ABSTRACT
Neurodegenerative disorders, including spinal cord injury (SCI), result in oxidative stress-induced cell damage. Morroniside (MR), a major active ingredient of the Chinese herb Shan Zhu Yu, has been shown to ameliorate oxidative stress and inflammatory response. Our previous study also confirmed that morroniside protects SK-N-SH cell line (human neuroblastoma cells) against oxidative impairment. However, it remains unclear whether MR also plays a protective role for oligodendrocytes that are damaged following SCI. The present study investigated the protective effects of MR against hydrogen peroxide (H2O2)-induced cell death in OLN-93 cells. MR protected OLN-93 cells from H2O2-induced injury, attenuated H2O2-induced increase in reactive oxygen species (ROS) and malondialdehyde (MDA) levels, and blocked the reduction of mitochondrial membrane potential (MMP) induced by H2O2. MR enhanced the activity of the antioxidant enzyme superoxide dismutase (SOD) and suppressed H2O2-induced downregulation of the antiapoptotic protein Bcl-2 and activation of the proapoptotic protein caspase-3. Finally, we found that LY294002, a specific inhibitor of the PI3K/Akt pathway, inhibited the protective effect of MR against H2O2-induced OLN-93 cell injury in the MTT and TUNEL assays. LY294002 also inhibited the expression of SOD and Bcl-2, and increased the expression of iNOS and c-caspase-3 induced by MR treatment. MR exerts protective effects against H2O2-induced OLN-93 cell injury through the PI3K/Akt signaling pathway-mediated antioxidative stress and antiapoptotic activities. MR may provide a potential strategy for SCI treatment or other related neurodegeneration.
1. Introduction
Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that extend membranous processes to form multilamellar structures around axons, facilitating conduction of nervous impulses [Citation1,Citation2]. Attributable to oligodendrocytes are known to be very sensitive to oxidative stress, oligodendrocytes are the first to be subjected to oxidative damage in many neurodegenerative diseases. Among them, the most typical one is spinal cord injury (SCI) [Citation3]. After SCI, oxidative stress induces massive death of oligodendrocytes and eventually results in degeneration and demyelination of axons, which results in neurologic function damage [Citation4,Citation5]. Thus, the rescue of oligodendrocytes, especially under oxidative stress, has potential therapeutic value in neurodegenerative diseases. In recent years, various pharmacological agents including antioxidant have been tested for the treatment of SCI [Citation6,Citation7]. However, there is still no recognized treatment for SCI.
Morroniside (MR) is the main active component of the extract of Calendula officinalis. It has been reported that MR may inhibit oxidative stress and inflammatory response in liver and kidney of diabetic rats [Citation8,Citation9]. Wang et al. demonstrated that MR possessed antioxidant and antiapoptotic properties, prevented peroxide-induced apoptosis in human neuroblastoma cells, and protected the brain from damage induced by focal cerebral ischemia in rats [Citation10–12]. Our previous study also confirmed that MR protected SK-N-SH cells against oxidative impairment by blocking the production of reactive oxygen species (ROS) while MR also inhibited Bax and stimulated Bcl-2 expression, thereby mitochondrial mediated apoptosis was blocked [Citation13]. However, it remains unclear whether MR also exerts protective effects on oligodendrocytes that are damaged in the SCI.
In view of the excellent antioxidant and anti-apoptotic abilities of MR, we speculate that MR can protect oligodendrocytes from oxidative stress in neurodegenerative diseases. Thus, we investigated whether MR exerts protective effects and explored the potential mechanism of MR against H2O2-induced injury of OLN-93 cells, which was established from spontaneously transformed rat brain oligodendrocyte cultures [Citation14]. Our results provide insights for the future application of MR in CNS diseases.
2. Materials and methods
2.1. Cell culture and treatment
OLN-93 cells (obtained from ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM: Hyclone, USA) with 10% fetal bovine serum (Gibco Life Science, Grand Island, NY, USA) under 37°C and 5% CO2, the medium was changed every 2 d. Pretreatment of OLN-93 cells with different concentrations (0, 100, 200, 300 and 400 μM) of MR (Phytomarker Ltd., Tianjin, China) for 24 h were performed. The medium was then removed, and the cells were washed once with DMEM. Subsequently, the cells were continuously cultured in DMEM containing H2O2 (100 μM) for 12 h. MR was dissolved in 1× PBS, H2O2 (all from Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) was mixed with medium, and LY294002 (AKT inhibitor, Selleck, USA) was dissolved in dimethyl sulfoxide (DMSO, final concentration of 0.1%) [Citation15]. OLN-93 cells were preincubated with LY294002 (25 μM) for 1 h and then pretreated with MR for 24 h [Citation16], then incubated in H2O2 for 12 h [Citation13]. All experiments were performed in triplicate.
2.2. Cell viability assay
The viability of the cells was assessed by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. OLN-93 cells were plated into 96-well plates at the density of 8 × 103 cells/well. The cells were pretreated for 24 h with different concentrations of MR prior to exposure to H2O2. After 12 h, 20 µl MTT stock solution (5 mg/ml, Solarbio, China) was added into every well for incubating in dark for 4 h (37°C). Then, 150 µl DMSO (Sigma, USA) was used to dissolve the formazan crystals, and their OD values were determined at the wavelength of 490 nm.
2.3. Cytotoxicity assay
For analyzing H2O2-induced cytotoxicity, lactate dehydrogenase (LDH) assay was executed. After the cells were exposed to 100 μM H2O2 in the presence of MR for 24 h, the medium was collected for analysis according to the manufacturer’s instructions of cytotoxicity assay kit (Sigma, USA). The OD values of each group were determined at the wavelength of 490 nm.
2.4. Monitoring mitochondrial membrane potential (MMP)
JC-1 staining was used to determine the change of MMP. OLN-93 cells from different treatment groups were flushed with DMEM and incubated with the fluorescent dye JC-1 (10 μg/ml, Beyotime Biotechnology) for 20 min at 37°C in dark. The cells were then washed two times with PBS. The samples were measured by fluorescence microscopy within 30 min. Aggregated JC-1 in intact mitochondria were evidence as red fluorescence with emission at 585 nm indicating high or normal MMP. Green fluorescence with emission at 529 nm indicates low MMP when JC-1 remains in the monomeric form in the cytoplasm. To quantify fluorescence intensity, 15 different images that were obtained from each group were analyzed using Image J software. The ratio of aggregated and monomeric JC-1 was used to quantify the change of MMP.
2.5. Apoptosis analysis
Hoechst 33342 as a kind of fluorescent nuclear dye (Beyotime Biotechnology), was used for detecting apoptosis in cells by observing cell morphology. Briefly, the samples were washed with 1× PBS, and then incubated in the medium with 1× Hoechst 33342 in dark for 10 min (37°C). The cells were washed with 1× PBS and then observed by fluorescence microscopy. To further investigate the antiapoptotic effect of MR, the TUNEL (TransGen Biotech, China) staining assay was performed. After the indicated treatment, the treated cells were fixed with cold 4% paraformaldehyde for 15 min at room temperature. The cells were then suspended in PBS containing 0.1% Triton X-100 for 5 min at room temperature. The cells were incubated with terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling for 1 h at 37°C in dark. After labeling, the cells were suspended in PBS containing 0.1% Triton X-100 for 5 min, and the process was repeated three times. The cells were counterstained with DAPI and examined by fluorescence microscopy.
2.6. Assessment of intracellular ROS production
The relative levels of intracellular ROS were measured using a commercial ROS detection kit (Beyotime, China). After drug treatment, OLN-93 cells were exposed to 2’,7’- dichlorofluorescein diacetate (DCFH-DA, 10 μM) in dark for 20 min (37°C). OLN-93 cells were rinsed three times with 1× PBS, and then observed by fluorescence microscopy. The intensity of 2’,7’-dichlorofluorescein (DCF) was detected at the 490 nm (excitation wavelengths) and 525 nm (emission wavelengths), via a fluorescence spectrophotometer.
2.7. Lipid peroxidation assay
Malondialdehyde as a stable end product of lipid peroxidation cascade was measured by MDA assay kit (MDA; Beyotime Biotechnology) to monitor the lipid peroxidation. OLN-93 cells were washed with ice-cold PBS, and the proteins were extracted after treatment with H2O2. Cell homogenates were centrifuged at 16,000 g (4°C) for 10 min. The supernatant was collected for MDA measurement, supernatant (100 μl) was added into a 5-ml tube and then mixed with the MDA working solution (200 μl). The mixture was incubated for 15 min (100°C), and centrifuged at 1000 g for 10 min. The MDA content in the supernatant was assayed by the thiobarbituric acid (TBA) reaction. The absorbance of the resultant-colored product was spectrophotometrically measured at 535 nm. The levels of TBA reactive species were expressed as μmol/g protein.
2.8 Western blot analysis
OLN-93 cells from different experimental groups were harvested in ice-cold lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium orthovanadate, sodium fluoride, EDTA, leupeptin; Beyotime Biotechnology). The protein of each group was electrophoresed via SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred to membranes. After membrane was blocked with milk (5%), antibodies against Bcl-2 (1:800, ProteinTech Group Inc., Chicago, IL, USA), Bax (1:10,000, ProteinTech Group Inc.), SOD2 (1:500, Wanleibio, China), iNOS (1:800, ProteinTech Group Inc.), AKT (1:500, Wanleibio), AKT-Phospho (p-AKT, 1:500, Wanleibio), and Caspase-3 (1:500, ProteinTech Group Inc.) were added and incubated overnight at 4°C, followed by incubation with fluorescent-conjugated secondary antibody (1:15,000, LI-COR, USA) for 1 h at room temperature. Gel images were captured and quantified using an Odyssey CLx infrared imaging system (ODYSSEY CLX, LI-COR). Relative densitometric values were calculated after normalization with β-actin.
2.9.. Statistical analysis
Data are presented as mean ± standard deviation of the mean (SD). The data of the two groups were analyzed by Student’s t test (for normally distributed data) or nonparametric Mann–Whitney U test (for non-normally distributed data). The data for three or more groups were analyzed by one-way ANOVA followed by Tukey’s post-hoc test to determine whether there were significant differences between individual groups. All experiments were repeated at least three times. Values of P< 0.05 were considered statistically significant.
3. Results
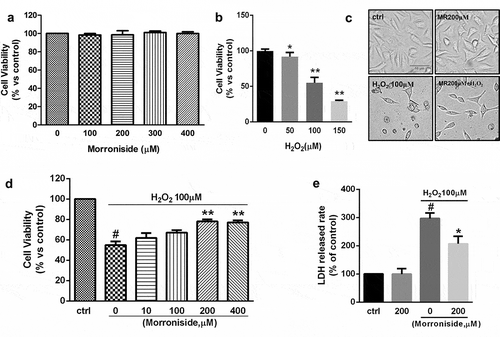
3.1. MR protects OLN-93 cells against H2O2-induced injury
To determine the suitable concentrations of MR and H2O2 for subsequent experiments, we treated OLN-93 cells with different concentrations of MR or H2O2, and measured the viability of the treated cells by MTT assays. As shown in , MR did not affect cell viability at 100, 200, 300 and 400 μM for 24 h (). Treatment of OLN-93 cells with H2O2 at 50, 100 and 150 μM for 12 h resulted in a significant decrease in cell viability compared with the control group (0 μM). We found that a concentration of 100 μM H2O2 decreased cell viability to 55% compared to the control group (). Pretreatment of MR (200 μM, 400 μM) significantly attenuated the decreased cell viability (78.04 ± 4.42%, 77.14 ± 4.18%, respectively) caused by 100 μM H2O2 (54.96 ± 7.85%, )). Based on these results, OLN-93 cells were pretreated with 200 μM MR for 24 h and then exposed to H2O2 (100 μM) for 12 h to induce oxidative stress in subsequent experiments. The LDH release assay showed a significant increase in LDH release after 12 h exposure of the cells to 100 μM H2O2 (296.7 ± 19.43%), and 200 μM MR significantly attenuated this increase in LDH release (207.1 ± 26.5%, P < 0.01; ). Additionally, morphological changes of OLN-93 cells were observed. OLN-93 cells treated for 12 h with 100 μM H2O2 lost the projections of cells, became round and swollen, or had shrunken cell body. These transformations were inhibited by administration with MR ().
Figure 1. MR protects OLN-93 cells against H2O2-induced death. (a) Effects of MR on the viability of OLN-93 cell. MR did not influence the viability of cell at concentrations of 100, 200, 300, and 400 μM; (b–d) Protective effects of MR pretreatment on H2O2-induced cytotoxicity in OLN-93 cells. Cells were pretreated with different concentrations of MR (100–400 µM) for 24 h and then incubated in the absence or presence of 100 µM H2O2 for 12 h. (b) Viability was assessed by the MTT reduction assay. (c) MR (200 µM) inhibited the release of lactate dehydrogenase (LDH) in OLN-93 cells induced by H2O2. (d) MR suppressed the changes of morphological induced by H2O2. Scale bar, 50 µm. #P< 0.01 vs. the control group; *P< 0.05, **P< 0.01 vs. the only H2O2 treatment group
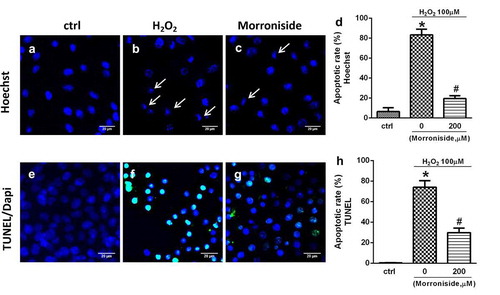
3. 2. MR protects OLN-93 cells from H2O2-induced apoptosis
Hoechst 33342 and TUNEL staining were used to investigate whether H2O2 induces apoptosis of OLN-93 cells. Hoechst 33342 staining showed that the nuclei from untreated OLN-93 cells were intact, large, and oval-shaped (). H2O2 (100 μM) treatment produced many apoptotic cells with condensed or fragmented nuclei as compared to the control group (83.23 ± 5.68% vs. 6.34 ± 3.93%; P< 0.01; )). H2O2-induced apoptosis of OLN-93 cells was rescued by pretreatment with 200 μM MR (19.42 ± 2.79% vs. 83.23 ± 5.68%; P< 0.01; )). Similar to the findings above, few TUNEL+ cells were detected among OLN-93 cells without H2O2 treatment (). However, H2O2 treatment increased the percentage of TUNEL+ cells (74 ± 12.99% vs. 0.38 ± 0.43%; P< 0.01; )), which was reduced by preincubation with 200 μM MR (29.78 ± 9.02% vs. 74 ± 12.99%; P< 0.01; )).
Figure 2. MR inhibits H2O2-induced apoptosis of OLN-93 cells. (a–d) Apoptotic nuclei of OLN-93 cells were stained by Hoechst 33342. The arrow indicates condensed nuclear cells. (a) Control group; (b) cells induced by H2O2 (100 µM) with condensed nuclei; (c) cells administrated with 200 µM MR for 24 h displayed attenuated sensitivity to H2O2 (100 µM), as evidenced by fewer cells with condensed nuclei. (e–h) Apoptotic cells were detected by TUNEL (green), and the nuclei were detected by DAPI (blue). (e) Control group; (f) cells induced by H2O2 (100 µM) with condensed nuclei; (g) cells were administrated with 200 µM MR followed by 100 µM H2O2 for 12 h. Quantitative analyses are shown in panels (d) and (h). Scale bar, 20 µm. *P< 0.01 vs. the control group; #P< 0.01 vs. the only H2O2 treatment group
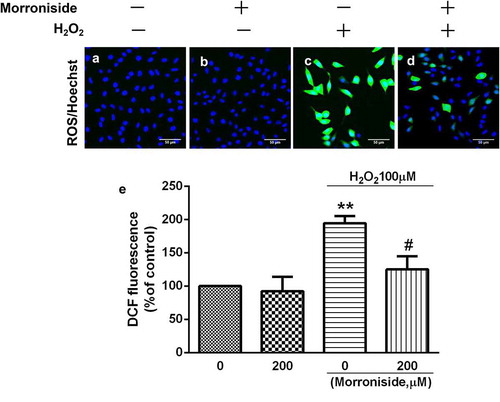
3.3. MR inhibits H2O2-induced elevation in intracellular ROS level
Intracellular ROS level was measured using the fluorescent dye dichlorofluorescein diacetate (H2-DCF-DA). A significant increase was observed in intracellular DCF fluorescence (194.6 ± 24.03%, P< 0.01; )) when OLN-93 cells were treated with 100 μM H2O2 for 12 h, demonstrating that the levels of ROS were increased. Nevertheless, administration with 200 μM MR inhibited this increase set against the H2O2-induced cells (125.2 ± 43.62% vs. 194.6 ± 24.03%, P< 0.05; )), thus indicating that MR had a protective effect against the free radical formation induced by H2O2.
Figure 3. MR inhibits the elevation of intracellular ROS level induced by H2O2. (a) Control group; (b) OLN-93 cells incubated with 200 µM MR; (c) cells exposed to 100 µM H2O2; (d) cells pretreated with 200 µM MR for 24 h prior to induced by H2O2 (100 µM) for 12 h. Scale bar, 50 µm. Quantitative analysis is shown in E. *P< 0.01 vs. the control group and the 200 µM MR group, respectively; #P< 0.05 vs. the only H2O2 treatment group
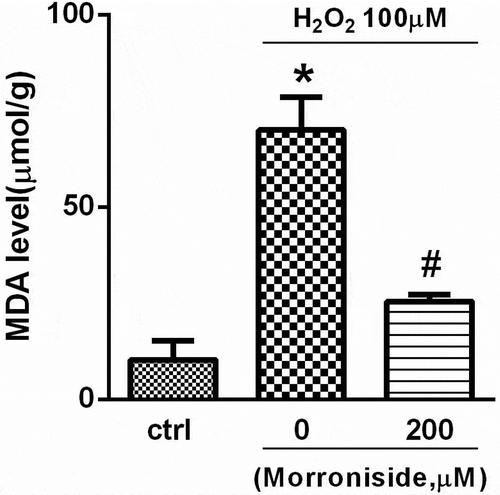
3.4. MR inhibits H2O2-induced lipid peroxidation in OLN-93 cells
The MDA assay was used to monitor lipid peroxidation levels in OLN-93 cells. We found that the MDA level was significantly higher in OLN-93 cells treated with 100 μM H2O2 for 12 h than in the control group (70.06 ± 16.96 μmol/g vs. 10.27 ± 10.05 μmol/g; P< 0.01; ). However, pretreatment with 200 μM MR reduced MDA levels (25.49 ± 3.62 μmol/g; P< 0.01; ).
3.5. MR rescued H2O2-induced MMP decrease in OLN-93 cells
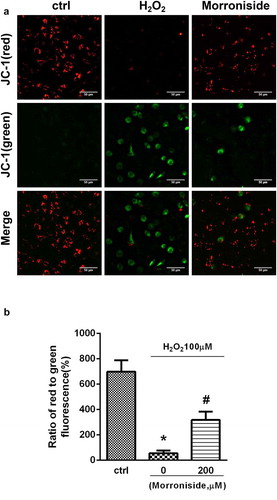
JC-1 dye was used to detect the decrease in MMP in OLN-93 cells. The cells were administered with H2O2 (100 μM) for 12 h, which resulted in a remarkable reduction of MMP, as indicated by a reduction in the intensity ratio of red/green fluorescence (698.3 ± 89.57 vs. 55.64 ± 21.25%, P< 0.01), ). This MMP decrease was rescued by administration with 200 μM MR (317.9 ± 65.96% vs. 55.64 ± 21.25%, P< 0.01) ().
Figure 5. Inhibition of H2O2-induced reduction in mitochondrial membrane potential (MMP) by MR. (a) Cells were administrated with 200 µM MR for 24 h prior to exposure to H2O2 (100 µM) for 12 h. Aggregated (red) or monomeric (green) cell morphological images were taken under a fluorescence microscope. (b) The ratio of aggregated and monomeric JC-1 was used to quantify the change of MMP. Bar diagram showing the loss of MMP by MR. Scale bar, 50 µm. *P< 0.01 vs. the control group; #P< 0.01 vs. the only H2O2 treatment group
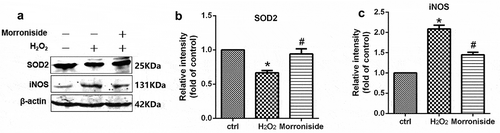
3.6. MR reverses H2O2-induced decrease in SOD2 activity and increase in iNOS level
The level of SOD2 and iNOS was measured to investigate the antioxidant potential of MR. As shown in , the activity of SOD2 in OLN-93 cells treated with 100 μM H2O2 for 12 h was significantly lower than that of the control group (0.66 ± 0.07-fold of control, P< 0.05). MR significantly enhanced SOD2 activity in the cells pretreated with H2O2 (0.94 ± 0.14 vs. 0.66 ± 0.07, P< 0.01) (). Moreover, treatment with 100 μM H2O2 induced an increase in iNOS protein level (2.08 ± 0.18-fold of control, P< 0.01), which was reversed by pretreatment with 200 μM MR (1.45 ± 0.12 vs. 2.08 ± 0.18, P< 0.01; )).
Figure 6. Effect of MR on the expression of oxidation-related proteins. (a) Cells were pretreated with 200 µM MR for 24 h, followed by incubation in the presence of 100 µM H2O2 for 12 h. The cells were analyzed by SDS-PAGE followed by western blot analysis. (b–c) The levels of SOD2 and iNOS were quantified by densitometric analysis.*P< 0.01 vs. the control group; #P< 0.01 vs. the only H2O2 treatment group
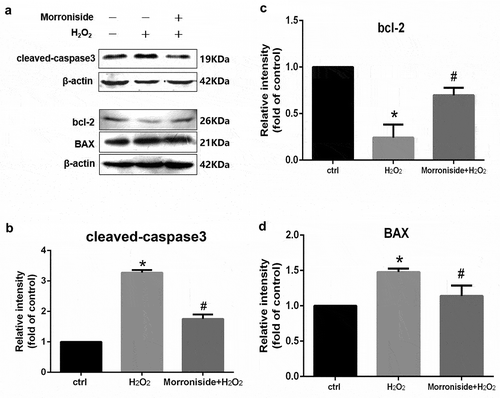
3.7. MR modulates the expression of apoptosis-related proteins
The expression levels of several apoptosis-associated proteins such as cleaved caspase-3 (c-caspase-3), BAX and anti-apoptotic protein (Bcl-2) were measured. OLN-93 cells administrated with H2O2 (100 μM) for 12 h revealed a significant elevation inthe level of c-caspase-3 and a significant decrease in the level of Bcl-2 relative to those of the control cells. Pretreatment with 200 μM MR suppressed H2O2-induced upregulation of c-caspase-3 level and downregulation of Bcl-2 level (P < 0.05; )).
Figure 7. Effect of MR on the expression of apoptosis-related proteins in OLN-93 cells. (a) Cells were pretreated with 200 µM MR for 24 h and then incubated in the presence of 100 µM H2O2 for 12 h. The cells were analyzed by SDS-PAGE followed by western blot analysis. (b–d) The levels of cleaved-caspase3, bcl-2, and Bax were quantified by the densitometric analysis. *P< 0.05 vs. the control group; #P< 0.05 vs. the only H2O2 treatment group
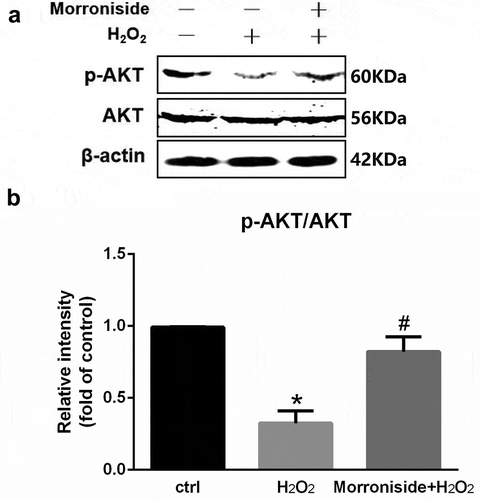
3.8. MR rescues H2O2-induced downregulation of AKT phosphorylation
To investigate whether the activation of the Akt pathway was involved in H2O2-induced death and MR-induced neuroprotection, the phosphorylation of AKT was determined. The results showed that H2O2 induced downregulation of AKT phosphorylation. However, pretreatment with 200 μM MR rescued H2O2-induced downregulation of AKT phosphorylation (P< 0.05; ).
Figure 8. MR rescues H2O2-induced downregulation of p-AKT in OLN-93 cells. (a) Cells were pretreated with 200 µM MR for 24 h and then incubated in the presence of 100 µM H2O2 for 12 h. The cells were analyzed by SDS-PAGE followed by western blot analysis. (b) Relative levels of p-AKT versus total AKT in each sample as determined by blot densitometry. Densitometric analysis of the immunoblot was expressed as a fold of control. *P< 0.05 vs. the control group; #P< 0.05 vs. the only H2O2 treatment group
3.9. LY294002 blocks the protective effect of MR on H2O2-induced OLN-93 cell death/apoptosis
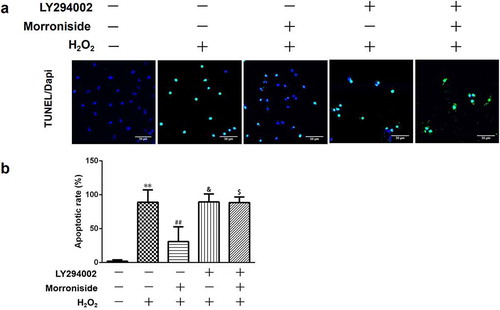
To further investigate whether MR-induced neuroprotection is mediated by the activation of the Akt pathway, OLN-93 cells were pretreated with LY294002 for 1 h before MR treatment to block the activation of Akt. As shown in , pretreatment with LY294002 showed a significant increase in the percentage of TUNEL+ cells (MR + H2O2, 30.93 ± 21.77% vs. LY294002+ H2O2, 89.43 ± 11.81%, P< 0.05), further supporting the involvement of Akt activation in MR-induced apoptosis of OLN-93 cells.
Figure 9. LY294002 blocks the protective role of MR on H2O2-induced apoptosis in OLN-93 cells. (a) OLN-93 cells were preincubated with LY294002 for 1 h and then pretreated with MR for 24 h, followed by incubation with H2O2 for 12 h. Apoptotic cells were detected by TUNEL (green), and the nuclei were detected by DAPI (blue). Quantitative analyses are shown in panel B. Scale bar, 50 µm. **P<0.01 vs. the control group; ##P<0.01 vs. the only H2O2 treatment group; &P<0.05 and $P<0.05 vs. MR+H2O2.
3.10. LY294002 blocks the inhibitory effect of MR on H2O2-induced ROS overproduction
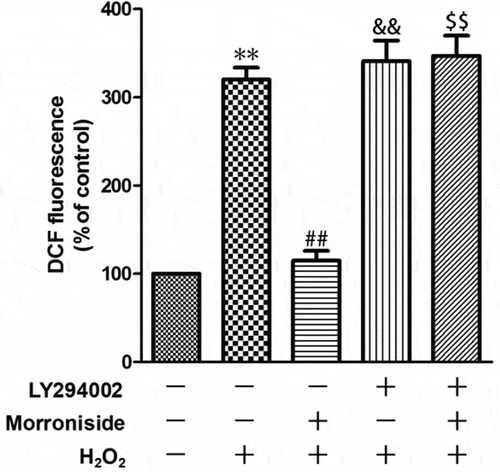
To investigate whether the inhibitory effect of MR on H2O2-induced ROS overproduction is mediated by the activation of the Akt pathway, OLN-93 cells were preincubated with LY294002 and then MR, followed by incubation with H2O2. The result showed that pretreatment with LY294002 significantly increased ROS level compared to the MR group (MR + H2O2, 114.8 ± 25.18% vs. LY294002+ H2O2, 340.8 ± 46.96%, P< 0.01; ).
Figure 10. LY294002 blocks inhibitory effects of MR on H2O2-induced ROS overproduction. OLN-93 cells were preincubated with LY294002 for 1 h and then pretreated with MR for 24 h, followed by incubation with H2O2 for 12 h. **P<0.01 vs. the control group; ##P<0.01 vs. the only H2O2 treatment group; &&P<0.01 and $$P<0.01 vs. MR+H2O2.
3.11 The Akt pathway is involved in the protective effect of MR on H2O2-induced apoptosis of OLN-93 cells through regulating the expression of oxidation- and apoptosis-related proteins
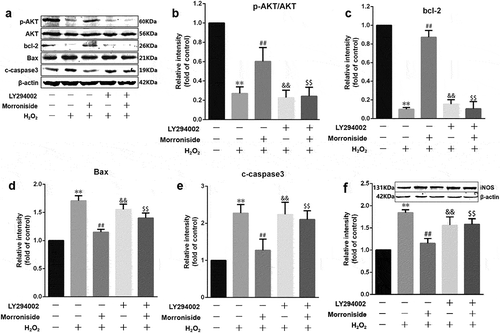
To determine the molecular mechanism underlying the protective effects of MR on OLN-93 cells from H2O2-induced injury, the levels of several oxidation- and apoptosis-associated proteins were investigated after LY294002 was administered. The results showed that LY294002 inhibited the expression of SOD and increased the expression of iNOS induced by MR treatment (). Similarly, LY294002 inhibited Bcl-2 expression and increased the levels of c-caspase-3 induced by MR ().
Figure 11. MR modulates the expression of apoptosis-related proteins by increasing Akt phosphorylation. Cells were preincubated with LY294002 for 1 h and then pretreated with MR for 24 h, followed by incubation with H2O2 for 12 h. The cells were analyzed by SDS-PAGE followed by western blot analysis (a and f). Relative levels of p-AKT versus total AKT in each sample as determined by blot densitometry (b). Densitometric analysis of the immunoblot was expressed as a fold of control (b–f). **P<0.01 vs. the control group; ##P<0.01 vs. the only H2O2 treatment group; &&P<0.05 and $$P<0.05 vs. MR+H2O2.
4. Discussion
SCI causes massive death of neurons and oligodendrocytes, and eventually results in the degeneration and demyelination of axons below the injury site [Citation4,Citation5]. The death of oligodendrocytes can occur over many days or weeks at sites distant from the injury [Citation17,Citation18]. Oligodendrocytes are particularly susceptible to oxidative stress because of their poor antioxidant defense system [Citation19,Citation20]; moreover, oxidative stress and ROS production play a predominant role in the mechanisms of secondary injury of SCI [Citation21]. MR, a major active ingredient of the Chinese herb Shan Zhu Yu, has been shown to ameliorate oxidative stress [Citation11,Citation22]. Our previous laboratory study demonstrated that MR protects SK-N-SH cell line (human neuroblastoma cells) against oxidative impairment by decreasing the production of ROS while inhibiting the expression of Bax and activating the expression of Bcl-2, thereby inhibiting the cell apoptosis mediated by mitochondria [Citation13]. However, it remains unclear whether MR also protects oligodendrocytes, the myelin-forming glial cells of the central nervous system (CNS), against oxidative damage. In the present study, OLN-93 cells were used as an in vitro model to investigate whether MR also plays a protective role in H2O2-induced injury.
In this study, we demonstrated that treatment with H2O2 on OLN-93 cells causes a rapid reduction in cytoactive and a significant elevation in LDH release as determined by the MTT assay and the LDH assay, respectively. However, pretreatment with MR increased cell viability and ameliorated the morphology of OLN-93 cells induced by H2O2. Moreover, our study demonstrated that MR protected OLN-93 cells from H2O2-induced apoptosis. Together, these findings suggest that MR protects OLN-93 cells from H2O2-induced injury.
Exposure of cells to exogenous H2O2 can induce excessive accumulation of ROS [Citation23,Citation24], resulting in the membrane lipid peroxidation and disruption of cell integrity [Citation25]. Overdose of ROS destroys the intracellular redox balance and leads to apoptosis [Citation26]. The present research proved that administration with MR decreased the production of intracellular ROS induced by H2O2 in OLN-93 cells, thus suggesting that MR reverses H2O2-induced injury by blocking ROS production. Previous researches have shown that H2O2 exerts toxic effects on various cells by causing oxidative damage to nucleic acids, proteins, and cell membrane lipids [Citation27–29]. Our data proved that MR treatment obviously attenuated the H2O2-induced increased MDA level, a marker of lipid peroxidation, in OLN-93 cells. Together, these results suggest that MR protects OLN-93 cells from H2O2-induced toxicity and inhibits oxidative damage induced by ROS.
Mitochondria are the main targets of ROS and mitochondrial calcium overloading trigger the activation of the mitochondrial permeability transition pore (mPTP), which reduces the MMP [Citation30]. The collapse of MMP coincides with the opening of the mPTPs, leading to the release of cytochrome c into the cytosol, where it forms the apoptosome by binding to apoptotic protease activating factor 1, which triggers other downstream events in the apoptotic cascade [Citation31,Citation32]. In our study, H2O2 treatment reduced the MMP, and this decrease was inhibited by administration with MR, demonstrating that MR plays a protective role in blocking the mitochondrial apoptotic pathway by rescuing MMP.
SOD is a major antioxidant enzyme. In most situations, intracellular defense systems, including SOD, suppress the production of ROS through metabolic processes [Citation33]. In this study, a remarkable reduction in the SOD activity was observed after H2O2 treatment, and the activity of SOD was upregulated by treatment with MR; this finding was consistent with the effect of MR on SK-N-SH cells [Citation13]. Thus, our finding indicates that MR attenuates H2O2-induced injury in OLN-93 cells by enhancing the level of antioxidants.
The accumulation of ROS in cells results in the collapse of the intracellular redox equilibrium and ultimately induces apoptosis [Citation34,Citation35]. Interestingly, we observed that MR obviously attenuated apoptosis triggered by H2O2 in OLN-93 cells. Caspase-3 is a member of the interleukin-1 β-converting enzyme family and is thought to be associated with the induction of apoptosis [Citation36]. Later studies suggested that H2O2 treatment activated the proapoptotic protein caspase-3, and this effect was blocked by pretreatment with MR. H2O2 modulates the levels of Bcl-2 and Bax, which play an important role in apoptosis regulation in various types of cells such as neurons [Citation37,Citation38], endothelial cells [Citation39,Citation40], and pancreatic acinar cells [Citation41]. A significant decrease was observed in the Bcl-2 level after H2O2 treatment, and MR suppressed the H2O2-induced downregulation of the Bcl-2 level. In conclusion, all the findings indicate that MR prevents the apoptosis of OLN-93 cells by regulating the expression of apoptosis-related proteins.
The PI3K/Akt pathway plays an important role in promoting cell survival and inhibiting apoptosis [Citation42]. The PI3K/Akt pathway is involved in the antioxidant role, which is also one of the protection mechanisms of cells against H2O2-induced cell damage [Citation43]. AKT is a serine/threonine kinase and the key mediator of PI3K-initiated signaling, and it can promote cell survival by regulating the expression of apoptosis-related proteins [Citation44]. PI3K activation results in the phosphorylation of Akt (p-Akt), and thus, p-Akt can be used as a marker of PI3K activation [Citation45]. Our data proved that H2O2 downregulated the phosphorylation of Akt, whereas MR blocked this downregulation, indicated the possibility that MR protects OLN93 cells from H2O2-induced injury by the activation of the PI3K/Akt pathway. Furthermore, LY294002 as a specific inhibitor of the PI3K/Akt pathway, we found that cell viability was decreased and TUNEL+ cells were increased following LY294002 treatment when compared with the MR group; this finding suggests that MR plays a protective role on OLN-93 cell death/apoptosis induced by oxidative stress through the PI3K/Akt signaling pathway. Finally, we determined the expression of several oxidation- and apoptosis-related proteins after LY294002 treatment. We found that LY294002 inhibited the expression of SOD and increased the expression of iNOS in MR treatment cells. Similarly, LY294002 inhibited the expression of the antiapoptotic protein Bcl-2 and increased the level of c-caspase-3 in MR group. Taken together, these results suggest that the Akt pathway was involved in the protective role of MR against H2O2-induced OLN-93 cell injury through regulating the expression of oxidation- and apoptosis-related proteins.
It is undeniable that our research still has some limitations. For instance, our data indicate that MR can protect cells against H2O2-induced cell injury by antioxidation and antiapoptotic. However, MR can also play a protective role by other means. The inactivation of PI3K/Akt pathway may partly explain the mechanism underlying MR neuroprotective effect on the SCI, but other signaling pathways should also be involved. In addition, the effect of MR on primary olidondrocytes and animal model should be performed in the future study.
In summary, the present study demonstrates that MR exhibits antioxidant and neuroprotective role against H2O2-induced cell injury in OLN-93 cells. MR regulates the expression levels of oxidation- and apoptosis-related proteins against oxidative stress, which is possibly mediated by the PI3K/Akt signaling pathway. This study provides new information on the neuroprotective effect of MR and thus indicates that MR may have potential for the treatment of SCI and other neurodegenerative disorders.
Impact statement
The study demonstrates that MR exhibits antioxidant and neuroprotective role against H2O2-induced cell injury in OLN-93 cells. MR regulates the expression levels of oxidation- and apoptosis-related proteins against oxidative stress, which is possibly mediated by the PI3K/Akt signaling pathway. This study provides new information on the neuroprotective effect of MR and thus indicates that MR may have potential for the treatment of SCI and other neurodegenerative disorders.
Disclosure
The authors declare no financial conflicts of interest.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67(6):451–467.
- Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39(6):823–831.
- Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22(4):371–378.
- Silva NA, Sousa N, Reis RL, et al. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57.
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–643.
- Donovan J, Kirshblum S. Clinical trials in traumatic spinal cord injury. Neurotherapeutics. 2018;15(3):654–668.
- Griffin JM, Bradke F. Therapeutic repair for spinal cord injury: combinatory approaches to address a multifaceted problem. EMBO Mol Med. 2020;12(3):e11505.
- Park CH, Noh JS, Kim JH, et al. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biol Pharm Bull. 2011;34(10):1559–1565.
- Yokozawa T, Yamabe N, Kim HY, et al. Protective effects of morroniside isolated from Corni Fructus against renal damage in streptozotocin-induced diabetic rats. Biol Pharm Bull. 2008;31(7):1422–1428.
- Wang W, Huang W, Li L, et al. Morroniside prevents peroxide-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells. Cell Mol Neurobiol. 2008;28(2):293–305.
- Wang W, Sun F, An Y, et al. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur J Pharmacol. 2009;613(1–3):19–23.
- Wang W, Xu J, Li L, et al. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res Bull. 2010;83(5):196–201.
- Zhang JX, Wang R, Xi J, et al. Morroniside protects SK-N-SH human neuroblastoma cells against H2O2-induced damage. Int J Mol Med. 2017;39(3):603–612.
- Richter-Landsberg C, Heinrich M. OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J Neurosci Res. 1996;45(2):161–173.
- Chen J, Li J, Miao Z, et al. XAV939, a small molecular inhibitor, provides neuroprotective effects on oligodentrocytes. J Neurosci Res. 2014;92(10):1252–1258.
- Hu JG, Fu SL, Wang YX, et al. Platelet-derived growth factor-AA mediates oligodendrocyte lineage differentiation through activation of extracellular signal-regulated kinase signaling pathway. Neuroscience. 2008;151(1):138–147.
- Assinck P, Duncan GJ, Plemel JR, et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J Neurosci. 2017;37(36):8635–8654.
- Dyck S, Kataria H, Akbari-Kelachayeh K, et al. LAR and PTPσ receptors are negative regulators of oligodendrogenesis and oligodendrocyte integrity in spinal cord injury. Glia. 2019;67(1):125–145.
- Matute C, Alberdi E, Domercq M, et al. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24(4):224–230.
- Tekkök SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21(12):4237–4248.
- Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100(3):639–649.
- Li X, Huo C, Wang Q, et al. Identification of new metabolites of morroniside produced by rat intestinal bacteria and HPLC-PDA analysis of metabolites in vivo. J Pharm Biomed Anal. 2007;45(2):268–274.
- Qi L, Jiang J, Zhang J, et al. Curcumin protects human trophoblast HTR8/SVneo cells from H2O2-induced oxidative stress by activating Nrf2 signaling pathway. Antioxidants (Basel). 2020;9:2.
- Jiang X, Nie B, Fu S, et al. EGb761 protects hydrogen peroxide-induced death of spinal cord neurons through inhibition of intracellular ROS production and modulation of apoptotic regulating genes. J Mol Neurosci. 2009;38(2):103–113.
- Xu H, Shen J, Liu H, et al. Morroniside and loganin extracted from Cornus officinalis have protective effects on rat mesangial cell proliferation exposed to advanced glycation end products by preventing oxidative stress. Can J Physiol Pharmacol. 2006;84(12):1267–1273.
- Xian D, Lai R, Song J, et al. Emerging perspective: role of increased ros and redox imbalance in skin carcinogenesis. Oxid Med Cell Longev. 2019;2019:8127362.
- Choi DJ, Cho S, Seo JY, et al. Neuroprotective effects of the Phellinus linteus ethyl acetate extract against H2O2-induced apoptotic cell death of SK-N-MC cells. Nutr Res. 2016;36(1):31–43.
- Gay NH, Phopin K, Suwanjang W, et al. Neuroprotective effects of phenolic and carboxylic acids on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Neurochem Res. 2018;43(3):619–636.
- Park HR, Lee H, Park H, et al. Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complement Altern Med. 2015;15:171.
- Rottenberg H, Hoek JB. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell. 2017;16(5):943–955.
- Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010;17(10):1540–1550.
- Yang Y, Zong M, Xu W, et al. Natural pyrethrins induces apoptosis in human hepatocyte cells via Bax- and Bcl-2-mediated mitochondrial pathway. Chem Biol Interact. 2017;262:38–45.
- Surai PF, Kochish II, Fisinin VI, et al. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants (Basel). 2019;8:7.
- Deng G, Su JH, Ivins KJ, et al. Bcl-2 facilitates recovery from DNA damage after oxidative stress. Exp Neurol. 1999;159(1):309–318.
- Yang M, Zhang J, Li Y, et al. Bioassay-guided isolation of dehydrocostus lactone from Saussurea lappa: a new targeted cytosolic thioredoxin reductase anticancer agent. Arch Biochem Biophys. 2016;607:20–26.
- Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–1364.
- Carteri RB, Kopczynski A, Rodolphi MS, et al. Testosterone administration after traumatic brain injury reduces mitochondrial dysfunction and neurodegeneration. J Neurotrauma. 2019;36(14):2246–2259.
- Jo HS, Kim DW, Shin MJ, et al. Tat-HSP22 inhibits oxidative stress-induced hippocampal neuronal cell death by regulation of the mitochondrial pathway. Mol Brain. 2017;10(1):1.
- Du W, An Y, He X, et al. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid Med Cell Longev. 2018;2018:1610751.
- Hwang HJ, Jung TW, Kim JW, et al. Protectin DX prevents H2O2-mediated oxidative stress in vascular endothelial cells via an AMPK-dependent mechanism. Cell Signal. 2019;53:14–21.
- Cho SO, Lim JW, Kim H. Oxidative stress induces apoptosis via calpain- and caspase-3-mediated cleavage of ATM in pancreatic acinar cells. Free Radic Res. 2019;1–11. DOI:10.1080/10715762.2019.1655145
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11(3):297–305.
- Wang X, McCullough KD, Franke TF, et al. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275(19):14624–14631.
- Li H, Tang Z, Chu P, et al. Neuroprotective effect of phosphocreatine on oxidative stress and mitochondrial dysfunction induced apoptosis in vitro and in vivo: involvement of dual PI3K/Akt and Nrf2/HO-1 pathways. Free Radic Biol Med. 2018;120:228–238.
- Fresno VJA, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204.