ABSTRACT
This study aimed to determine the mechanism underlying the regulation of gout by the HOX transcript antisense RNA (HOTAIR) long non-coding RNA (lncRNA). The expression levels of HOTAIR, miR-20b, and Nlrp3 were estimated by qRT-PCR and western blotting. The methylation level of HOTAIR was detected by methylation-specific PCR. The recruitment of DNA methyltransferase 1 (DNMT1) to the lncRNA HOTAIR promoter was confirmed by a ChIP assay. RNA immunoprecipitation and RNA pull-down assays were used to confirm the interaction between HOTAIR and miR-20b. LncRNA HOTAIR and Nlrp3 expression was upregulated, and that of miR-20b was downregulated in synovial fluid mononuclear cells (SFMCs) collected from patients with gouty arthritis and monosodium urate (MSU)-stimulated THP-1 cells. Interleukin (IL)-1β level increased substantially upon stimulation by MSU crystals. The methylation percentage of HOTAIR was reduced in SFMCs from patients with gouty arthritis and MSU-stimulated THP-1 cells. DNMT1 expression was downregulated in MSU-stimulated THP-1 cells, and DNMT1 knockdown increased lncRNA HOTAIR expression. In addition, the interaction of HOTAIR with miR-20b was confirmed. HOTAIR knockdown suppressed Nlrp3 expression and the secretion of inflammatory cytokines via miR-20b regulation. Finally, in vivo experiments showed that HOTAIR knockdown alleviated ankle swelling in a mouse model of gouty arthritis. These findings suggest that lncRNA HOTAIR knockdown suppresses inflammatory cytokine secretion by upregulating miR-20b and downregulating NLRP3, thereby alleviating ankle swelling in gouty arthritis.
Introduction
Gouty arthritis is a type of inflammatory arthritis caused by the precipitation of uric acid and generation of monosodium urate (MSU) crystals in the joints, and has shown increased incidence recently, especially in developed countries [Citation1,Citation2]. MSU crystals deposited in the joints activate an acute inflammatory response, which induces acute gout attacks [Citation3]. Gouty arthritis usually includes both acute and chronic forms of inflammation. Acute gouty arthritis is characterized by a rapid onset of pain in the affected joints, with the onset of pain being self-limiting and usually lasting 3–14 days [Citation4]. Studies have shown that monocytes/macrophages are involved in the regulation of MSU-induced inflammatory response in patients with gouty arthritis, since monocytes/macrophages in synovial fluid of patients with acute gout attacks are known to produce higher levels of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β [Citation5]. In vitro experiments have also shown that MSU stimulation activates caspase-1 and promotes mature IL-1β secretion and TNF-α production in human monocytes and macrophages [Citation6]. Altogether, these studies indicate that monocytes/macrophages are involved in the MSU-induced inflammatory response in gouty arthritis.
Nucleotide-binding oligomerization domain-like receptor pyrin domain-containing protein 3 (NLRP3) is a member of the NLR family, which forms NLRP3 inflammasome with apoptosis-associated speck-like protein and caspase-1 [Citation7]. The NLRP3 inflammasome plays an important role in regulating inflammation and immunity, which requires NF-κB signaling and toll-like receptor stimulation [Citation8]. Recent studies have shown that Nlrp3 expression is increased in peripheral blood mononuclear cells and synovial cells from patients with gouty arthritis, and MSU activates the NLRP3 inflammasome in macrophages, which subsequently triggers IL-1β release [Citation9]. In a mouse model of gouty arthritis, the NLRP3 inflammasome inhibitor OLT1177 was found to reduce joint swelling and downregulate NLRP3 and IL-1β expression level in synovial samples [Citation10]. On the contrary, NLRP3 knockdown inhibited the activation of NLRP3 inflammasome and reduced the secretion of IL-1β and IL-18 in RAW264.7 macrophages [Citation11]. These studies indicate that the NLRP3 inflammasome in macrophages contributes to gouty arthritis. However, the mechanistic basis of the formation and regulation of the NLRP3 inflammasome in macrophages remains unclear.
MicroRNAs are a type of small noncoding RNA involved in the regulation of the inflammatory response in arthritis, such as gouty arthritis, rheumatoid arthritis, and osteoarthritis [Citation12,Citation13]. Increasing evidence indicates that microRNAs are aberrantly expressed in MSU-induced macrophages and joint tissues, where these act as proinflammatory regulators to modulate the production of inflammatory cytokines in gouty arthritis [Citation14–16]. MiR-20b is an inflammation-related miRNA aberrantly expressed in monocytes/macrophages in acute inflammatory lung injury and tuberculosis [Citation17,Citation18]. MiR-20b is known to reduce inflammatory gene expression in fibroblasts and T cells, thereby inhibiting pro-inflammatory signaling [Citation19]. Importantly, NLRP3 has been reported to be a direct target of miR-20b, and miR-20b overexpression reduces inflammatory response and inhibits the NLRP3/caspase-1/IL-1β pathway [Citation20]. However, whether miR-20b is involved in the regulation of Nlrp3 expression and inflammatory response in MSU-induced macrophages is largely unknown.
Recently, long non-coding RNAs (lncRNAs) have been identified as important modulators that mediate arthritis through regulating chondrocyte extracellular matrix degradation, inflammatory signaling, migration, and invasion of fibroblast-like synoviocytes [Citation21,Citation22]. The HOX transcript antisense RNA (HOTAIR) lncRNA is expressed at high levels in osteoarthritis articular chondrocytes, and blood mononuclear cells and serum exosome of rheumatoid arthritis patients, which leads to macrophage migration [Citation23,Citation24]. LncRNA HOTAIR is also upregulated in the synovial fluid of temporomandibular joint osteoarthritis patients and increases chondrocytes apoptosis [Citation25]. However, the role of lncRNA HOTAIR in gouty arthritis is not known. Bioinformatics-based analysis performed using the DIANA TOOLS software has identified putative binding sites for the interaction of HOTAIR and miR-20b. Therefore, we hypothesized that lncRNA HOTAIR promotes inflammatory response in gouty arthritis through miR-20b downregulation and Nlrp3 and IL-1β upregulation.
Materials and methods
Clinical samples and isolation of peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs)
Patients with acute gouty arthritis (n = 15) and healthy controls (n = 10) were recruited from The First Affiliated Hospital of Zhengzhou University. All patients provided informed consent. The clinical characteristics of gout patients are shown in . The experiments were approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University. Peripheral blood and synovial samples were collected from patients with acute gouty arthritis, and only peripheral blood samples were collected from healthy controls. Synovial samples were collected by needle aspiration of actively inflamed large or medium joints by an experienced rheumatologist. Polarized light microscopy was used to confirm the presence of monosodium urate (MSU) crystals in synovial samples. Peripheral blood and synovial samples were collected in tubes containing heparin. PBMCs were isolated from peripheral blood, and SFMCs were isolated from 7 to 11-mL aliquots of synovial samples using Histopaque-1077 (Sigma-Aldrich, USA) according to the manufacturer’s instructions.
Table 1. The clinical characteristics of patients with gout
Preparation of MSU crystals
MSU crystals were prepared according to a previous report [Citation26]. Uric acid (1 g; Sigma Aldrich USA) was dissolved in 180 ml of 0.01 M NaOH and heated to 70°C. The pH of the solution was maintained at 7.2, and the solution was cooled by continuous stirring at room temperature for 24 h. The MSU crystals were collected in a sterilized container, washed with ethanol, dried, and re-suspended in PBS by sonication. The MSU crystals prepared this way contained < 0.005 EU/mL endotoxin, as detected by a Pierce Chromogenic Endotoxin Quant Kit (Thermo Fisher Scientific, USA).
Cell culture and MSU stimulation
Human monocyte THP-1 was purchased from the American Type Culture Collection (ATCC, USA). THP-1 cells were cultured in RPMI 1640 medium (ThermoFisher Scientific, USA) supplemented with 0.05 mM 2-mercaptoethanol (Sinopharm Chemical Reagent, China) and 10% fetal bovine serum (ThermoFisher Scientific, USA) at 37°C in a humidified incubator containing 5% CO2. Stimulation with MSU was performed as previously reported [Citation26]. THP-1 cells were seeded in 6-well plates at a density of 1.5 × 106 cells/mL. The plated cells were first treated with 100 ng/mL Phorbol 12-myristate 13-acetate (Sigma-Aldrich, USA) for 3 h to enhance the phagocytic properties and induce a constitutive production of pro-IL-1β. Then, the THP-1 cells were stimulated with 100 μg/mL MSU for 24 h [Citation27].
Cell transfection
Adenovirus-mediated DNA methyltransferase 1 (DNMT1) overexpression (Ad-DNMT1) and scramble sequence set (Ad-NC; negative control), adenovirus-mediated DNMT1 knockdown (si-DNMT1) and scramble sequence set (si-NC), adenovirus-mediated HOTAIR knockdown (si-HOTAIR) and si-control, adenovirus-mediated miR-20b inhibitor, and lentivirus-mediated HOTAIR knockdown (Lenti-si-HOTAIR) and si-control were prepared by GeneChem (Shanghai, China). The viruses were packaged in HEK 293 T cells according to standardized protocols. THP-1 cells were seeded into a 24-well plate at a density of 2 × 104 cells/well until 70% confluence, and then transfected with Ad-DNMT1, si-DNMT1, si-HOTAIR, or negative controls for 72 h.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from PBMCs, SFMCs, or THP-1 cells using TRIzol reagent (ThermoFisher Scientific, USA) according to the manufacturer’s instructions. For the quantification of HOTAIR and miR-20b expression, total RNA was reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) according to the manufacturer’s instructions. qRT-PCR was performed using the SuperScript™ III Platinum™ SYBR™ Green One-Step qRT-PCR Kit (Invitrogen, USA) according to the manufacturer’s instructions. The relative expression of HOTAIR and miR-20b were quantified as a function of the threshold cycle (Ct) and analyzed by the 2−ΔΔCT method. GAPDH or U6 were used as internal controls. The primers used are listed in .
Table 2. The primer sequence
Western blotting
Proteins from PBMCs, SFMCs, or THP-1 cells were extracted using RIPA Lysis and Extraction Buffer (ThermoFisher Scientific, USA) and centrifuged at 12,000 × g for 10 min at 4°C. Protein concentration was measured using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, USA) according to the manufacturer’s instructions. Protein samples were mixed with sample buffer, boiled for 10 min, separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to PVDF membranes (Invitrogen, USA). The membranes were blocked with 5% nonfat dry milk for 1 h at 25°C, and incubated with the primary antibodies against NLRP3 (1:500; Abcam, USA, ab214185), DNMT1 (1:1000; Abcam, USA, ab87654), and β-actin (1:500; Abcam, Mass, USA, ab8227) overnight at 4°C. Then, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibodies (1:1000; Abcam, USA, ab205718) for 2 h at room temperature. Blots were visualized using the Pierce ECL Western Blotting Substrate (ThermoFisher Scientific, USA) on the Gel Imaging System (Bio-Rad, USA).
Enzyme-linked immunosorbent assay (ELISA)
IL-1β and TNF-α secretion in the supernatants of THP-1 cells was detected by the Human IL-1β ELISA kit (ImmunoWay Biotechnology, USA) and TNF alpha Human ELISA Kit (Invitrogen, USA) according to the manufacturers’ instructions.
Analysis of methylation levels
The methylation level of the HOTAIR promoter was detected by methylation-specific PCR. HOTAIR DNA was isolated with the DNA Extract All Reagents Kit (Applied Biosystems, USA), and HOTAIR DNA methylation was performed using the EpiJET DNA Methylation Analysis Kit (ThermoFisher Scientific, USA) according to the manufacturer’s instructions.
Chromatin immunoprecipitation assay (ChIP)
A ChIP assay was conducted using the Pierce™ Agarose ChIP Kit (ThermoFisher Scientific, USA) according to the manufacturer’s instructions. Chromatin was extracted and DNA was cut into 0.2–1 kb fragments. THP-1 cells were cross-linked using 1% formaldehyde, and the chromatin was immunoprecipitated with the DNMT1 antibody (Abcam). Isotype-specific IgG was used as a control. DNA was re-suspended in 50 μL of TE buffer and amplified by qRT-PCR. The primers for the HOTAIR promoter are shown in .
RNA immunoprecipitation (RIP) and RNA pull-down assays
RIP and RNA pull-down assays were conducted to confirm the interaction between HOTAIR and miR-20b. RIP assay was performed using the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Merck Millipore, USA) according to the manufacturer’s instruction. HEK 293 T cell lysate was prepared from 1.5 × 107 cells using 100 μL of RIP lysis buffer containing 0.25 μL of RNase inhibitor and 0.5 μL of 200X protease inhibitor cocktail. Then, the cell lysate was centrifuged, and the supernatant was collected and incubated with beads for 1.5 h at 4°C. The RNA–protein complex generated this way was pulled-down using protein A/G magnetic beads. Western blotting was used to detect AGO2 and qRT-PCR was used to check for lncRNA HOTAIR and miR-20b in the precipitates. An RNA pull-down assay was further conducted to confirm the interaction between HOTAIR and miR-20b. Biotin-labeled lncRNA HOTAIR was transcribed with T7 RNA Polymerase (Invitrogen, USA) and Biotin RNA Labeling Mix (Roche, Switzerland). HEK 293 T cells transfected with biotin-labeled lncRNA HOTAIR were collected and sonicated for 48 h. Beads were added to the resultant cell lysates and incubated for 2 h at room temperature. Western blotting was used to check for AGO2 in the cell lysates, and qRT-PCR was used to detect miR-20b in the HOTAIR pull-down complex.
Animal model experiments
Ten-week-old male C57BL/6 mice were used in this experiment according to previous literature [Citation28]. Mice were purchased from the Laboratory Animal Center of Zhengzhou University and maintained in a standard feeding environment without limitation to food and water. The protocol for these animal experiments was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University. MSU crystals (2 mg) suspended in 10 μL PBS were intraarticularly injected into both ankle joints of the mice to establish a mouse model of gouty arthritis. After treatment with MSU crystals for 24 h, lentivirus-mediated si-HOTAIR (Lenti-si-HOTAIR) and si-control (Lenti-NC) (1 × 109 plaque-forming units) were intra-articularly injected into one ankle joint and the contralateral ankle, respectively, of mice with gouty arthritis. The extent of swelling at 8th, 12th, 15th, and 19th day after Lenti-si-HOTAIR or Lenti-NC injection in ankles was measured using a precision caliper. Mice were euthanized by CO2 inhalation, and ankle tissues were collected 19 days after Lenti-si-HOTAIR or Lenti-NC injection.
Hematoxylin-eosin (HE) staining
Joints were collected from mice with gouty arthritis, fixed with 10% formalin, and embedded in paraffin. The joints were then cut into 4-μm thick sections and stained with a Hematoxylin and Eosin Staining Kit (Beyotime Biotechnology, China) for histological examination. Images were acquired using a microscope (Olympus, Japan).
Statistical analysis
All data are presented as mean±standard deviation. Differences between the two groups were determined by Student’s t-test, and those among groups were determined by one-way analysis of variance. P< 0.05 was considered statistically significant.
Results
Increased expression of lncRNA HOTAIR in SFMCs from patients with acute gouty arthritis
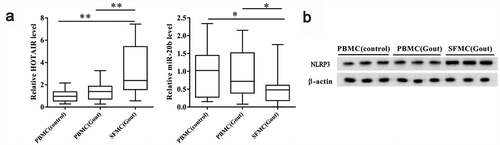
We analyzed lncRNA HOTAIR, miR-20b, and Nlrp3 expression levels in PBMCs and SFMCs. LncRNA HOTAIR and miR-20b expression in SFMCs from gout patients was significantly upregulated and downregulated, respectively, compared to those in PBMCs from healthy controls and gout patients (P< 0.05; ). There were no significant changes in lncRNA HOTAIR and miR-20b expression in PBMCs collected from healthy controls and gout patients. In addition, the NLRP3 protein levels in SFMCs from gout patients were upregulated compared to those in PBMCs from healthy controls and gout patients ().
Figure 1. LncRNA HOTAIR, miR-20b, and Nlrp3 expression levels in SFMCs from patients with acute gouty arthritis. Samples were collected from patients with acute gouty arthritis (n = 15) and healthy controls (n = 10), for which peripheral blood and synovial samples were collected from patients with acute gouty arthritis and peripheral blood samples were collected from healthy controls. PBMCs were isolated from peripheral blood, and SFMCs were isolated from synovial samples. A. Compared with PBMC (control) and PBMC (Gout) groups, lncRNA HOTAIR expression was up-regulated in the SFMC (Gout) group, whereas miR-20b expression was down-regulated in the SFMC (Gout) group. There were no significant changes in lncRNA HOTAIR and miR-20b expressions between PBMC (control) and PBMC (Gout) groups. B. Western blot showed that the NLRP3 protein level was up-regulated in SFMC (Gout) group than PBMC (control) and PBMC (Gout) groups. *P< 0.05, compared with PBMC (control) or PBMC (Gout)
Increased expression of lncRNA HOTAIR in MSU-stimulated THP-1 cells
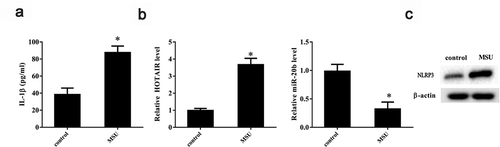
Given our observation that lncRNA HOTAIR was upregulated in SFMCs from gout patients, we checked whether MSU (a key factor in gout pathogenesis) could promote lncRNA HOTAIR expression in THP-1 cells in vitro. As shown in , IL-1β level increased appreciably in THP-1 cells on stimulation with MSU crystals (P< 0.05), indicating that an in vitro gouty arthritis model was successfully established. LncRNA HOTAIR expression was also significantly upregulated due to stimulation with MSU crystals (P< 0.05) (), whereas miR-20b expression was significantly downregulated (P< 0.05) (). Further, NLRP3 protein level was upregulated on MSU stimulation ().
Figure 2. LncRNA HOTAIR, miR-20b, and Nlrp3 expressions in THP-1 cells stimulated with MSU crystals. THP-1 cells were treated with 100 ng/mL PMA for 3 h, then THP-1 cells were stimulated with 100 μg/mL MSU crystals. A. Compared with the control group, the IL-1β level was up-regulated in the MSU group. B. Compared with the control group, lncRNA HOTAIR expression was up-regulated in the MSU group, and miR-20b expression was down-regulated in the MSU group. C. NLRP3 protein level was up-regulated in the MSU group. *P< 0.05, compared with control. Data are pooled from three individual experiments
DNA methylation-regulated lncRNA HOTAIR expression
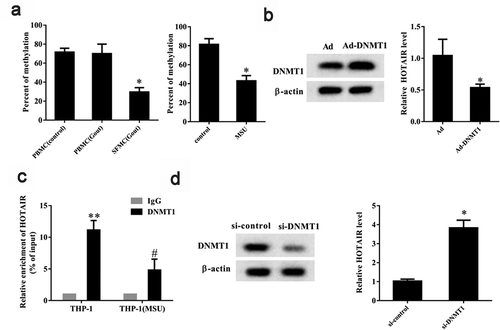
The abnormal expression of DNMT1-associated lncRNAs in colon cancer cells have been reported to affect their clonogenic potential, suggesting DNMT-mediated DNA methylation can regulate lncRNAs associated with pathological processes [Citation29]. Therefore, we focused on the potential role of DNA methylation in the regulation of lncRNA HOTAIR expression. We found, using methylation-specific PCR, that the DNA methylation level in SFMCs from gout patients was significantly lower (P< 0.05) than that in PBMCs from healthy controls and gout patients (). This suggests that the promoter region of HOTAIR was hypomethylated in SFMCs. DNA methylation in MSU-stimulated THP-1 cells showed a stark decrease compared to that in control (P < 0.05) (), also indicating that the promoter region of HOTAIR was hypomethylated in MSU-stimulated THP-1 cells. To gain more insights into the role of DNMT1 in the regulation of HOTAIR expression, MSU-stimulated THP-1 cells were transfected with Ad-DNMT1 or Ad-NC. We found that Ad-DNMT1 upregulated DNMT1 protein level and significantly downregulated lncRNA HOTAIR expression (P< 0.05) (). A ChIP assay showed that the recruitment of DNMT1 to lncRNA HOTAIR promoter decreased in MSU-stimulated THP-1 cells compared to control (P< 0.05) (). Further, si-DNMT1 decreased DNMT1 protein level and promoted lncRNA HOTAIR expression in THP-1 cells (P< 0.05) (). These findings suggested that DNMT-mediated DNA methylation could regulate lncRNA HOTAIR expression.
Figure 3. DNA methylation regulated lncRNA HOTAIR expression. A. The percent of methylation was detected in PBMC (control), PBMC (Gout), and SFMC (Gout) groups by methylation-specific PCR. Compared with the control group, the percent of methylation was low in the MSU group. *P< 0.05, compared with PBMC (control), PBMC (Gout), or control. B.THP-1 cells stimulated with MSU crystals were transfected with Ad-DNMT1 (DNA methyltransferase) or adenovirus (Ad). Western blot showed that the DNMT1 protein level was up-regulated in the Ad-DNMT1 group than the Ad group. qRT-PCR showed that lncRNA HOTAIR expression was down-regulated in the Ad-DNMT1 group than the Ad group. *P< 0.05, compared with Ad. C. ChIP assay showed that the recruitment of DNMT1 to lncRNA HOTAIR promoter decreased in the THP-1 (MSU) group than the THP-1 group. **P< 0.01, compared with IgG in THP-1 group; #P< 0.05, compared with DNMT1 in THP-1 group. D. THP-1 cells were transfected with si-DNMT1 or si-control. DNMT1 protein level was down-regulated in the si-DNMT1 group than the si-control group. LncRNA HOTAIR expression was up-regulated in the si-DNMT1 group than the si-control group. *P< 0.05, compared with si-control. Data are pooled from three individual experiments
HOTAIR knockdown inhibited inflammatory cytokine secretion in monocytes
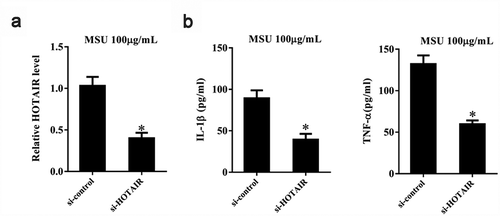
MSU crystals have been earlier shown to activate the NLRP3 inflammasome, thereby resulting in the production of active IL-1β [Citation6]. In addition, MSU is known to induce TNF-α release [Citation30]. Therefore, we investigated the effect of HOTAIR on IL-1β and TNF-α secretion in MSU-stimulated THP-1 cells. si-HOTAIR significantly downregulated lncRNA HOTAIR expression (P< 0.05) (). Moreover, IL-1β and TNF-α levels were significantly decreased by si-HOTAIR treatment (P< 0.05) ().
Figure 4. HOTAIR knockdown inhibited the secretion of inflammatory cytokines by monocytes. THP-1 cells stimulated with MSU crystals were transfected with adenovirus-mediated si-HOTAIR or si-control. A. LncRNA HOTAIR expression was down-regulated in the si-HOTAIR group than si-control. B. IL-1β and TNF-α levels were reduced in the si-HOTAIR group than si-control. *P< 0.05, compared with si-control. Data are pooled from three individual experiments
Regulation of miR-20b by lncRNA HOTAIR
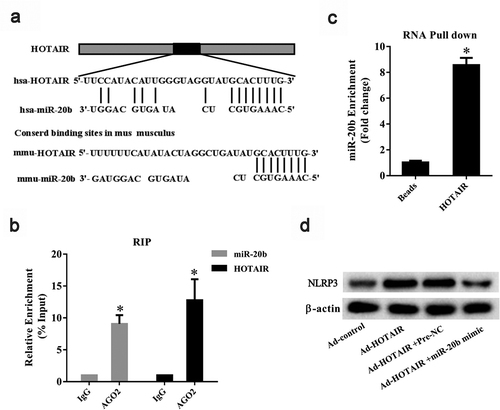
Predictive analysis using the bioinformatics software DIANA TOOLS (http://diana.imis.athena-innovation.gr/DianaTools/index.php) showed the presence of putative binding sites between HOTAIR and miR-20b (). As shown in , AGO2 could be detected in precipitates using the AGO2 antibody. In addition, HOTAIR and miR-20b were enriched in the AGO2 complex compared to the IgG group (P< 0.05) () and miR-20b was enriched in the HOTAIR pull-down complex (P< 0.05) (). Ad-HOTAIR was observed to upregulate NLRP3 protein level in THP-1 cells, and a miR-20b mimic abolished this effect of Ad-HOTAIR ().
Figure 5. The regulation role of lncRNA HOTAIR on miR-20b. A. Binding sites between HOTAIR and miR-20b were predicted by bioinformatics software. B. RIP assay was used to confirm the interaction between HOTAIR and miR-20b. qRT-PCR showed that HOTAIR and miR-20b were enriched in the AGO2 complex. *P< 0.05, compared with IgG. C. RNA pull-down was used to confirm the interaction between HOTAIR and miR-20b. miR-20b was enriched in HOTAIR pull-down complex *P< 0.05, compared with beads. D. The protein level of NLRP3 in THP-1 cells was detected using Western blot. Data are pooled from three individual experiments
LncRNA HOTAIR regulated Nlrp3 expression and inflammatory cytokine secretion in monocytes through miR-20b
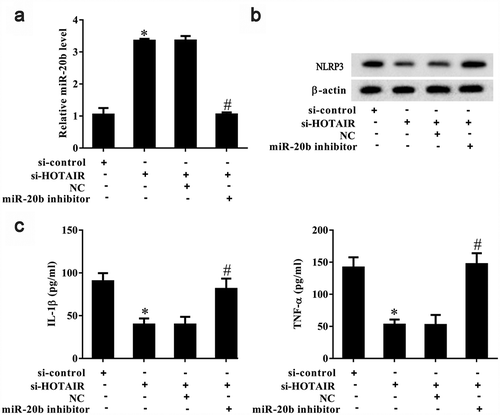
The above results suggest that lncRNA HOTAIR regulated the secretion of inflammatory cytokines in monocytes (). In addition, miR-20b is known to target NLRP3 in HEK293 cells and inhibit the activation of the NLRP3/caspase-1/IL-1β pathway [Citation20]. As shown in , si-HOTAIR significantly increased miR-20b expression in MSU-stimulated THP-1 cells (P< 0.05), and miR-20b inhibition reversed this trend. si-HOTAIR was also observed to significantly downregulate Nlrp3 expression in MSU-stimulated THP-1 cells, which was abolished in the presence of a miR-20b inhibitor (). si-HOTAIR significantly inhibited IL-1β and TNF-α expression in MSU-stimulated THP-1 cells, and a miR-20b inhibitor could suppress this inhibitory effect (P< 0.05) ().
Figure 6. LncRNA HOTAIR regulated Nlrp3 expression and inflammatory cytokines secreted by monocytes through miR-20b. THP-1 cells stimulated with MSU crystals were divided into si-control, si-HOTAIR, si-HOTAIR+NC, and si-HOTAIR+miR-20b inhibitor groups. A. si-HOTAIR increased miR-20b expression, and miR-20b inhibitor reversed the si-HOTAIR-induced promotion effect. B. si-HOTAIR down-regulated NLRP3 expression and miR-20b inhibitor reversed the si-HOTAIR-induced inhibition effect. C. si-HOTAIR decreased IL-1β and TNF-α levels, and miR-20b inhibitor reversed the si-HOTAIR-induced inhibition effect. *P< 0.05, compared with si-control; #P< 0.05, compared with si-HOTAIR+NC. NC: Negative control. Data are pooled from three individual experiments
HOTAIR knockdown suppressed ankle swelling in a mouse model of gouty arthritis
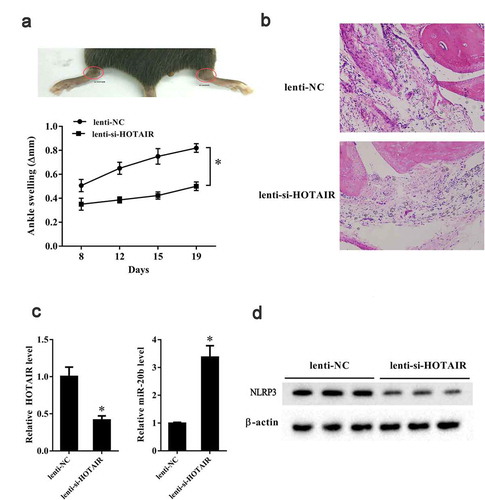
We established an in vivo gouty arthritis mouse model by intra-articular injection of MSU crystal into C57BL/6 mice. Then, Lenti-si-HOTAIR and Lenti-NC were intra-articularly injected into an ankle joint and a contra-lateral ankle and the mouse synovial tissues were isolated. In vivo experiments showed that ankle swelling at 8th, 12th, 15th, and 19th day in ankles injected with Lenti-si-HOTAIR was significantly reduced than that in the contra-lateral ankles injected with Lenti-NC (P< 0.05) (). HE staining showed reduced recruitment of inflammatory cells to the joint in the Lenti-si-HOTAIR group compared to the Lenti-NC group (). LncRNA HOTAIR and miR-20b expression in ankles injected with Lenti-si-HOTAIR was downregulated and upregulated, respectively, compared to that in the contra-lateral ankles injected with Lenti-NC (P< 0.05) (P< 0.05) (). Lenti-si-HOTAIR injection also resulted in decreased NLRP3 expression in ankles in gouty arthritis mice ().
Figure 7. HOTAIR knockdown suppressed ankle swelling in a gouty arthritis mouse model. C57BL/6 mice were intra-articularly injected with 2 mg MSU in 10 μl PBS. After 24 h, lentivirus-mediated si-HOTAIR and si-control were intra-articularly injected into one ankle joint and contra-lateral ankle, respectively. Ten C57BL/6 mice were randomly divided into the following two groups: Lenti-NC group (n = 5) and Lenti-si-HOTAIR group (n = 5). A. Ankle swelling was measured at the 8th, 12th, 15th, 19th day using a precision caliper. B. HE staining of the joint tissue in the two groups (200X) and circles indicated the lymphocytes. C. LncRNA HOTAIR expression was down-regulated in the Lenti-si-HOTAIR group. C. miR-20b expression was up-regulated in the Lenti-si-HOTAIR group. D. Nlrp3 expression was down-regulated in the Lenti-si-HOTAIR group. *P< 0.05, compared with Lenti-NC
Discussion
An increasing number of studies have shown that lncRNAs play important roles in regulating the progression of arthritis [Citation31,Citation32]. In order to identify whether lncRNA HOTAIR was aberrantly expressed in gouty arthritis and determine its effect on this inflammatory disease, qRT-PCR was used to check for the expression of lncRNA HOTAIR in SFMCs from patients with gouty arthritis and MSU-stimulated THP-1 cells. We also explored the effect of lncRNA HOTAIR on the secretion of inflammatory cytokines (IL-1β and TNF-α) in MSU-stimulated THP-1 cells and found that HOTAIR knockdown suppressed IL-1β and TNF-α secretion. In vivo experiments showed that HOTAIR knockdown reduced ankle swelling in a mouse model of gouty arthritis. These findings suggest that lncRNA HOTAIR knockdown could inhibit the secretion of inflammatory cytokines and alleviate ankle swelling in gouty arthritis in vivo.
Recent studies have reported altered DNA methylation during the progression of arthritis [Citation33,Citation34]. DNA methylation is catalyzed by DNA methyltransferases (DNMTs), including DNMT1 (responsible for maintaining methylation), DNMT3a, and DNMT3b (responsible for de novo methylation) [Citation35]. Genome-wide evaluation of DNA methylation loci have shown that hypermethylation and hypomethylation of genes changed gene expression in fibroblast-like synoviocytes from rheumatoid arthritis patients, suggesting that methylated genes play critical roles in the pathogenesis of rheumatoid arthritis [Citation36]. DNMT1, DNMT3a, and DNMT3b expression has been reported to be drastically downregulated in cytokine-stimulated fibroblast-like synoviocytes [Citation33]. In the present study, we found reduced methylation level of HOTAIR in SFMCs from gouty arthritis patients and MSU-stimulated THP-1 cells. Moreover, DNMT1 expression was remarkably downregulated in MSU-stimulated THP-1 cells, and DNMT1 knockdown increased lncRNA HOTAIR expression. These findings collectively suggest that the HOTAIR gene could be regulated by DNA methylation, therefore affecting the expression of lncRNA HOTAIR.
LncRNAs have been shown to regulate the progression of arthritis via competitive binding to miRNAs, and bioinformatics software could be used to predict these putative binding sites [Citation37,Citation38]. In this study, we identified binding sites between lncRNA HOTAIR and miR-20b using bioinformatics-based analysis, which were then experimentally validated using RIP and RNA pull-down assays. In addition, HOTAIR knockdown significantly increased miR-20b expression and decreased IL-1β and TNF-α secretion in MSU-stimulated THP-1 cells, which indicated that lncRNA HOTAIR could negatively regulate miR-20b in monocytes in gouty arthritis. To the best of our knowledge, no other studies have investigated the role of lncRNA HOTAIR/miR-20b in regulating the secretion of inflammatory cytokines in an in vitro gouty arthritis model. Therefore, the present study is expected to enrich existing literature and provide guidance in the treatment of gouty arthritis.
MiRNAs play vital roles in regulating the progression of gouty arthritis. For example, miR-146a knockout mice have been observed to exhibit more severe ankle joint swelling than wild type mice, and miR-146a knockout is known to promote the secretion of pro-inflammatory cytokines in MSU-stimulated macrophages and peritoneal cells [Citation16]. A previous study has demonstrated the suppression of IL-1β protein expression by miR-920 in MSU-stimulated THP-1 cells, suggesting that miR-488 and miR-920 regulated pro-inflammatory cytokines in MSU-stimulated THP-1 cells [Citation39]. In the present study, we found miR-20b downregulation in SFMCs from gouty arthritis patients and MSU-stimulated THP-1 cells. Treatment with a miR-20b inhibitor remarkably increased NLRP3 protein level and IL-1β and TNF-α secretion in MSU-stimulated THP-1 cells, suggesting that miR-20b could negatively regulate NLRP3 in an in vitro gouty arthritis model. Therefore, lncRNA HOTAIR might regulate Nlrp3 expression and inflammatory cytokine secretion in monocytes via negative regulation of miR-20b.
Conclusion
In conclusion, this study demonstrated that lncRNA HOTAIR and Nlrp3 were upregulated and miR-20b was downregulated in SFMCs from gouty arthritis patients and MSU-stimulated THP-1 cells. In vitro experiments showed that lncRNA HOTAIR expression was regulated by DNA methylation, and HOTAIR knockdown suppressed Nlrp3 expression and inflammatory cytokine secretion via miR-20b regulation. In vivo experiments showed that HOTAIR knockdown alleviated ankle swelling in a mouse model of gouty arthritis.
Highlights
1. We have reported, for the first time, the interaction between lncRNA HOTAIR and miR-20b.
2. HOTAIR knockdown suppressed NLRP3 expression via regulation of miR-20b.
3. This work represents the first report that HOTAIR knockdown alleviated ankle swelling in a mouse model of gouty arthritis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Luo Y, Wang L, Peng A, et al. Metabolic profiling of human plasma reveals the activation of 5-lipoxygenase in the acute attack of gouty arthritis. Rheumatology (Oxford). 2018;58(2):1–7.
- Kuo C, Grainge MJ, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–662.
- Hsu D-Z, Chen S-J, Chu P-Y, et al. Therapeutic effects of sesame oil on monosodium urate crystal-induced acute inflammatory response in rats. SpringerPlus. 2013;2:659.
- Neogi T. Clinical practice. Gout N Engl J Med. 2011;364(5):p. 443–52.
- Jeong JH, Hong S, Kwon OC, et al. CD14(+) cells with the phenotype of infiltrated monocytes consist of distinct populations characterized by anti-inflammatory as well as pro-inflammatory activity in gouty arthritis. Front Immunol. 2017;8:1260.
- Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241.
- Haneklaus M, O’Neill L. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62.
- Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40(3):620–623.
- Chang WC, Jan Wu Y-J, Chung W-H, et al. Genetic variants of PPAR-gamma coactivator 1B augment NLRP3-mediated inflammation in gouty arthritis. Rheumatology (Oxford). 2017;56(3):457–466.
- Marchetti C, Swartzwelter B, Koenders MI, et al. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther. 2018;20(1):169.
- Xie Q, SHEN -W-W, ZHONG J, et al. Lipopolysaccharide/adenosine triphosphate induces IL‑1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells. Int J Mol Med. 2014;34(1):341–349.
- Gao J, Kong R, Zhou X, et al. MiRNA-126 expression inhibits IL-23R mediated TNF-α or IFN-γ production in fibroblast-like synoviocytes in a mice model of collagen-induced rheumatoid arthritis. Apoptosis. 2018;23(11):607–615.
- Xu D, Song M, Chai C, et al. Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4, J. Cell Physiol. 2019;234(2):1502–1511
- Jin H, Kim TJ, Choi JH, et al. MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis. Arthritis Res Ther. 2014;16(2):1-9.
- Ma T, Liu X, Cen Z, et al. MicroRNA-302b negatively regulates IL-1β production in response to MSU crystals by targeting IRAK4 and EphA2. Arthritis Res Ther. 2018;20(1):34.
- Zhang Q, Qing YF, Yin CC, et al. Mice with miR-146a deficiency develop severe gouty arthritis via dysregulation of TRAF 6, IRAK 1 and NALP3 inflammasome. Arthritis. Res. Ther. 2018;20(1):45.
- Rao R, Nagarkatti P, Nagarkatti M. Role of miRNA in the regulation of inflammatory genes in staphylococcal enterotoxin B-induced acute inflammatory lung injury and mortality. Toxicol Sci. 2015;144(2):284–297.
- Zhang D, Yi Z, Fu Y. Downregulation of miR-20b-5p facilitates Mycobacterium tuberculosis survival in RAW 264.7 macrophages via attenuating the cell apoptosis by Mcl-1 upregulation.. J Cell Biochem. 2019;120(4):5889–5896.
- Figueiredo Neto M, Figueiredo M. Combination of Interleukin-27 and MicroRNA for enhancing expression of anti-inflammatory and proosteogenic genes. Arthritis. 2017;2017:6365857.
- Lou J, Wang Y, Zhang Z, et al. MiR-20b inhibits mycobacterium tuberculosis induced inflammation in the lung of mice through targeting NLRP3. Exp Cell Res. 2017;358(2):120–128.
- Liu Q, Zhang X, Dai L, et al. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 2014;66(4):969–978.
- Chew C, Conos SA, Unal B, et al. Noncoding RNAs: master regulators of inflammatory signaling. Trends Mol Med. 2018;24(1):66–84.
- Dou P, Hu R, Zhu W, et al. Long non-coding RNA HOTAIR promotes expression of ADAMTS-5 in human osteoarthritic articular chondrocytes. Pharmazie. 2017;72(2):113–117.
- Song J, Kim D, Han J, et al. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med. 2015;15(1):121–126.
- Zhang C, Wang P, Jiang P, et al. Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene. 2016;586(2):248–253.
- Reber L, Marichal T, Sokolove J, et al. Contribution of mast cell-derived interleukin-1β to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 2014;66(10):2881–2891.
- Liu YF, Wen CYZ, Chen Z, et al. Effects of Berberine on NLRP3 and IL-1β Expressions in Monocytic THP-1 Cells with monosodium urate crystals-induced inflammation. Biomed Res Int. 2016;2016(6):2503703.
- Jin HM, Kim T-J, Choi J-H, et al. MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis. Arthritis Res Ther. 2014;16(2):p. R88.
- Merry C, Forrest ME, Sabers JN, et al. DNMT1-associated long non-coding RNAs regulate global gene expression and DNA methylation in colon cancer. Hum Mol Genet. 2015;24(21):6240–6253.
- Meng Z, Hudson AP, Schumacher HR, et al. Monosodium urate, hydroxyapatite, and calcium pyrophosphate crystals induce tumor necrosis factor-alpha expression in a mononuclear cell line. J Rheumatol. 1997;24(12):2385–2388.
- Yang C, Li J-P, Yen J-C, et al. lncRNA NTT/PBOV1 axis promotes monocyte differentiation and is elevated in rheumatoid arthritis. Int J Mol Sci. 2018;19:9.
- Zou Y, Xu S, Xiao Y, et al. Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J Clin Investig. 2018;128:10.
- Nakano K, Boyle D, Firestein G. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J Immunol. 2013;190(3):1297–1303.
- Glossop J, Glossop JR, Emes RD, et al. Genome-wide DNA methylation profiling in rheumatoid arthritis identifies disease-associated methylation changes that are distinct to individual T- and B-lymphocyte populations. Epigenetics. 2014;9(9):1228–1237.
- Margot J, Ehrenhofer-Murray A, Leonhardt H. Interactions within the mammalian DNA methyltransferase family. BMC Mol Biol. 2003;4:7.
- Nakano K, Whitaker JW, Boyle DL, et al. DNA methylome signature in rheumatoid arthritis. Ann Rheum Dis. 2013;72(1):110–117.
- Sun T, Yu J, Han L, et al. Knockdown of long non-coding RNA RP11-445H22.4 alleviates LPS-Induced injuries by regulation of MiR-301a in osteoarthritis. Cell Physiol Biochem. 2018;45(2):832–843.
- Li Y, Li S-H, Liu Y, et al. Long Noncoding RNA CIR Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis by Acting as a Sponge For Mir-27b. Cell Physiol Biochem. 2017;43(2):602–610.
- Zhou W, Wang Y, Wu R, et al. MicroRNA-488 and −920 regulate the production of proinflammatory cytokines in acute gouty arthritis. Arthritis Res. Ther. 2017;19(1):203.
