ABSTRACT
Diabetic cutaneous wounds are one of the complications of diabetes mellitus (DM) and are difficult to cure at present. Autologous dermal fibroblasts (DFs) have shown great promise in skin regeneration and repair. However, whether exosomes derived from autologous dermal fibroblasts (DF-Ex) can be used to accelerate diabetic cutaneous wound healing is unclear. In this study, human umbilical vein endothelial cells (HUVECs) were treated with high glucose. We found that DF-Ex could reverse the damage produced by high glucose in HUVECs in vitro. A high-fat diet and streptozotocin were used to establish a rat model of type 2 diabetes mellitus (T2DM), and a diabetic cutaneous wound model was established in the T2DM rats. We discovered that subcutaneous injections of DF-Ex could significantly promote re-epithelialization, collagen deposition, skin cell proliferation, angiogenesis and inhibit inflammation to accelerate diabetic cutaneous wound healing. We further explored the underlying mechanism and found that DF-Ex exerted positive effects by activating the Akt/β-catenin pathway. This research revealed that DF-Ex may provide a new treatment strategy for diabetic cutaneous wound healing.
1. Introduction
Diabetes mellitus (DM) is a metabolic disease that threatens life and health, and type 2 diabetes mellitus (T2DM) accounts for 95% of diabetes cases [Citation1]. DM easily leads to many complications, and diabetic cutaneous wounds, also called diabetic ulcers, are one such complication [Citation2,Citation3]. Hyperglycemia-induced metabolic abnormalities include alterations in protein and lipid metabolism, peripheral neuropathy, and endothelial dysfunction [Citation4]. Such abnormalities lead to the occurrence of diabetic ulcers and impede the healing process. Currently, treatments for diabetic ulcers include dressing changes, antibiotic application, growth factor application and debridement [Citation5,Citation6]. However, there are many disadvantages with these approaches. Therefore, it is urgent to develop a more effective therapy.
In recent years, stem cell therapy, particularly mesenchymal stem cell (MSC) therapy, has attracted interest in the field of tissue regeneration and repair. MSCs are derived from a variety of sources, such as bone marrow, adipose tissue, human umbilical cord, and menstrual blood [Citation7]. Some studies show that MSCs can repair tissues in multiple disease models, such as in models for DM, liver fibrosis, acute and chronic renal injury, and cutaneous wound healing [Citation1,Citation8–10]. There is growing evidence that MSCs play an important role in promoting tissue regeneration and repair primarily through the paracrine pathway. Extracellular vesicles (EVs), especially exosomes, are key components of the paracrine pathway, and have promising prospects for regenerative medicine. Exosomes originating from the interior budding of the late endosomal membrane are a type of small EVs (30–150 nm in diameter) [Citation11,Citation12]. They can be secreted by nearly all kinds of living cells and exist in various bodily fluids [Citation13]. The characteristic lipid bilayer membranes of exosomes allow them to deliver bioactive molecules (including proteins, DNAs, mRNAs, miRNAs, etc.) to target cells/tissues and support tissue repair [Citation11,Citation12]. Compared with cell therapy, parental cell-derived exosomes possess various advantages including high stability, ease of storage and transport, low immunogenicity and low tumorigenesis [Citation11–14].
Skin is composed of the epidermis and dermis, with its homeostasis achieved through constant crosstalk among the major skin components: epidermal keratinocytes, dermal fibroblasts (DFs), immune cells, nerves, endothelial cells, and intradermal adipocytes [Citation15–17]. The wound healing process is divided into three stages including inflammation, proliferation and matrix remodeling [Citation18]. Some studies have reported that exosomes extracted from human urine-derived MSCs, adipose-derived MSCs and menstrual blood-derived MSCs play important roles in facilitating diabetic cutaneous wound healing [Citation19–21]. The exosomes derived from MSCs (MSC-Ex) promote skin repair mainly by promoting skin cell proliferation, inhibiting the inflammatory response and improving the wound microenvironment [Citation13]. DFs, located in the dermis of the skin, are essential for skin remodeling. They can produce abundant collagens and cytokines which are primary constituents in dermal extracellular matrix [Citation15]. Therefore, DFs are essential for skin regeneration and damage repair. However, whether exosomes derived from DFs (DF-Ex) play a role in the repair of diabetic ulcers is unclear. Therefore, in the current study, the role of DF-Ex in diabetic cutaneous wound healing was investigated. Neovascularization could provide damaged tissues with nutrition and oxygen, and is considered to be a crucial step in the healing process [Citation17,Citation22]. Therefore, we chose human umbilical vein endothelial cells (HUVECs) for the in vitro experiments in the current study. Recent studies have already confirmed a direct association between poor angiogenesis and high glucose levels [Citation4,Citation23].
In the current study, we administered DF-Ex to HUVECs under normal/high glucose conditions, and found that DF-Ex could promote proliferation, migration and tube formation in HUVECs under both conditions in vitro, which indicated that DF-Ex could promote neovascularization. In vivo, a subcutaneous injection of DF-Ex could significantly accelerate diabetic cutaneous wound healing. The Akt/β-catenin pathway was activated with DF-Ex treatment, which may be the underlying mechanism for DF-Ex in promoting skin repair. Our findings suggested that DF-Ex administration could be an alternative therapy for diabetic ulcers.
2. Materials and methods
All experimental protocols were approved by the Medical Ethics Committee of Jiangsu University (2012258).
2.1. Cell culture
DFs were isolated from the skins of new-born rats as previously described [Citation24]. In brief, the skin specimens were cut into small pieces (1–2 mm) using surgical scissors, and then placed in a 10 cm well, and cultured in α-MEM medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Bioind) and 1% penicillin-streptomycin (Bioind). The medium was changed every 3 d. DFs growing around the skin pieces were dissociated 7–9 d later, and 3–7 passages of DFs were used for the experiments. DFs were photographed using a microscope (Olympus) and identified using FAP and Vimentin antibodies through western blotting. HUVECs were purchased from the cell bank of the Chinese Academy of Sciences and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 10% FBS. Human dermal microvascular endothelial cells (HDMECs), cultured in Endothelial Cell Medium, were purchased from ScienCell Research Laboratories. All cells were cultured under high glucose (H.G., 30 mM glucose) or normal glucose (5.5 mM glucose) at 37°C with 5% CO2. In addition, cells treated with 30 mM mannitol were used as an osmolarity control for high glucose.
2.2. Isolation and characterization of exosomes
DF-Ex were isolated from cell supernatants as previously described [Citation25]. The final exosomes were passed through a 0.22 μm filter (Millipore) and stored at −70°C. The protein content of the DF-Ex, as a quantification of exosomes, was determined using a BCA protein assay kit (CWBIO). The final concentration of DF-Ex used for treating skin cells was 400 μg/mL in vitro, and total 2 mg of DF-Ex was applied to treat each animal in vivo. The morphology of the DF-Ex was observed using a transmission electron microscope (FEI Tecnai 12, Philips). Size distribution was identified through nanosight tracking analysis (Zeta View, Particle Metrix). The exosomal markers CD9, CD63, ALIX and the negative control Calnexin were determined using western blotting.
2.3. Western blotting
Cells, exosomes and skin tissues were lysed in RIPA buffer (Pierce) containing proteinase inhibitors (Pierce). The total protein concentration was detected through an A280 (nm) UV absorption method using NanoDrop One (Thermo Fisher Scientific). An equal amount of 150 μg of total proteins was separated on a 12% SDS-polyacrylamide gel through SDS-page electrophoresis, and then transferred onto polyvinylidene fluoride membranes (PVDF, Millipore). After blocking with 5% nonfat milk for 2 h, the membranes were incubated with primary antibodies at 4°C overnight, with β-actin as the internal control. Membranes were washed with TBST and then incubated with an HRP-conjugated goat anti-rabbit/mouse IgG secondary antibody (Invitrogen, #31460/#31430, 1:2000) for 1 h at room temperature. Subsequently the membranes were washed using TBST three times. The bands on the membrane were visualized using an ECL detection system (General Electric Company) and analyzed using ImageJ software. The primary antibodies are as follows: FAP (Santa Cruz Biotechnology, #sc-65398, 1:500), Vimentin (Abcam, #ab8978, 1:500), CD9 (Proteintech, #60232-1-Ig, 1:500), CD63 (Proteintech, #25682-1-AP, 1:500), ALIX (CST, #2171S, 1:500), Calnexin (Abclonal, #A15631, 1:500), PCNA (CST, #13110, 1:1000), Bcl-2 (Bioworld, #BS70205, 1:500), Bax (CST, #2772S, 1:500), VEGF-A (Bioworld, #BS6496, 1:500), p-Akt (Ser473) (Abclonal, #AP0140, 1:1000), t-Akt (Abclonal, #A7270, 1:1000), β-catenin (CST, #8480S, 1:1000), β-actin (Bioworld, #AP0060, 1:2000).
2.4. Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells using Trizol reagents (Glibo) in accordance with standard protocols, and the RNA concentration was measured using a NanoDrop One (Thermo Fisher Scientific). A reverse transcription kit (Vazyme) was used to synthesize cDNA from 1 μg total RNA in accordance with the manufacturer’s protocols. The qRT-PCR was performed using a SYBR Green PCR kit (CWBIO) with a PCR machine (Applied Biosystems), and the primer sequences of the target genes are listed in Supplementary Table 1 Relative gene expression was calculated using the 2–ΔΔCT method and β-actin was used as a housekeeping gene for normalization.
2.5. Immunofluorescence (IF)
A total of 1 × 104 cells was seeded on cover glass in 12-well plates, and 12 h later, different treatments were applied for 48 h: under high glucose or normal glucose conditions, with/without DF-Ex. The cells were then washed with PBS and fixed in 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton-X100 for 30 min, blocked with 5% bovine serum albumin for 30 min, and incubated with Vimentin (Abcam, #ab8978, 1:100), CD31 (Bioworld, #BS1206, 1:100), p-Akt (Ser473) (Abclonal, #AP0140, 1:100), and β-catenin (CST, #8480S, 1:100) antibodies overnight at 4°C, followed by incubation with a FITC-conjugated goat anti-rabbit/mouse IgG secondary antibody (SAB, #L35073/#L35071, 1:200) for 1 h at room temperature. The nuclei were stained using Hoechst 33342 (Sigma-Aldrich, #B2261, 1:300). Images were taken using a fluorescence microscope (Nikon) or a confocal microscope (General Electric Company).
2.6. Exosome labeling and internalization
Diluted exosomes (1 mL) were incubated with 5 μL of the membrane dye DiI (red, Invitrogen) for 30 min at 37°C.The labeled exosome suspension was centrifuged at 1000 × g for 30 min so that the unconjugated DiI could pass through a 100-kDa MWCO hollow fiber membrane (Millipore). A total of 1 × 104 cells was seeded on cover glass in 12-well plates and incubated with DiI-labeled exosomes for 24 h at 37°C. The cells were then fixed in 4% paraformaldehyde and stained with a specific cell marker (CD31 for HUVECs, Vimentin for DFs) and Hoechst 33342 (Sigma-Aldrich) for IF. Images were obtained using a confocal microscope (General Electric Company).
2.7. Cell proliferation assay
The proliferation of cells stimulated by high glucose and DF-Ex was evaluated using a Cell Counting Kit-8 (CCK-8) assay and a cell colony formation assay. The CCK-8 assay was performed to evaluate cell viability in accordance with the manufacturer’s instructions. Cells were seeded into 96-well plates with 1500 cells per well and treated with 10 μL of CCK-8 reagent (Vazyme) every 24 h. The absorbance values at 450 nm were analyzed using an Absorbance Reader (BioTek). A cell colony formation assay was another way to assess cell activity. A total of 2 × 103 cells was seeded into 35 mm wells. The medium was replaced every 3 d, and 10 d later, cells in each well were fixed in 4% paraformaldehyde and stained using crystal violet staining solution. The size and number of cell colonies were observed and photographed.
2.8. Cell migration assays
The migration ability of the cells was evaluated using a transwell assay and a scratch wound healing assay. Cells were firstly treated with high glucose and DF-Ex in 6-well plates for 48 h. For the transwell assay, 2 × 104 cells from different groups in 200 μL of serum-free medium were plated into the upper chambers of a 24-well transwell plate (pore size = 8 μm, Corning), with 600 μL of complete medium in the lower chambers. After incubation at 37°C with 5% CO2 for 16 h, the cells from the upper surface of the filter membranes were removed using a cotton swab, while the cells that migrated to the lower surface of the filter membrane were fixed in 4% paraformaldehyde and stained using a crystal violet staining solution. The stained cells were observed, photographed and counted using a microscope (Nikon), and at least three fields of view were assayed for each group. For the scratch wound healing assay, 2 × 105 cells from different groups were seeded into 6-well plates. After the cells had attached, the monolayer of cells was scratched using a 200 μL pipette tip, and washed with PBS to remove the floating cells. Plates were photographed at 0 h and 24 h after wounding. The rate of the migration area was calculated as follows: Migration area (%) = (A0 – At)/A0 × 100%, where A0 represented the area of the initial wound and At represented the remaining area of the wound at the metering point.
2.9. Tube formation assay
HUVECs were firstly treated with high glucose and DF-Ex in 6-well plates for 48 h. In brief, 200 μL of cold Matrigel (BD Bioscience) per well was added to a 24-well plate with a pre-cooled tip and it was allowed to polymerize at 37°C for 30 min. A total of 6.5 × 104 cells from different groups was plated onto the Matrigel in 500 μL of complete medium. After incubation for 12 h at 37°C, the tubular structures were viewed using a phase-contrast microscope (Olympus), and random photomicrographs were taken per well.
2.10. Diabetic wound healing evaluation
T2DM animal models were established in the current study as previously described [Citation1]. Male Sprague Dawley rats (8 weeks old, 180–220 g) were purchased from the Laboratory Animal Center of Jiangsu University and were fed 45% high fat diets (HFD) for 5 weeks. After fasting for 12 h without food and water, the HFD fed rats were injected with streptozotocin (35 mg/kg in 0.1 M citrate-buffered saline, pH 4.5) via the tail vein to induce DM. Blood glucose was measured 3 d later using blood glucose test strips (Roche) and a blood glucose level above 16.7 mM was considered to indicate DM. Diabetic rats were maintained with normal diets for another 2 months to establish a stable diabetic animal model and blood glucose levels were confirmed again just before the wound was created. After anesthesia using a 10% chloral hydrate solution (250 μL/100 g) through intraperitoneal injection, round full-thickness cutaneous wounds (2 cm) were created on the hair-removed back of each rat. Rats were then randomly assigned into two groups, which were either injected subcutaneously at four sites at the wound edge with DF-Ex (2 mg in 200 μL of PBS) or 200 μL of PBS (control). Rats were maintained individually with normal diets and water. To observe the process of wound healing, wounds were photographed using a digital camera at day 0, 6, 12 and 15 after operation. All rats were sacrificed, and skin specimens harvested at day 15 were analyzed through hematoxylin/eosin (HE) staining, Sirius Red staining, immunohistochemistry (IHC), immunohistofluorescence (IHF) and western blotting. Wound sizes at day 0, 6, 12, and 15 were analyzed using ImageJ software. The percent of wound closure was calculated using the formula: wound closure (%) = (A0 – At)/A0 × 100%, where A0 was the wound size at day 0 and At was the wound size at each certain day. The scheme of the whole process is shown in Supplementary .
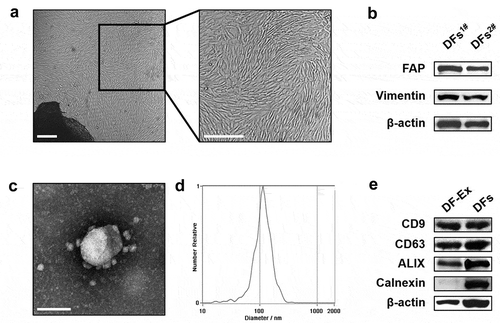
Figure 1. Isolation and characterization of DFs and DF-Ex. (a) The morphology of primary DFs was observed using an optical microscope (scale bar = 250 μm). (b) DFs were identified with FAP and Vimentin through western blotting. (c) The morphology of DF-Ex was observed using a transmission electron microscope (scale bar = 100 nm). (d) The size distribution of DF-Ex was detected through nanosight tracking analysis. (e) The exosomal markers were analyzed through western blotting
2.11. Histologic, IHC and IHF analysis
Skin tissues were obtained from sacrificed rats at day 15. The samples were fixed in 4% paraformaldehyde, gradually dehydrated, embedded in paraffin, and then cut into 4-μm sections. Sections underwent HE staining and Sirius Red staining in accordance with standard protocols. IHC was performed in accordance with the manufacturer’s instructions (Boster, #SA1020). Primary antibodies such as PCNA (CST, #13110, 1:200), CD31 (Abcam, #ab182981, 1:200), IL-6 (Bioworld, #BS6419, 1:200) and p-Akt (Ser473) (Abclonal, #AP0140, 1:200) were incubated with sections for IHC. Skin sections stained with HE, Sirius Red, and IHC were observed using a digital slide scanner (Pannoramic MIDI, 3D Histech). Skin sections were subjected to IHF in accordance with standard protocols. Sections were incubated with a β-catenin (CST, #8480S, 1:100) antibody at 4°C overnight, then incubated with a FITC-conjugated goat anti-rabbit IgG secondary antibody (SAB, #L35073, 1:200) for 1 h, and subsequently stained with Hoechst 33342 (Sigma-Aldrich, #B2261, 1:300) before observation using a fluorescence microscope (Nikon).
2.12. Statistical analysis
All data are presented as means ± standard deviation (SD) and were analyzed using GraphPad Prism software (version 5.0). T-tests were used to compare means between two different groups, while one-way analysis of variance (ANOVA) was used to determine the significance level between three or more groups. P value ≤ 0.05 was considered to be statistically significant. Asterisks *, **, and *** stood for P < 0.05, P < 0.001, and P < 0.0001 respectively.
3. Results
3.1. Isolation and characterization of DFs and DF-Ex
To investigate the roles of DF-Ex in wound healing, DFs were firstly acquired from the skins of new-born SD rats and were grown in clusters ()). FAP and Vimentin were used to identify DFs ()). The cell culture supernatant was collected from DFs of 3–7 passages. DF-Ex were extracted and purified through differential centrifugation from the cell culture supernatant, and were subsequently characterized through transmission electron microscopy, nanosight tracking analysis and western blotting. We found that DF-Ex exhibited a sphere-shaped morphology ()). The diameters of most DF-Ex ranged from 100 to 120 nm, and the majority were approximately 110 nm ()). Exosomal markers such as CD9, CD63 and ALIX were abundant in the DF-Ex while the negative control Calnexin was absent in the DF-Ex ()). Taken together, the results indicate that DFs and DF-Ex were successfully acquired for further research.
3.2. DF-Ex protected skin cells from high glucose damage
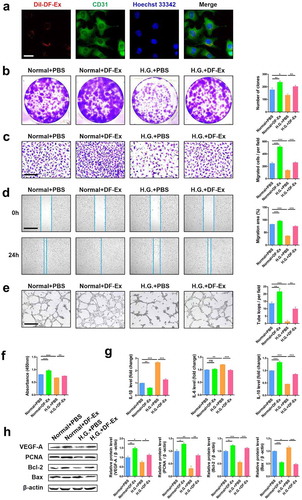
Exosomes have a characteristic lipid bilayer that can easily fuse with the cytomembrane of recipient cells. Using a confocal microscope, we observed that DF-Ex were internalized by HUVECs ()). The proliferation (), migration () and tube formation ()) abilities of HUVECs declined under high glucose conditions. However, with DF-Ex treatment, the damaging effects of high glucose were obviously reversed. DF-Ex also enhanced the proliferation, migration and tube formation of HUVECs under normal culture conditions (). Given that HUVECs treated with high glucose were under conditions of high osmolarity, we used an osmolarity control of 30 mM mannitol for high glucose, and found that high osmolarity had no effects on the proliferation and migration of HUVECs (Figure S2A–C). In addition, to exclude the possibility that something co-isolated with DF-Ex may have an influence on HUVECs, we used DF-Ex depleted medium as a control and found no effects either (Figure S2A–C). Compared with normal glucose conditions, the expression levels of pro-inflammatory cytokines including IL-1β and IL-6 were higher, while the expression level of anti-inflammatory cytokine IL-10 was lower under high glucose conditions. DF-Ex treatment down-regulated the levels of IL-1β and IL-6, and up-regulated the level of IL-10 to inhibit the inflammatory response ()). Vascular endothelial growth factor A (VEGF-A), an important factor for angiogenesis, decreased under high glucose conditions compared with normal glucose conditions, and recovered with DF-Ex treatment ()). The same changes were found in proliferative-and-apoptotic related proteins (PCNA, Bcl-2, Bax) ()). The protein levels of PCNA, Bcl-2 and Bax revealed that DF-Ex promoted HUVEC proliferation ()), consistent with the results of the cell colony formation assay and CCK-8 assay (). Therefore, these results confirmed that high glucose conditions did harm HUVECs. However, DF-Ex could protect HUVECs from high glucose damage by prompting proliferation, migration and tube formation, and inhibiting apoptosis and the inflammatory response.
Figure 2. DF-Ex reversed damaging effects of high glucose on HUVECs. (a) The internalization of DiI-labeled DF-Ex by HUVECs was observed using a confocal fluorescence microscope (scale bar = 20 μm). (b) The proliferation of HUVECs treated with/without high glucose or DF-Ex was determined through a cell colony formation assay (n = 3). (c) A transwell assay indicated the migration ability of HUVECs with/without high glucose or DF-Ex treatment (scale bar = 200 μm, n = 3). (d) A scratch wound healing assay revealed the motility of HUVECs with/without high glucose or DF-Ex treatment (scale bar = 1000 μm, n = 3). (e) The tube formation ability of HUVECs treated with/without high glucose or DF-Ex was determined through a tube formation assay (scale bar = 400 μm, n = 3). (f) The cell viability of HUVECs with different treatments was detected through a CCK-8 assay (n = 3). (g) qRT-PCR for the relative mRNA levels of inflammatory cytokines (IL-1β, IL-6, IL-10) in HUVECs (n = 3). (h) The expression levels of proliferation (PCNA, Bcl-2), apoptosis (Bax) and angiogenesis (VEGF-A) related proteins were detected through western blotting (n = 3). (b–h) *p < 0.05, **p < 0.01, ***p < 0.001, ns: no significance
Considering that HUVECs are macrovascular cells, we also verified the effects of high glucose and DF-Ex on HDMECs (a microvascular cell line). The proliferation (Figure S3A) and migration (Figure S3B, C) of HDMECs were impaired under high glucose conditions, but were enhanced with DF-Ex treatment. The results with HDMECs were consistent with the results with HUVECs.
Moreover, we also found that high glucose and DF-Ex treatment had similar influences on DFs. After being self-internalized by DFs (Figure S4A), DF-Ex enhanced the migration ability, collagen levels (collagenIα, collagenIIIα) and cytokine levels (VEGF-A) of DFs under normal/high glucose conditions and partly reversed the damaging effects of high glucose on DFs (Figure S4B, C).
3.3. DF-Ex accelerated cutaneous wound healing in T2DM rats
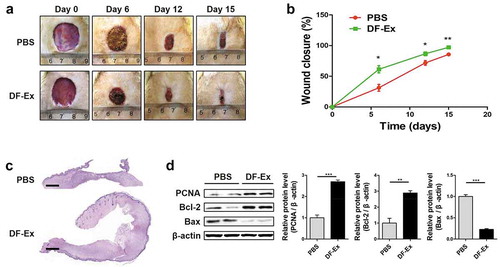
A round full-thickness cutaneous wound was created on the hair-removed back of each diabetic rat using surgical scissors, and subsequently, DF-Ex or PBS (control) were injected subcutaneously at four sites of the wound edges. From images taken at day 0, 6, 12, and 15, the wound size in the DF-Ex group was obviously smaller than that in the control group, and the wounds treated with DF-Ex had nearly closed at day 15 ()). An analysis of the wound closure rates confirmed that the wounds treated with DF-Ex contracted significantly faster than the wounds treated with PBS at day 6, 12, and 15 ()). From general observations of the HE stained sections, skin wounds treated with DF-Ex had a complete re-epithelialization and a horny layer covered over the epidermis. In contrast, skin wounds treated with PBS showed a low extent of re-epithelialization ()). The protein levels of PCNA, Bcl-2 and Bax revealed that DF-Ex enhanced the proliferation of skin cells to accelerate the wound healing process ()).
Figure 3. DF-Ex accelerated cutaneous wound healing in diabetic rats. (a) Representative images of wound healing process at day 0, 6, 12, and 15. (b) Wound closure rates of two groups were analyzed at day 6, 12, and 15 (n = 6). (c) Images of whole skin sections with HE staining harvested at day 15 (scale bar = 1000 μm). (d) The protein levels of PCNA, Bcl-2 and Bax were analyzed through western blotting at day 15 (n = 3). (b, d) *p < 0.05, **p < 0.01, ***p < 0.001
3.4. DF-Ex promoted skin reconstruction, collagen deposition, cell proliferation, neovascularization and inhibited inflammation
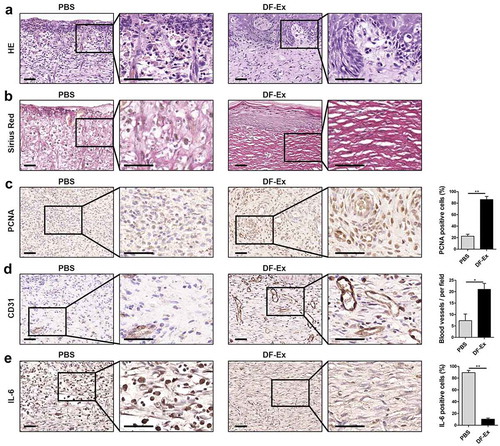
Skin specimens harvested at day 15 from sacrificed rats underwent a series of histological analyses. Skin sections from the PBS group were infiltrated with a mass of inflammatory cells (nuclear hyperchromatism) and red blood cells, without epidermis and adnexal glands. On the contrary, in the skin sections treated with DF-Ex, complete re-epithelialization and new-forming appendages appeared, while inflammatory cells and red blood cells could hardly be seen ()). Collagen deposition analysis was performed through Sirius Red staining. Large amounts of collagen fibers in the DF-Ex group demonstrated a wavy shape and appeared in a well-organized manner, while in the PBS group, the collagen fibers were much fewer and in a disordered state ()). This discovery suggested that DF-Ex up-regulated the collagen expression levels of DFs (Figure S4C). We observed very few PCNA-positive cells in wound beds treated with PBS. In contrast, in the DF-Ex group, a large number of PCNA-positive cells were discovered in the wound ()). Newly formed blood vessels were detected through IHC for CD31. Compared with the blood vessels in the PBS group, blood vessels in the DF-Ex group were presented in a larger number, and a bigger size with an integrated lumen structure in the dermis ()). Skin cells treated with PBS showed a high positive rate of IL-6, while skin cells treated with DF-Ex showed a low positive rate of IL-6 ()), implying that DF-Ex regulated the inflammatory response in the damaged skin. These findings revealed that DF-Ex significantly enhanced cutaneous wound healing by promoting re-epithelialization, collagen deposition, skin cell proliferation and angiogenesis, and inhibiting inflammation.
Figure 4. Histological analysis of diabetic cutaneous wound healing at day 15. (a) HE staining was used to detect skin structure in the wounds. (b) The distribution of collagen fibers in skin tissues was analyzed through Sirius Red staining. (c) IHC was used to detect the expression level of PCNA in the wounds. (d) IHC was used to detect the expression level of CD31 in the wounds. (e) IHC was used to detect the expression level of the inflammatory cytokine IL-6 in the wounds. (a–e) Sections in the rectangle are magnified on the right, scale bar = 50 μm. (c–e) n = 3, *p < 0.05, **p < 0.01
3.5. DF-Ex promoted diabetic cutaneous wound healing by activating the Akt/β-catenin pathway
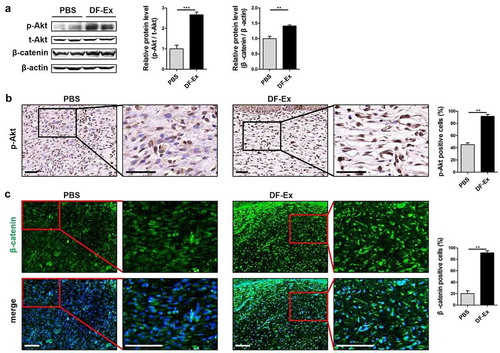
Studies have confirmed that Akt signaling is directly involved in regulating skin cell proliferation, and therefore, the activation of Akt was monitored [Citation26]. The ratio of p-Akt/t-Akt in HUVECs was reduced by high glucose, and was increased with DF-Ex treatment, as assessed through western blotting analysis ()). The fluorescence intensity of p-Akt was much higher in the DF-Ex group than in the PBS group and high glucose group ()). According to our previous results, β-catenin signaling is activated in the cutaneous wound healing process [Citation27], and herein, we also detected the activation of β-catenin. We found that β-catenin was inhibited by high glucose and activated by DF-Ex in HUVECs ()). β-catenin nuclear translocation could be clearly seen in the DF-Ex group ()). Similar to HUVECs that underwent DF-Ex treatment in vitro, the activation of Akt and β-catenin was also found in skin specimens from animals that underwent DF-Ex treatment ().
Figure 5. DF-Ex alleviated high glucose damage to HUVECs through the Akt/β-catenin pathway in vitro. (a) The expression levels of p-Akt, t-Akt and β-catenin in HUVECs were detected through western blotting (β-actin as the internal control for β-catenin, t-Akt as the internal control for p-Akt, n = 3). (b) The expression and localization of p-Akt in HUVECs was analyzed through IF (scale bar = 100 μm, n = 3). (c) The expression and localization of β-catenin in HUVECs was detected through IF (scale bar = 100 μm, n = 3). (a–c) *p < 0.05, **p < 0.01, ***p < 0.001, ns: no significance
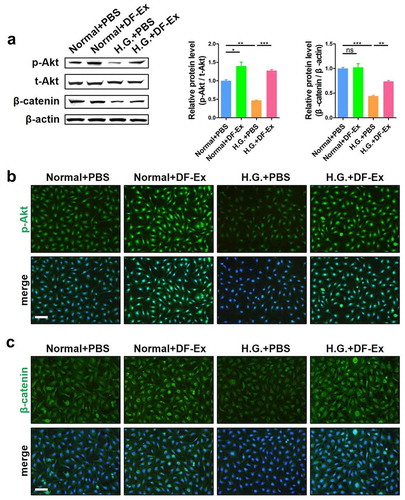
Figure 6. DF-Ex accelerated cutaneous wound healing in T2DM rats through the Akt/β-catenin pathway in vivo. (a) The protein levels of p-Akt, t-Akt and β-catenin in skin tissues were analyzed through western blotting (n = 3). (b) Activation of the Akt pathway in vivo was assessed through IHC (scale bar = 50 μm, n = 3). (c) The expression and localization of β-catenin in skin tissues was assessed through IHF (scale bar = 100 μm, n = 3). (a–c) **p < 0.01, ***p < 0.001
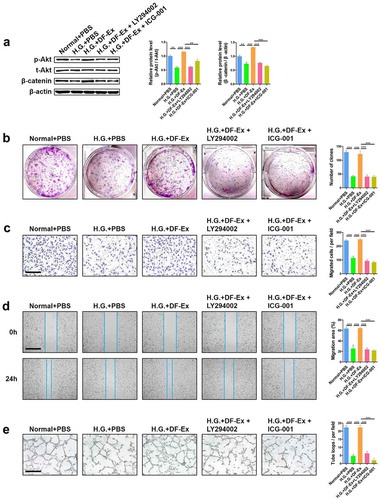
To further confirm that DF-Ex exerted effects through the Akt/β-Catenin pathway, experiments to interfere with the activity of Akt or β-catenin were performed. LY294002 was used to suppress the activation of Akt and ICG-001 was used to suppress the activation of β-catenin ()). We had already verified that DF-Ex protected HUVECs from high glucose damage. However, with additional LY294002 or ICG-001, the positive effects of DF-Ex on the proliferation, migration and tube formation of HUVECs under high glucose conditions were diminished ()). Taken together, these results demonstrated that DF-Ex promoted diabetic cutaneous wound healing by activating the Akt/β-catenin pathway.
Figure 7. Interference in activation of the Akt/β-catenin pathway made DF-Ex ineffective. (a) LY294002 (Beyotime, #S1737, 10 μM) and ICG-001 (Selleck, #S2662, 10 μM) were used to suppress the activation of Akt and β-catenin respectively. Western blotting was used to detect the expression levels of p-Akt, t-Akt and β-catenin in HUVECs with different treatments (n = 3). (b) A cell colony formation assay was used to detect the proliferation ability of HUVECs with different treatments (n = 3). (c) A transwell assay indicated the migration ability of HUVECs with different treatments (scale bar = 200 μm, n = 3). (d) A scratch wound healing assay revealed the motility of HUVECs with different treatments (scale bar = 1000 μm, n = 3). (e) The tube formation ability of HUVECs with different treatments was determined through a tube formation assay (scale bar = 400 μm, n = 3). (a–e) **p < 0.01, ***p < 0.001
4. Discussion
Diabetic ulcers are one of the most malignant complications of DM, and frequently result in amputation or death through systemic infection. Therefore, diabetic ulcers are becoming a global burden [Citation3]. Current clinical therapies cannot solve this problem thoroughly. Therefore, a new effective treatment method is urgently needed. Recently, exosomes from different sources have been reported to accelerate diabetic cutaneous wound healing [Citation19–21], which provided us with a new idea that exosomes derived from autologous DFs would be useful. Compared with other cell sources, DFs have some outstanding advantages. DFs are easy to isolate and culture with vigorous growth, and with no need for any other additional recombinant growth factor in vitro.
Wound healing starts with DF proliferation [Citation28]. Resident DFs neighboring the wound begin to proliferate and migrate to the wound clot 3–4 d after the wound occurs [Citation22]. Therefore, the importance of DFs is without parallel in this context. However, the functions of DFs are impaired under high glucose conditions [Citation29], which is one vital factor for delayed diabetic cutaneous wound healing. In the current study, we isolated DFs that have significant ability for proliferation from new-born healthy rats, which may represent a new cell therapy to accelerate diabetic cutaneous wound healing. In consideration of the weaknesses of cell therapy such as short cell lifespan and low cell activity, we extracted exosomes derived from DFs as a cell-free therapy. DFs can produce a lot of cytokines and growth factors for endothelial cells and keratinocytes, and these bioactive molecules may be packaged by exosomes and delivered to target cells to prompt the activities of recipient cells [Citation11,Citation13,Citation15–17,Citation28].
In this research, the proliferation, migration and tube formation of HUVECs were impaired under high glucose conditions. However, we found that DF-Ex treatment could reverse the effects of high glucose. Moreover, DF-Ex could also alleviate the inflammatory reaction of HUVECs under high glucose conditions. Aside from this, DF-Ex augmented the migration ability and the cytokine levels of DFs treated with high glucose, which indicated that DF-Ex extracted from healthy DFs could benefit DFs demonstrating poor growth. After the effects of DF-Ex were analyzed in vitro, we applied DF-Ex in cutaneous wounds in T2DM rats. Our research showed that DF-Ex prominently promoted skin re-epithelialization, collagen deposition, skin cell proliferation, angiogenesis, and alleviated the inflammatory response. So far, we have confirmed that DF-Ex plays a vital role in accelerating diabetic cutaneous wound healing.
The activation of the Akt/β-Catenin Pathway was involved in the DF-Ex positive effects in our study. It is well known that the Akt signaling pathway is closely related to cell proliferation, migration, and tube formation [Citation26,Citation27,Citation30,Citation31]. Zhang et al. suggested that exosomes derived from human umbilical cord MSCs (hucMSC-Ex) could reverse acute thermal injury-induced apoptosis in skin cells mainly through activation of the Akt pathway [Citation27]. Ma et al. reported that exosomes derived from Akt-modified hucMSCs could improve cardiac regeneration and promote angiogenesis [Citation30]. Wnt family proteins (including Wnt1, Wnt2, Wnt3, Wnt3a, Wnt4 and Wnt8) transfer β-catenin from the cytoplasm into the cell nucleus to trigger downstream signaling pathways [Citation32]. Wnt/β-catenin signaling participates in the development of multiple tissues and organs including skins. Zhang et al. showed that hucMSC-Ex mediated the Wnt4/β-catenin pathway to enhance angiogenesis in cutaneous wounds [Citation25]. Ibrahim et al. used lentiviral transduction to augment Wnt/β-catenin signaling in fibroblasts to convert fibroblasts into therapeutically potent exosome factories [Citation33]. In the current study, the subtype of Wnt that mediates β-catenin is still unknown and needs to be further investigated. Some studies have revealed that the Wnt/β-catenin signaling could be directly or indirectly regulated by Akt [Citation31,Citation34]. In terms of the current results, the relationship between Akt and β-catenin signaling is unclear and needs requires further study. In fact, apart from bioactive proteins, nucleic acid molecules including miRNAs, circRNAs, lncRNAs and piRNAs may also play important roles in the positive effects of DF-Ex on skin repair. Therefore, in future work, a sequencing chip will be applied to thoroughly discover the specific molecular mechanism of DF-Ex.
Exosomes derived from autologous DFs were used to augment cutaneous wound healing in T2DM rats. In the near future, we may be able to use exosomes derived from allogeneic human fibroblasts to treat patients with diabetic ulcers. Human fibroblasts can be acquired from juvenile or adult foreskin [Citation35,Citation36], a kind of medical waste, and waste materials may be changed into things of value in that way. Considering that the Akt and β-catenin signaling pathways were both activated by DF-Ex in vivo and in vitro, a new perspective arises that modifying DFs through overexpression of Akt and β-catenin simultaneously may enhance the positive effects of DF-Ex on diabetic cutaneous wound healing. Biomaterials also represent a potential pathway to healing chronic wounds. Biomaterials can coordinate the temporal delivery of multiple bioactive molecules, protect sensitive biologics from degradation, and provide supportive matrices that encourage tissue growth [Citation5]. For example, Wang et al. fabricated an injectable adhesive thermosensitive multifunctional polysaccharide-based dressing with sustained pH-responsive exosome release [Citation20]. The combined application of DF-Ex and biomaterials will enable a novel therapeutic approach to diabetic ulcers.
5. Conclusion
In summary, our findings indicated that DF-Ex effectively augmented the functional properties of skin cells and accelerated diabetic cutaneous wound healing in T2DM rats through activation of the Akt/β-catenin pathway, which may provide an alternative treatment for diabetic ulcers.
Authors’ contributions
Xinye Han and Peipei Wu contributed equally to this work.
Supplemental Material
Download MS Word (1.8 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Sun Y, Shi H, Yin S, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving beta-cell destruction. ACS Nano. 2018;12(8):7613–7628.
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188.
- Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724.
- Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):29.
- Pop MA, Almquist BD. Biomaterials: a potential pathway to healing chronic wounds? Exp Dermatol. 2017;26(9):760–763.
- Choi SM, Lee KM, Kim HJ, et al. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018;66:325–334.
- Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5–14.
- Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med. 2015;30(5):580–589.
- Peng X, Xu H, Zhou Y, et al. Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury. Exp Biol Med (Maywood). 2013;238(8):960–970.
- Zhang B, Shi Y, Gong A, et al. Huc umbilical cord exosome-delivered 14-3-3zeta orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34(10):2485–2500.
- Cabral J, Ryan AE, Griffin MD, et al. Extracellular vesicles as modulators of wound healing. Adv Drug Deliv Rev. 2018;129:394–406.
- Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232.
- Wu P, Zhang B, Shi H, et al. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301.
- Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15(4):193–203.
- Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev. 2018;7(2):e307.
- Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23(8):917–927.
- Rognoni E, Watt FM. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 2018;28(9):709–722.
- Ferreira ADF, Gomes DA. Stem cell extracellular vesicles in skin repair. Bioengineering (Basel). 2018;6(1). DOI:10.3390/bioengineering6010004
- Chen CY, Rao SS, Ren L, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8(6):1607–1623.
- Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13(9):10279–10293.
- Dalirfardouei R, Jamialahmadi K, Jafarian AH, et al. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–568.
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276(5309):75–81.
- Huang Z, Li N, Shan Y, et al. Hsa-miRNA-29a protects against high glucose-induced damage in human umbilical vein endothelial cells. J Cell Biochem. 2019;120(4):5860–5868.
- Morimoto N, Takemoto S, Kanda N, et al. The utilization of animal product-free media and autologous serum in an autologous dermal substitute culture. J Surg Res. 2011;171(1):339–346.
- Zhang B, Wu X, Zhang X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl Med. 2015;4(5):513–522.
- Zhang W, Bai X, Zhao B, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370(2):333–342.
- Zhang B, Wang M, Gong A, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–2168.
- Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–281.
- P Kwesiga M, Cook E, Hannon J, et al. Investigative study on nitric oxide production in human dermal fibroblast cells under normal and high glucose conditions. Med Sci (Basel). 2018;6:4.
- Ma J, Zhao Y, Sun L, et al. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med. 2017;6(1):51–59.
- Chen YG, Li Z, Wang XF. Where PI3K/Akt meets Smads: the crosstalk determines human embryonic stem cell fate. Cell Stem Cell. 2012;10(3):231–232.
- Shi H, Xu X, Zhang B, et al. 3,3ʹ-Diindolylmethane stimulates exosomal Wnt11 autocrine signaling in human umbilical cord mesenchymal stem cells to enhance wound healing. Theranostics. 2017;7(6):1674–1688.
- Ibrahim AGE, Li C, Rogers R, et al. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat Biomed Eng. 2019;3(9):695–705.
- Chen EY, Mazure NM, Cooper JA, et al. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–2433.
- Lako M, Hill RP, Gledhill K, et al. Generation and characterization of multipotent stem cells from established dermal cultures. PLoS ONE. 2012;7(11):e50742.
- Budel L, Djabali K. Rapid isolation and expansion of skin-derived precursor cells from human primary fibroblast cultures. Biol Open. 2017;6(11):1745–1755.
