 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
WHAMM (WAS Protein Homolog Associated with Actin, Golgi Membranes, and Microtubules) is involved in Golgi membrane association, microtubule binding, and actin nucleation as a nucleation-promoting factor, which activates the actin-related protein 2/3 complex (the Arp2/3 complex). However, the role of WHAMM in mammalian oocyte maturation is poorly understood. The presence of WHAMM mRNA and protein during all stages of mouse oocyte maturation has been verified. It is mainly co-localized with the actin cage permeating the spindle during mouse oocyte maturation. Through the knockdown of WHAMM, we confirmed that it regulates spindle formation and affects the localization of the microtubule-organizing center (MTOC) during the early stages of spindle formation. Moreover, depletion of WHAMM impaired the formation of the spindle actin and chromosome alignment, which might be the cause of chromosomal aneuploidy and abnormal, asymmetric division. Treatment with brefeldin A (BFA), an inhibitor of vesicle transport from the endoplasmic reticulum (ER) to the Golgi apparatus, induced abnormal and dispersed localization of WHAMM. Taken together, these findings show that WHAMM is an essential component of the actin cytoskeleton machinery and plays a crucial role in oocyte maturation, presumably by controlling the formation of spindles with normal length by activating the formation of the spindle actin via the Arp2/3 complex.
Introduction
During mammalian oocyte maturation, oocytes undergo two asymmetric divisions, which is crucial for further development, including fertilization and embryogenesis. Because the plane of cell division is determined by the position of the spindle [Citation1], migration of the spindle to the cellular cortex is one of the essential steps for asymmetric cell division in mammalian gametes and is a probable link to the remodeling of the actin cytoskeleton [Citation2]. The major changes taking place in the actin cytoskeleton during oocyte maturation include cortical actin cap formation [Citation3] and thickening of the inner-cortex, called the “subcortex” [Citation4,Citation5]. Recent reports show that the spindle actin (also called “actin cage”) covers the entire spindle and forms structures that resemble meiotic spindle; thus, it regulates microtubule dynamics, protects the meiotic spindle, and regulates chromosome alignment in spindles of mammalian oocytes [Citation6,Citation7]. There are three types of actin exist in mouse oocyte; cortical actin, spindle actin, cytoplasmic actin mesh. Different types of cytoskeleton were controlled by distinct kind of actin nucleators and regulating factors, such as various NRFs or GTPase and so on. However, it is unclear how actin-binding proteins affect spindle actin formation, protection, and depolymerization of each type of actin cytoskeleton.
While formin/spire forms the cytoplasmic actin mesh [Citation8,Citation9], the Arp2/3 complex-mediated actin nucleation of branched actin is considered to be responsible for cortical actin cap formation and cortex thickening in maturing oocyte [Citation4,Citation5,Citation10,Citation11]. Type I nucleation promoting factors (NPFs) [Citation12], which include the Wiskott–Aldrich syndrome (WAS) family of proteins, such as n-WASP, WAVE2, WASH, WHAMM (WASP homolog associated with actin, membranes, and microtubules), and JMY, activated the Arp2/3 complex. WASH, WHAMM, and JMY are involved in cell migration, endocytosis, and binding to actin monomers [Citation13]. The roles of various NPFs in oocyte maturation have been previously reported [Citation4,Citation14–16]. The understanding of mechanisms governing spindle migration in oocytes has remarkably progressed. However, the mechanism underlying spindle formation and/or protection by cage actin is still elusive.
The WHAMM protein comprises the WH2, connector, and acidic motifs (WCA) [Citation17]. WHAMM directly binds to the Arp2/3 complex via its acidic C-terminal domains, which are homologous to the regions found within the NPFs. Furthermore, it binds to monomeric actin via its proline-rich region containing putative sites for Profilin binding, and it attaches to microtubules through its coiled-coil region [Citation17,Citation18]. WHAMM also has been shown to be regulated in autophagy, where it is involved in autophagosome biogenesis and autophagic lysosome reformation by the Arp2/3 complex [Citation19]. WHAMM and the Arp2/3 complex are required for actin assembly in various cells via recruitment by Rab1a, which is essential for actin assembly [Citation20]. WHAMM, unlike other NPFs, also interacts with membranes of cis-Golgi; it is also found in the tubulovesicular ER-Golgi intermediate compartment (ERGIC) [Citation17]. WHAMM interacts with both actin and microtubule cytoskeletons to control membrane tubulation and dynamics during transport from the endoplasmic reticulum (ER) to the Golgi apparatus [Citation17]. These findings demonstrate its role in regulating the cytoskeleton by coordinating actin and microtubule dynamics. WHAMM is localized at the periphery of spindles and plays a role in spindle migration in maturing oocytes [Citation21]. Besides its involvement in tubulovesicular structures and spindle movement, it was unclear that what type of actin cytoskeleton or which parts of oocytes were related with WHAMM and functional and mechanistic roles of WHAMM in other cells or oocytes are still not known.
In this study, we investigated the functional roles of WHAMM in oocyte maturation by using WHAMM knockdown mediated via WHAMM-specific dsRNA, and we especially focused on the mechanisms of spindle actin polymerization, meiotic spindle formation, chromosome alignment, and asymmetric cell division. Results from this study show that WHAMM is localized around the spindle, and depletion of WHAMM causes failure of spindle actin formation, abnormal spindle assembly during early oocyte maturation, and extrusion of the greater polar body.
Material and methods
Oocyte collection and culture and brefeldin A treatment
All animal experiments were approved and performed according to the guidelines outlined by the Animal Research Committee of Chungbuk National University (approval no. CBNUR-1026-16-01). Germinal vesicle (GV)-intact oocytes were collected from the ovaries of 6–8-week-old imprinting control region (ICR) mice and were cultured in M16 medium (Sigma), using paraffin oil at 37°C and 5% CO2. Oocytes were collected in the M2 medium, separately, for immunostaining and microinjection after culturing for various time periods. Cell cycle progress was arrested at the GV stage using a microinjection of 4 µM milrinone. Polar body extrusion and cytokinesis were observed using a stereomicroscope. The longest diameters of oocytes and polar bodies were measured using the ImageJ software.
BFA was dissolved in dimethyl sulfoxide (DMSO, Sigma) to make 1000 fold of stock solution at 5 mM. The stock was stored at −20°C and diluted in M16 medium until final working concentration (5 or 10 µM) before treatment.
Real-time quantitative PCR analysis
WHAMM expression was detected using real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis, and data were analyzed using the 2−ΔΔCT method [Citation22]. Total RNA was extracted from 30 oocytes using the Dynabead mRNA DIRECT Kit (Life Technologies, Foster City, CA). First-strand cDNA was generated using a cDNA Synthesis Kit (Takara, Kyoto, Japan) and Oligo (dT)12–18 primers. The PCR primers used to amplify WHAMM are listed in Table 1. qRT-PCR was performed using SYBR Green at a final reaction volume of 20 μl (qPCR kit, Finnzymes, Vantaa, Finland). PCR conditions were as follows: 94°C for 10 min, followed by 39 cycles at 95°C for 15 s, 60°C for 15 s, and 72°C for 45 s, with a final extension step at 72°C for 5 min. Finally, relative gene expression was quantified by normalization to the levels of GAPDH mRNA. Experiments were conducted in triplicate.
Microinjection
dsRNAs were microinjected into GV-stage mouse oocytes, as described previously [Citation24]. Briefly, a 734 bp fragment of the WHAMM gene (corresponding to 947–1680 bp of NM_001004185.3) was amplified from first-strand cDNA, which was generated from RNA extracted from MII oocytes using gene-specific primers containing the T7 promoter sequence (supplementary Table S1). In vitro transcription was performed using a Megashortscript T7 Kit (Life Technologies). WHAMM knockdown was achieved by diluting WHAMM dsRNA to obtain a final concentration of 1 μg/μl, and its microinjection into the cytoplasm of a fully grown GV-stage oocyte was performed using a FemtoJet® 4i electronic microinjector (Eppendorf AG, Hamburg, Germany) and an ECLIPSE TE300 inverted microscope (Nikon UK Ltd, Kingston upon Thames, Surrey, UK) equipped with an MM0-202 N hydraulic three-dimensional micromanipulator (Narishige Inc. Sea Cliff, NY). After injection, oocytes were incubated in M16 medium containing 4 μM milrinone for 15 h to ensure knockdown. They were then transferred into fresh M16 medium and cultured for further experiments. Control oocytes were microinjected with 5–10 pl of dsRNA against GFP.
Immunostaining and confocal microscopy
Oocytes were fixed in 4% paraformaldehyde dissolved in phosphate-buffered saline (PBS) for 30 min at 20°C. They were then transferred into a membrane permeabilization solution (0.5% Triton X-100 in 0.1% polyvinyl alcohol in phosphate-buffered saline, PVA-PBS) and incubated for 1 h. For the quantification of cortical and cytoplasmic actin mesh, membrane permeabilization was omitted to preserve actin density. For immunostaining, after 1 h incubation in the blocking buffer (PBS containing 1% bovine serum albumin), oocytes were incubated overnight at 4°C with a rabbit anti-WHAMM antibody (1:200 dilution) (Merck Millipore, Massachusetts, United States). After three washes with the washing buffer (PBS containing 0.1% Tween 20 and 0.01% Triton X-100), oocytes were labeled with the Alexa-Fluor-488-conjugated goat anti-rabbit-IgG (1:100) for 2 h at room temperature. An anti-pericentrin antibody was used for MTOC staining. To stain cytoplasmic actin mesh or cortical actin, oocytes were treated for 3 h with phalloidin–TRITC (10 mg/ml), which binds to F-actin. Quantification of actin mesh density was carried out using a single section in the center of the oocyte image acquired by a 63x oil-immersion objective lens. Cytoplasmic regions devoid of cortical actin and spindle actin were selected as a square (10 × 10 µm), then average grayscale of phalloidin channel in the selected region was measured using ImageJ. Intensity of fluorescence calculated by mean value (intensity/pixel) and normalized as percent of mean values of the control group.
For mouse monoclonal anti-α-tubulin–FITC antibody staining (1:200 dilution) (Sigma-Aldrich, Missouri, United States), oocytes were incubated overnight at 4°C, washed three times with the washing buffer, for 2 min each, and then incubated with Hoechst 33,342 stain (10 mg/ml in PBS) for 15 min. Samples were finally examined using a confocal laser-scanning microscope (Zeiss LSM 710 META, Jena, Germany) with a 40 × objective lens used for the observation of fixed oocytes.
To normalize variations in WHAMM intensity between experimental repeats (N = 3), the intensity values of each oocyte were normalized as percent of mean values of the control group of each experimental repeat.
For chromosome spreading samples, zona pellucida of oocyte was eliminated by Tyrode’s solution (T1788, Sigma). After PVA-PBS washing, oocytes were transferred on the glass slide and hypotonic fixed (1% PFA in distilled water PH9.2 with 0.1% Triton X-100 and 3 mM dithiothreitol). The samples were dried at RT and placed in blocking solution for 1 h. after that, samples co-stained by anti-ACA (Antibodies Incorporates) and Hochest 33,342 for labeling the centromere and DNA, respectively.
Time-lapse microscopy of oocyte maturation
Time-lapse imaging was performed for WHAMM knockdown and negative control oocytes. dsRNA or mRNA was microinjected into GV-stage oocytes, as described previously in this section. For visualization of the spindle, tubulin-GFP mRNA was injected in combination with dsRNA, and for visualization of chromosomes, H2B–mCherry mRNA was also injected. Images were acquired at intervals of 300 s, for 7–8 h, using the Lumascope 620 (Etaluma Inc, Carlsbad, CA) inverted microscope installed inside an incubator, which was maintained at 37°C and 5% CO2.
Data analysis
At least three replicate experiments were performed for each treatment. Statistical analyses were performed using Welch’s t-test, Pearson’s chi-square test, Fisher’s exact test, or an analysis of variance (ANOVA), followed by Tukey’s multiple comparisons of means by R (R Development Core Team, Vienna, Austria). Data are expressed as the meanS.E.M., and differences were considered statistically significant at P < 0.05.
Results
WHAMM localizes around the meiotic spindle during mouse oocyte maturation
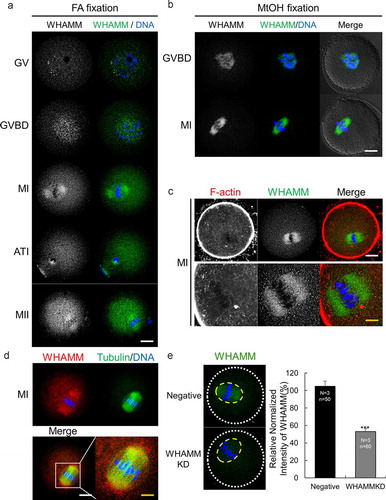
The localization of WHAMM was observed in mouse oocytes at various stages of maturation using immunostaining with a WHAMM-specific antibody. At the germinal-vesicle (GV) and germinal vesicle breakdown (GVBD) stages, before meiotic spindle formation, WHAMM was localized near the chromosomes. In metaphase I (MI), anaphase-telophase I (ATI), and metaphase II (MII) stage oocytes, WHAMM mainly appeared to have a spindle-shaped structure (). The distribution of WHAMM protein was similar between samples fixed with paraformaldehyde () and those fixed with methanol (). WHAMM was localized in the spindle actin region (Supplementary Figure 1), as seen via the co-staining of WHAMM and the cytoplasmic actin mesh (). Through the co-immunostaining for WHAMM and alpha-tubulin, we confirmed that WHAMM placed with meiotic spindle in MI stage mouse oocytes (). mRNA expression levels of WHAMM were determined using qRT-PCR, which revealed that WHAMM is expressed at all stages in immature GV, GVBD, MI, ATI, and MII oocytes (Supplementary Figure 1a).
Figure 1. Expression and localization of WHAMM during mouse oocyte maturation. (a) Subcellular localization of WHAMM during mouse oocyte maturation. Immunostaining was performed using an anti-WHAMM antibody. WHAMM was detected near the chromosomes in the GV and GVBD stages, or around the spindle after the MI stage. (b) Immunostaining of WHAMM fixed with methanol. After fixation, immunostaining was performed as described in (A). (a and b) Blue, DNA; green, WHAMM; white; DIC. Scale bar: 20 μm. (c) Co-staining of WHAMM and cytoplasmic actin mesh. WHAMM localizes in the spindle actin region. Blue, DNA; red, F-actin; green, WHAMM. Scale bar: 20 μm. (d) Co-staining of WHAMM and alpha-tubulin. WHAMM is localized around the meiotic spindle. Blue, DNA; green, Tubulin; red, WHAMM. Scale bar: white, 20 μm; yellow, 4 μm. (e) Quantification of WHAMM intensity in Negative and WHAMM dsRNA-injected oocytes after 8 h meiotic resumption. WHAMM protein level in yellow circle was measured using a confocal microscope and by staining with anti-WHAMM antibody and was normalized using relative intensity in WHAMM dsRNA-injected groups against of Negative control. ***P < 0.001. Experimental replication was performed 3times (N = 3). n values are as indicated
Knockdown of WHAMM impairs asymmetric division of oocytes and decreases the density of the spindle actin
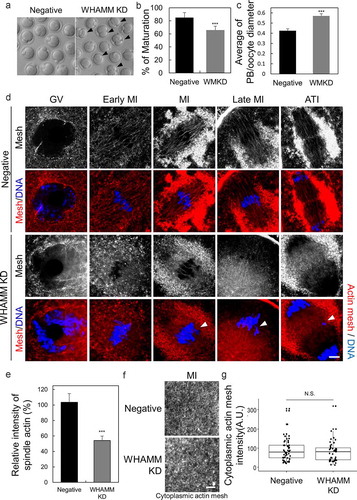
We examined whether the depletion of WHAMM affects polar body extrusion or spindle actin density during oocyte maturation. Oocytes were injected with WHAMM-specific dsRNA at the GV stage to knockdown the expression of WHAMM (hereafter defined as WHAMM KD group). Following the microinjection, mRNA levels of WHAMM were decreased to 5% as compared to those of negative control-injected oocytes (Supplementary Figure 2a). Also, WHAMM immunostaining showed that WHAMM dsRNA injection decreased WHAMM protein levels in oocytes at the MI stage (). The percentage of oocyte maturation to the MII stage was significantly lower in the WHAMM KD group than that in the negative control group (). Following dsRNA injection, asymmetric division and polar body size were observed 12 h after the maturation period. Depletion of WHAMM frequently resulted in symmetrical cell division and extruded an abnormally large polar body as indicated by the arrows in ). The ratio of the diameter of the polar body to that of the oocyte (polar-body/oocyte cytoplasm) was significantly increased in the WHAMM KD group (). For evaluation of the impact of WHAMM knockdown on the formation of spindle actin, which is crucial for cell division, oocytes were stained with phalloidin at the GV, early MI, MI, late MI, and ATI stages (). WHAMM dsRNA-injected oocytes failed to form the typical structure of the spindle actin observed in the control dsRNA-injected group (). Furthermore, WHAMM KD oocytes showed impaired chromosome alignment, thus resulting in chromosome lagging as indicated with a white arrow in ). Intensity of actin cage (spindle actin) mesh was significantly decreased () in WHAMM KD oocytes than controls. However, the cytoplasmic actin mesh, except for around the spindle and chromosome, was not significantly different between the control and WHAMM KD oocytes ().
Figure 2. WHAMM is essential for asymmetric division and formation of spindle actin in maturing mouse oocytes. (a) Maturation status of oocytes injected with dsRNA against WHAMM, or that for the negative control. Impairment of asymmetric division by knockdown of WHAMM. Arrows indicate the extrusion of large polar bodies. Scale bar: 50 μm. (b) Maturation ratio 12 h after meiotic resumption was examined. Oocytes yielding a large polar body are also considered as MII. ***P < 0.001. (c) WHAMM knockdown increases the polar body (Manseau and Schupbach) size. The ratio of the diameter of the polar body to that of the oocyte in control and WHAMM-knockdown oocytes is shown. ***P < 0.001. (D, E, and F) Phalloidin-stained cytoplasmic actin mesh in oocytes injected with negative control dsRNA (Negative) and WHAMM-specific dsRNA (WHAMM KD). Oocytes were sampled at the GV, early MI, MI, late MI, or ATI stages (0, 5, 6, 8, and 9.5 h, respectively, after meiotic resumption) under the same conditions. Knockdown of WHAMM impairs the formation of complete spindle actin. The arrow indicates misaligned chromosomes by disruption of spindle actin. Blue, DNA; red F-actin. Scale bar: 7 μm. (e) Spindle actin intensity was represented percent (%) of intensity relative to mean of control. The number of oocytes used to analysis in control and WHAMM KD was 10, 11, respectively. ***P < 0.001. (f and g) The knockdown of WHAMM impairs the formation of spindle actin but does not affect cytoplasmic actin mesh. (g) Quantification of phalloidin fluorescence intensity of cytoplasmic actin at 8 h (MI) after meiosis resumption in oocytes injected with dsRNA. Fluorescence intensities of each oocyte were normalized to the mean intensity of control oocytes and presented as percent (%) of intensity relative to mean of control. Boxes show the interquartile range; whiskers show 1.5× the interquartile range; the line represents the median. N.S.: not statistically significant (P > 0.05)
WHAMM affects spindle organization by clustering of MTOC during early maturation
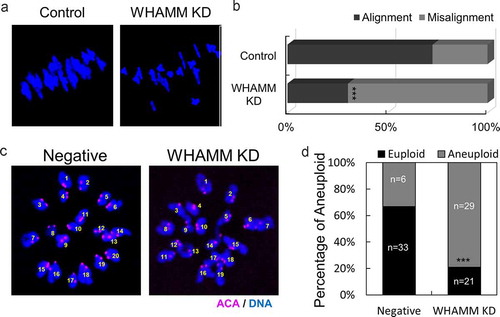
In order to determine whether the defect in meiotic spindle formation was induced by WHAMM, live-cell imaging was performed following the injection of alpha-tubulin-GFP and H2B-mCherry to visualize spindle and chromatin, respectively. The length of the spindles, 5 h after meiotic resumption was significantly longer, and incomplete spindles assembled in WHAMM dsRNA-injected oocytes, as compared to those in control cells (). Next, to investigate the cause of abnormal spindle length, we performed immunostaining using pericentrin as a marker for MTOC. MTOCs clustered at both ends of the spindles in control oocytes and in WHAMM KD groups. However, MTOCs failed to gather at spindle poles and thus caused a failure in the formation of normal bipolar spindles (). Lagging chromosomes were observed in WHAMM knockdown oocytes, as indicated by the white arrow (). While the chromosomes of control oocytes were aligned in the metaphase plate, WHAMM KD oocytes showed a significantly higher ratio of misalignment than the control oocytes (control: 26% vs. WHAMM KD: 70%; ). To investigate the whether the lagging chromosome of WHAMM KD oocytes disrupts chromosomal configuration, we proceed chromosome spread. Finally, chromosomal aneuploidy was significantly increased in WHAMM KD oocytes after polar-body extrusion than in the negative control group ().
Figure 3. Knockdown of WHAMM impairs formation of normal length spindle, localization of the MTOC, and chromosome alignment during early spindle formation. (a) Time-lapse microscopy of maturing control (Negative) and WHAMM-knockdown oocytes. Spindles or chromatin were visualized upon injection of Complementary RNA (cRNA) encoding alpha-tubulin-GFP (green) or H2B-mCherry (Schmitt and Nebreda), respectively. The length of the spindles 5 h after meiotic resumption is marked with a white line. Scale bar: 10 μm. (b) The length of the spindle was determined by quantifying the distance between the two spindle poles in control and WHAMM KD oocytes. n values are indicated. Boxes show interquartile range; whiskers show 1.5x the interquartile range; the line represents the median. ***P < 0.001. (c) WHAMM dsRNA and control dsRNA-injected oocytes were sampled at 3.5, 4, 4.5, 5.5, and 6 h after meiotic resumption. Co-staining of the MTOC using the anti-pericentrin antibody, meiotic spindles, and chromosomes is represented. Localization of pericentrin was impaired and failed to accumulate at spindle poles in WHAMM KD oocytes, as indicated by the arrow. Defective spindle morphology and chromosome alignment in WHAMM KD oocytes are shown. Blue, DNA; red, pericentrin; green, alpha-tubulin. Scale bar: 10 μm. (d) Relative area of MTOC at MI stage oocytes injected by WHAMM dsRNA or control dsRNA. MTOC was stained by the anti-pericentrin antibody. The area of pericentrin was increased in WHAMM KD oocytes than that of the negative control group, as indicated by the arrow. Boxes show interquartile range; whiskers show 1.5x the interquartile range; the line represents the median. ***P < 0.001
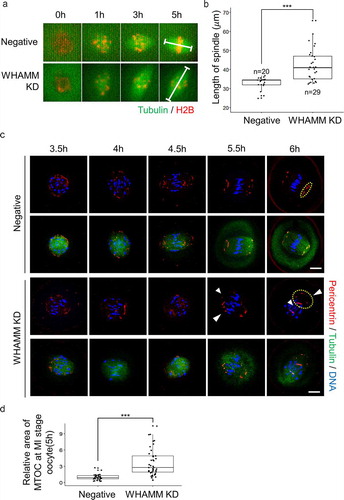
Figure 4. Depletion of WHAMM disrupts chromosome alignment of MI stage oocytes and increases aneuploidy at MII stage oocytes. (a) Chromosome spread of negative and WHAMM-specific dsRNAs-injected oocytes fixed at the MI stage. Blue, DNA. (b) Percentage of distance between the chromosomes in negative and WHAMM knockdown MI oocytes. The chromosomal misalignment of WHAMM was increased in WHAMM KD oocytes. (c) Chromosome spreads of MII stage oocytes and immunostained by anti-ACA antibody for detecting the kinetochore. Pink, ACA; Blue, DNA. (d) Percentage of aneuploidy (including the extra and missing chromosome) of WHAMM KD or negative control at MII stage oocytes. n values are indicated. ***P < 0.001
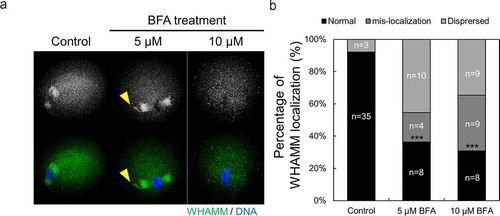
Localization of WHAMM is affected by BFA treatment
In order to identify the function of WHAMM in ER membrane dynamics, the change in localization of WHAMM was examined following BFA treatment, which blocks actin-dependent vesicle transport by indirectly inhibiting vesicle transport from the ER to the Golgi apparatus. WHAMM protein was localized around meiotic spindles and near the chromosome in control oocytes. However, BFA treatment-induced abnormal localization of WHAMM in the 5 μM BFA-treated group (). In case of the 10 μM BFA-treated oocytes, WHAMM failed to accumulate near the chromosomes and dispersed into the cytoplasm of oocyte (). Taken together, we confirmed that WHAMM maintains its normal localization around of spindle and chromosome by binding with ER and golgi structure.
Figure 5. Localization of WHAMM is affected by ER to Golgi apparatus transport. (a and b) Representative images were stained for DNA (blue), cortical actin (Schmitt and Nebreda), and WHAMM (green), showing dispersed localization of WHAMM in control and 50 or 100 μM BFA-treated oocytes. Oocytes were fixed 8 h after meiotic resumption and then immunostained. BFA treatment caused abnormal WHAMM localization and failed accumulation of WHAMM near the chromosome. Scale bar: 20 μm. (c) Abnormal localization of WHAMM increased in BFA-treated oocytes compared to the control group. n values are indicated. ***P < 0.001
Discussion
Actin nucleators that form the cortical and cytoplasmic actin in oocytes are the Arp2/3 [Citation15,Citation16,Citation25] complex and formin [Citation26,Citation27]/spire [Citation9, Citation23, Citation24], respectively [Citation28]. Formation of the spindle actin is significantly decreased in formin knockout mouse oocytes, which leads to the increased chromosome lagging [Citation6]. In addition, abnormal morphology of the meiotic spindle and chromosome misalignment have been reported upon treatment with the formin inhibitor, SMIFH2, or upon knockdown of the formin family proteins, mDIA1 and mDIA2 [Citation29]. In this study, we confirmed that WHAMM is localized surrounding the chromosomes, has similar morphology to that of the spindle, and co-localizes with the spindle actin during mouse oocyte maturation. Considering that WHAMM is a nucleation-promoting factor of the Arp2/3 complex [Citation17], results of our study suggest that the spindle actin is also formed by actin nucleation mediated by the Arp2/3 complex, which is independently promoted by WHAMM or along with the Arp2/3 complex, besides that by formins.
Actin filaments permeating the spindle are very similar in shape to it and are known as spindle actin in maturing mouse oocytes [Citation6] as shown in supplementary Figure 1. Spindle actin, also called actin cage, is crucial for spindle migration and chromosome segregation [Citation6]. Reduced spindle actin formation results in chromosome lagging and increased instability of the meiotic spindle [Citation6] in mouse oocytes. In addition, when cage actin is maintained by treatment with SiR-Actin, the K-fiber bundle of the spindle fails to divide in the MII stage during oocyte maturation [Citation6]. These results indicate that the spindle actin is essential in the spindle formation and maintenance and in chromosome segregation [Citation6]. In this study, depletion of WHAMM using dsRNA injection was shown to impair spindle actin formation, which is crucial for cell division and which causes defective polar body extrusion during mouse oocyte maturation. Thus, it is clear that the asymmetric cell division of oocytes is regulated by WHAMM, which controls spindle actin during oocyte maturation.
WHAMM binds directly to microtubules, actin, the Arp2/3 complex, as well as to ER and Golgi transporter vesicles, and co-localizes with the Golgi membrane during the tabulation process in the cell [Citation13,Citation17]. It might play a crucial role in the interaction with microtubules and clotting factors in oocytes and also in the formation of the meiotic spindle. In the early stages of oocyte maturation, the meiotic spindle is formed from the MTOC. The MTOC is scattered around cytoplasm, followed by oocyte maturation and formation of the bipolar spindle, where MTOC is clustered at both ends of the spindle [Citation30] in maturing mouse oocytes. However, the functional role of WHAMM in the clustering of the MTOC and spindle formation in mouse oocyte maturation has not been investigated. In this study, WHAMM knockdown impaired migration and clustering of the MTOC, which probably resulted in abnormally long meiotic spindles. The depletion of WHAMM significantly increased chromosome disalignment at the MI stage, and finally, it caused chromosomal aneuploidy and failure of asymmetric division at the MII stage. Collectively, our results confirm that WHAMM has a direct impact on spindle actin and spindle formation by regulating MTOC clustering and migration, and accurate segregation of chromosomes during mouse oocyte maturation.
In this study, treatment with BFA, an inhibitor of vesicle transport from the ER to the Golgi apparatus, caused dispersal or mis-localization of WHAMM. In previous study, BFA treatment disrupts meiotic spindle positioning and asymmetric division in maturing oocyte [Citation31]. The ER surrounds the spindle [Citation32], similar to the localization of WHAMM, as confirmed in this study. Taken together, these results indicate that WHAMM is distributed near the chromosomes and has cage actin spindle-like morphology and is involved in linking ER membrane and microtubules in oocyte cytoplasm during oocyte maturation. Considering that WHAMM is associated with the ER membrane tubulation [Citation13,Citation17], MTOC clustering might be caused by actin filament-mediated transport. Furthermore, it is necessary to elucidate whether these functional roles of WHAMM are associated with the Arp2/3 complex.
Supplemental Material
Download MS Word (158.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- White SJ, Jacobs RS. Effect of stypoldione on cell cycle progression, DNA and protein synthesis, and cell division in cultured sea urchin embryos. Mol Pharmacol. 1983;24:500–508.
- Yi K, Li R. Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation. Cytoskeleton. 2012;69:727–737.
- Maro B, Johnson MH, Pickering SJ, et al. Changes in actin distribution during fertilization of the mouse egg. J Embryol Exp Morphol. 1984;81:211–237.
- Chaigne A, Campillo C, Gov NS, et al. A soft cortex is essential for asymmetric spindle positioning in mouse oocytes. Nat Cell Biol. 2013;15:958–966.
- Chaigne A, Campillo C, Voituriez R, et al. F-actin mechanics control spindle centring in the mouse zygote. Nat Commun. 2016;7:10253.
- Mogessie B, Schuh M. Actin protects mammalian eggs against chromosome segregation errors. Science. 2017;357. DOI:10.1126/science.aal1647
- Plessner M, Knerr J, Grosse R. Centrosomal actin assembly is required for proper mitotic spindle formation and chromosome congression. iScience. 2019;15:274–281.
- Azoury J, Lee KW, Georget V, et al. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–1519.
- Pfender S, Kuznetsov V, Pleiser S, et al. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol. 2011;21:955–960.
- Deng M, Suraneni P, Schultz RM, et al. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar-body extrusion in mouse oocytes. Dev Cell. 2007;12:301–308.
- Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–317.
- Stradal TE, Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol. 2006;18:4–10.
- Rottner K, Hanisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–661.
- Dehapiot B, Carriere V, Carroll J, et al. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev Biol. 2013;377:202–212.
- Sun SC, Wang ZB, Xu YN, et al. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PloS One. 2011;6:e18392.
- Yi K, Unruh JR, Deng M, et al. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol. 2011;13:1252–1258.
- Campellone KG, Webb NJ, Znameroski EA, et al. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell. 2008;134:148–161.
- Disanza A, Scita G. Cytoskeletal regulation: coordinating actin and microtubule dynamics in membrane trafficking. Curr Biol. 2008;18:R873–875.
- Kast DJ, Zajac AL, Holzbaur EL, et al. WHAMM directs the Arp2/3 complex to the ER for autophagosome biogenesis through an actin comet tail mechanism. Curr Biol. 2015;25:1791–1797.
- Russo AJ, Mathiowetz AJ, Hong S, et al. Rab1 recruits WHAMM during membrane remodeling but limits actin nucleation. Mol Biol Cell. 2016;27:967–978.
- Huang X, Ding L, Pan R, et al. WHAMM is required for meiotic spindle migration and asymmetric cytokinesis in mouse oocytes. Histochem Cell Biol. 2013;139:525–534.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408.
- Manseau LJ, Schupbach T. cappuccino and spire: two unique maternal-effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes Dev. 1989;3:1437–1452.
- Jo YJ, Jang WI, Namgoong S, et al. Actin-capping proteins play essential roles in the asymmetric division of maturing mouse oocytes. J Cell Sci. 2015;128:160–170.
- Yi K, Rubinstein B, Unruh JR, et al. Sequential actin-based pushing forces drive meiosis I chromosome migration and symmetry breaking in oocytes. J Cell Biol. 2013;200:567–576.
- Dumont J, Million K, Sunderland K, et al. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev Biol. 2007;301:254–265.
- Leader B, Lim H, Carabatsos MJ, et al. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol. 2002;4:921–928.
- Namgoong S, Kim NH. Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle. 2016;15:1830–1843.
- Kim HC, Jo YJ, Kim NH, et al. Small molecule inhibitor of formin homology 2 domains (SMIFH2) reveals the roles of the formin family of proteins in spindle assembly and asymmetric division in mouse oocytes. PloS One. 2015;10:e0123438.
- Clift D, Schuh M. A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat Commun. 2015;6:7217.
- Wang L, Wang ZB, Zhang X, et al. Brefeldin A disrupts asymmetric spindle positioning in mouse oocytes. Dev Biol. 2008;313:155–166.
- FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol. 2007;305:133–144.
