th2 cells producing IL-4 was reduced in Combination group compared with Blockade and MWA group (, ##P < 0.01, vs. Blockade; ^^P < 0.01, vs. MWA). Furthermore, neither anti-PD-1/anti-CTLA-4 nor MWA used alone affected the polarization of Th1 or Th2 cells (). In addition, an evidently higher Th1/Th2 ratio was observed in Combination group in comparison with Blockade and MWA group (, ###P < 0.001, vs. Blockade; ^^^P < 0.001, vs. MWA). The data from the experiments revealed that MWA with anti-PD-1/anti-CTLA-4 could cause the polarization of Th1 cells.
Figure 5. Treatment of MWA with anti-PD-1/anti-CTLA-4 caused Th1 cell polarization. The proportion of IFN-γ-producing Th1 cells and Th2 cells producing Interleukin-4 (IL-4) was quantified after flow cytometry analysis. The ratio of Th1/Th2 cells was then measured. ^^P < 0.01, ^^^P < 0.001, vs. MWA; ##P < 0.01, ###P < 0.001, vs. Blockade. Each experiment was independently conducted in triplicate
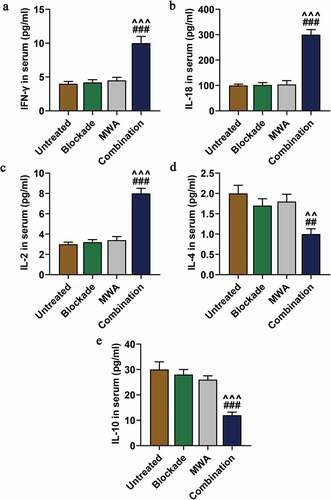
MWA combined with anti-PD-1/anti-CTLA-4 increased Th1-type cytokines but reduced Th2-type cytokines
The plasma concentrations of cytokines were detected by ELISA to further measure the levels of Th1 and Th2. As shown, the levels of Th1-type cytokines (IFN-γ, IL-18, IL-2) in Combination group were significantly up-regulated than those in Blockade and MWA group (###P < 0.001, vs. Blockade; ^^^P < 0.001, vs. MWA). The levels of Th2-type cytokines IL-4 and IL-10 were significantly down-regulated in Combination group compared to the Blockade and MWA group (, e, ##P < 0.01, ###P < 0.001, vs. Blockade; ^^P < 0.01, ^^^P< 0.001, vs. MWA). These results demonstrated that MWA used with anti-PD-1/anti-CTLA-4 treatment could increase Th1-type cytokines but reduce Th2-type cytokines.
Figure 6. MWA combined with anti-PD-1/anti-CTLA-4 increased Th1-type cytokines but reduced Th2-type cytokines. A: Serum concentrations of Th1-type cytokine IFN-γ in the mice of Untreated group, Blockade group, MWA group and Combination group were measured. ^^^P < 0.001, vs. MWA; ###P < 0.001, vs. Blockade. B: Serum concentrations of Th1-type cytokine IL-18 in mice of Untreated group, Blockade group, MWA group and Combination group were measured. ^^^P < 0.001, vs. MWA; ###P < 0.001, vs. Blockade. C: Concentrations of Th1-type cytokine IL-2 in mice serum of Untreated group, Blockade group, MWA group and Combination group were measured. ^^^P < 0.001, vs. MWA; ###P < 0.001, vs. Blockade. D: Serum concentrations OF Th2-type cytokine IL-4 in mice of Untreated group, Blockade group, MWA group and Combination group were measured. ^^P < 0.01, vs. MWA; ##P < 0.01, vs. Blockade. E: Serum concentrations of Th2-type cytokine IL-10 in mice of Untreated group, Blockade group, MWA group and Combination group were measured. n = 5. ^^^P < 0.001, vs. MWA; ###P < 0.001, vs. Blockade. Each experiment was independently conducted in triplicate
Discussion
MWA has a high performance in treating HCC without promoting local tumor recurrence [Citation23]. MWA has also been used as a safe technique treating liver cancer [Citation24]. After MWA treatment, the lymphocyte infiltration of the tumor is found increased and improved the host’s immune function [Citation25]. Further research found that in destroying tumor by MWA, intracellular tumor-specific antigens are released and captured by antigen-presenting cells in lymphatic tissues [Citation26], which stimulates T cells in response to tumors [Citation20]. Thus, MWA may be used as a novel potential therapeutic method for HCC treatment.
Liang et al. demonstrated that MWA-induced T-cell responses are not effective enough in preventing the patients from tumor recurrence [Citation26], because only weak T-cell responses were induced by MWA [Citation19]. In our study, MWA increased survival rate in comparison with Untreated group, but all the mice died on 30 days after the MWA treatment, which as a matter of fact was not quite satisfactory. Thus, an effective combination of MWA with other therapies should be developed for HCC treatment.
PD-1 and CTLA-4 could induce immune response [Citation27]. However, based on currently available data, the inhibitory effects of PD-1 and CTLA-4 on poorly immunogenic tumors are highly limited, and such findings are consistent with our results [Citation28]. Studies have reported that the combination of MWA with PD-1 and CTLA-4 blockade can synergistically enhance the efficacy of anti-breast cancer through an enhanced specific immune response [Citation20]. In the current study, we found that the injection of anti-PD-1/anti-CTLA-4 antibody after the MWA treatment increased survival time of the mice and protected most mice from tumor recurrence, indicating that the combined therapy could ameliorate therapeutic benefits and could be further applied in preventing HCC recurrence. Furthermore, the present experiment found that intratumorally infiltrated T cells was increased after the combined treatment, which was similar to a previous study [Citation29]. In addition, we also observed that the combined treatment further enhanced the MWA-induced infiltration of CTL instead of Th cells. Moreover, current findings also showed that the combined therapy not only enhanced the peripheral T-cells responses, but also resulted in the systemic tumor-specific immunity. The enhanced anti-tumor immunity may lead to the rejection of tumor in recurrence simulation.
The role of CD4+ Th cell in the synergistic effects of immune system was detected. Generally, Th cells further differentiate into two major cell subtypes, that is, Th1 and Th2 cells [Citation30]. In Shim et al.’s research, the protective immunity of host against intracellular bacteria is mediated by both cellular (Th1 cells) and humoral (Th2 cells) immune responses [Citation31]. In our study, we discovered the ratio of Th1 to Th2 cells in the Combination treatment group was evidently higher than those in other groups, suggesting that the disturbing Th1/Th2 balance to Th1-dominated immunity could promote T-cell responses in combination group. Furthermore, the expressions of IFN-γ, IL-18, IL-2, and the Th1-type cytokines were up-regulated in the mice of Combination group, while the expressions of IL-4 and IL-10, the Th2-type cytokines were down-regulated. As shown in previous study, the Combination of IL-18 and IL-2 cytokines synergistically enhanced the proliferation and cytolytic activity of peripheral blood mononuclear cells [Citation32], induced T cells to differentiate into Th1 cells [Citation33], and stimulated IFN-γ production from Th1 cells [Citation34]. The experimental results suggested that the combined treatment could facilitate the production of several Th1-type cytokines to activate the response triggered by Th1 cells, which is possibly and potentially the mechanism underlying the effects of Combination treatment.
The current study was the first to discover that MWA in combination with anti-PD-1/anti-CTLA-4 is effective in treating HCC. The experimental results y showed that the combined treatment could induce the infiltration of T-cells and systemic T-cell responses. Anti-PD-1/anti-CTLA-4 treatment increased the release of multiple Th1-type cytokines, and led to the T cell response to Th1 polarization. In addition, the combined treatment could ameliorate the systemic T-cell responses and synergistically resulted in strong tumor-specific immune responses. Finally, the combined treatment evidently increased the survival of the mice injected with Hepa1-6 HCC cells and protected most mice from tumor recurrence. These experimental data suggested that MWA used with anti-PD-1/anti-CTLA-4 could be a potential therapeutic method for treating HCC patients. Furthermore, experiments with multiple HCC cells may more convincingly confirm the role of microwave ablation combined with cellular immunity in the treatment of HCC. In addition, adding a positive control to the IHC experiment may be an effective basis for evaluating positive staining, and we will add this study in future experiments.
Disclosure of Conflict-of-Interest
The authors declare no conflicts of interest.
Additional information
Funding
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012 Mar 31;379(9822):1245–1255. PubMed PMID: 22353262; eng.
- Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, et al. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016 Jun;25(2):74–85. PubMed PMID: 27312032; eng.
- Guan Q, Gu J, Zhang H, et al. Correlation between vascular endothelial growth factor levels and prognosis of hepatocellular carcinoma patients receiving radiofrequency ablation. Biotechnol Biotechnol Equip. 2015 Jan 2;29(1):119–123. PubMed PMID: 26019624; PubMed Central PMCID: PMCPMC4434046. eng.
- Wu H, Chen B, Peng B. Effects of intratumoral injection of immunoactivator after microwave ablation on antitumor immunity in a mouse model of hepatocellular carcinoma. Exp Ther Med. 2018 Feb;15(2):1914–1917. PubMed PMID: 29434784; PubMed Central PMCID: PMCPMC5776511. eng.
- Wang Y, Han X, Li YD, et al. Effects of tumor-specific antigen induced by lentinan on murine H22 hepatocellular carcinoma immunoprophylaxis. Eur Rev Med Pharmacol Sci. 2015 Dec;19(23):4516–4524. PubMed PMID: 26698247; eng.
- Vogl TJ, Nour-Eldin NA, Albrecht MH, et al. Thermal ablation of lung tumors: focus on microwave ablation. RoFo: Fortschritte Auf Dem Gebiete Der Rontgenstrahlen Und Der Nuklearmedizin. 2017 Sep;189(9):828–843. PubMed PMID: 28511267; eng.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014 Mar;14(3):199–208. PubMed PMID: 24561446; eng.
- Wei Z, Ye X, Yang X, et al. The efficacy and safety of microwave ablation in patients with retroperitoneal metastases. Int J Hyperthermia. 2018 Nov;34(7):1053–1060. PubMed PMID: 29082799; eng.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. JVIR. 2010 Aug;21(8 Suppl):S192–203. PubMed PMID: 20656229; PubMed Central PMCID: PMCPMC3065977. eng.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018 May;3(5):317–325. PubMed PMID: 29503247; eng.
- Vogl TJ, Panahi B, Albrecht MH, et al. Microwave ablation of pancreatic tumors. MITAT. 2018 Feb;27(1):33–40. PubMed PMID: 29278340; eng.
- den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004 Jun 1;64(11):4024–4029. PubMed PMID: 15173017; eng.
- Yu Z, Geng J, Zhang M, et al. Treatment of osteosarcoma with microwave thermal ablation to induce immunogenic cell death. Oncotarget. 2014 Aug 15;5(15):6526–6539. PubMed PMID: 25153727; PubMed Central PMCID: PMCPMC4171648. eng.
- Dong BW, Zhang J, Liang P, et al. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int J Hyperthermia. 2003 Mar-Apr;19(2):119–133. PubMed PMID: 12623635; eng.
- Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007 May;56(5):739–745. PubMed PMID: 17195077; eng.
- Lo B, Abdel-Motal UM. Lessons from CTLA-4 deficiency and checkpoint inhibition. Curr Opin Immunol. 2017 Dec;49:14–19. PubMed PMID: 28806575; eng.
- Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016 Aug;13(8):473–486. PubMed PMID: 27141885; eng.
- Taggart D, Andreou T, Scott KJ, et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8(+) T cell trafficking. Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1540–e1549. PubMed PMID: 29386395; PubMed Central PMCID: PMCPMC5816160. eng.
- Li L, Wang W, Pan H, et al. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer. J Transl Med. 2017 Jan 31;15(1):23. PubMed PMID: 28137271; PubMed Central PMCID: PMCPMC5282633. eng.
- Zhu J, Yu M, Chen L, et al. Enhanced antitumor efficacy through microwave ablation in combination with immune checkpoints blockade in breast cancer: A pre-clinical study in a murine model. Diagn Interv Imaging. 2018 Mar;99(3):135–142. PubMed PMID: 29398572; eng.
- Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY). 2015 Nov 27;350(6264):1079–1084. PubMed PMID: 26541610; PubMed Central PMCID: PMCPMC4721659. eng.
- Gide TN, Quek C, Menzies AM, et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and Anti-PD-1/Anti-CTLA-4 combined therapy. Cancer Cell. 2019 Feb 11;35(2):238–255.e6. PubMed PMID: 30753825; eng.
- Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. 2014 Mar;83(3):552–558. PubMed PMID: 24418287; eng.
- Poggi G, Tosoratti N, Montagna B, et al. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7(25):2578–2589. PubMed PMID: 26557950; eng.
- Wiowski TT, Hänsler J, Neureiter D, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003 Oct 1;63(19):6496–6500. PubMed PMID: 14559842; eng.
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005 Apr;235(1):299–307. PubMed PMID: 15731369; eng.
- Beavis PA, Henderson MA, Giuffrida L, et al. Dual PD-1 and CTLA-4 checkpoint blockade promotes antitumor immune responses through CD4(+)Foxp3(-) cell-mediated modulation of CD103(+) dendritic cells. Cancer Immunol Res. 2018 Sep;6(9):1069–1081. PubMed PMID: 30018045; eng.
- Nolan E, Savas P, Policheni AN, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Transl Med. 2017 Jun 7;9(393):eaal4922. PubMed PMID: 28592566; PubMed Central PMCID: PMCPMC5822709. eng.
- Behm B, Di Fazio P, Michl P, et al. Additive antitumour response to the rabbit VX2 hepatoma by combined radio frequency ablation and toll like receptor 9 stimulation. Gut. 2016 Jan;65(1):134–143. PubMed PMID: 25524262; eng.
- Biedermann T, Rocken M, Carballido JM. TH1 and TH2 lymphocyte development and regulation of TH cell-mediated immune responses of the skin. J Invest Dermatol Symp Proc. 2004 Jan;9(1):5–14. PubMed PMID: 14870978; eng.
- Shim S, Soh SH, Im YB, et al. Elicitation of Th1/Th2 related responses in mice by chitosan nanoparticles loaded with Brucella abortus malate dehydrogenase, outer membrane proteins 10 and 19. Int J Med Microbiol IJMM. 2019 Oct 18:151362. DOI:10.1016/j.ijmm.2019.151362. PubMed PMID: 31676233; eng.
- Son YI, Dallal RM, Mailliard RB, et al. Interleukin-18 (IL-18) synergizes with IL-2 to enhance cytotoxicity, interferon-gamma production, and expansion of natural killer cells. Cancer Res. 2001 Feb 1;61(3):884–888. PubMed PMID: 11221875; eng.
- Chang J, Segal B, Nakanishi K, et al. The costimulatory effect of IL18 on the induction of antigen-specific IFN-γ production by resting T cells is IL12 dependent and is mediated by up-regulation of the IL12 receptor β2 subunit. Eur J Immunol. 2000;30:1113–1119. .
- Sedlak C, Patzl M, Saalmuller A, et al. IL-12 and IL-18 induce interferon-gamma production and de novo CD2 expression in porcine gammadelta T cells. Dev Comp Immunol. 2014 Nov;47(1):115–122. PubMed PMID: 25036760; eng.
