ABSTRACT
The members of the tumor necrosis factor receptor (TNFR) family have been demonstrated to play critical roles in various cancers. However, little is known about the function of the Receptor Expressed in Lymphoid Tissues (RELT) in cancers, which is a member of the TNFR family, especially in the esophageal squamous cell carcinoma (ESCC). In this study, we found that RELT expression was increased in ESCC tissues and was consequently associated with poor overall survival of ESCC patients. Moreover, RELT overexpression was found to promote cell growth, cell cycle progression, and suppressed cell apoptosis in vitro; it also decreased the expression of p27 and caspase 3, and increased the expression of survivin. In addition, RELT contributed to the tumorigenesis of ESCC in vivo. Furthermore, we suggest that RELT may function in the pathogenesis of ESCC by activating the nuclear factor κB (NF-κB) pathway. An inhibitor of NF-κB reversed the RELT-induced malignancy in the ESCC cells. Altogether, our findings identified that RELT served as an oncogene in ESCC through the NF-κB pathway, suggesting that RELT may be developed as a novel biomarker for the diagnosis and treatment of the ESCC.
Introduction
Esophageal cancer is a malignancy of the digestive tract and is known for its high global mortality rates among the various types of cancers. Esophageal squamous cell carcinoma (ESCC) is a major histological type of esophageal cancer [Citation1,Citation2]. Although comprehensive treatments, including surgery, chemotherapy, radiotherapy, and targeted therapy, have improved ESCC treatments over the past decade, the existing 5-year survival rate remains unsatisfactory [Citation3]. Therefore, identification of new molecular biomarkers for the detection and elucidation of the potential mechanisms of tumor pathogenesis is of great significance to improve the prognosis of ESCC.
The tumor necrosis factor receptor (TNFR) superfamily is composed of 19 ligands and 29 receptors, which play critical roles in cell proliferation, cell differentiation, and cell death [Citation4–6]. The transmembrane TNFR activates the intracellular signal pathways by binding to the corresponding tumor necrosis factor (TNF) ligands [Citation7,Citation8]. Currently, many TNFR and TNFR-associated factors (TRAFs) are at various stages of preclinical or clinical development as targets for the treatment of cancer, inflammation, and immune diseases [Citation9]. The Receptor Expressed in Lymphoid Tissues (RELT) is a member of the TNFR family, which is mainly distributed in the lymphoid tissues and has two identified homologous binding partners, the RELT-like protein1 (RELL1) and the RELT-like protein 2 (RELL2). Although the TNFRs activate nuclear factor κB (NF-κB) and the mitogen-activated protein kinase (MAPK) by engaging TRAFs, RELT has been found to activate p38 and the c-Jun N-terminal kinase (JNK) through the Ste20-related protein proline-alanine-rich kinase (SPAK) [Citation10]. RELT has also been found to activate the transcription factor NF-κB and selectively bind to the TNF receptor-associated factor 1 (TNFRAF1) [Citation11,Citation12]. However, it has been reported that up-regulation of RELT in the 293 cells does not activate NF-κB [Citation10]. Therefore, the effects of RELT on NF-κB need to be further studied.
Researches have shown that an autoantibody against RELT may be a promising biomarker for the early diagnosis of breast cancer [Citation13], as RELT expression increases significantly when breast cancer cells undergo an epithelial-mesenchymal transformation [Citation14]. Besides, RELT has not been studied exclusively in various cancers. In the present study, we aimed to explore the role of RELT in ESCC. We evaluated the expression of RELT in the ESCC samples from The Cancer Genome Atlas (TCGA) database and compared them with the levels in the normal esophageal samples. Moreover, we explored the underlying mechanism of RELT in the pathogenesis of ESCC both in vitro and in vivo.
Materials and methods
Database analysis
RELT expression data (RNA-sequencing) for ESCC patients and clinical information were collected from The Cancer Genome Atlas (TCGA) and analyzed using the R software (version 3.4.1). The difference in the levels of RELT expression between the ESCC and the normal tissues was compared by the Student’s t-test. Survival analyses were performed using the Kaplan–Meier plotter along with the log-rank p test.
Cell lines and ESCC clinical samples
ECA-109, KYSE140, TE-1, TE11, TE-14, and the human esophageal epithelial cell (HEEC) lines were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China) and cultured in the RPMI-1640 medium (Invitrogen, USA) with 10% fetal bovine serum (Hyclone, China). The cells were maintained at 37°C with 5% CO2. Moreover, the NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC; 50 μmol, Sigma-Aldrich, USA), or the vehicle (culture medium plus the solvent) were added to the cells for additional experiments [Citation15].
A total of twelve pairs of primary ESCCs and corresponding samples were collected at the Henan Provincial People’s Hospital in 2019. All patients were diagnosed by pathologists and had not received any chemotherapy or radiotherapy prior to the resection. This study was approved by the Ethics Committee of the Henan Provincial People’s Hospital.
Real-time RT-PCR
Total RNA was isolated from cells and tissues using the TRIzol reagent (Invitrogen, USA) and used to synthesize cDNAs with the Reverse Transcription kit (Fermentas, USA) following the manufacturer’s instructions. The primers used were as follows: RELT, 5' CTT GTC TGT GGG CAG GTT C 3' (forward) and 5' GTT CTC CTC GGC CTT GTT C 3' (reverse); GAPDH, 5' AAT CCC ATC ACC ATC TTC 3' (forward) and 5' AGG CTG TTG TCA TAC TTC 3' (reverse). Real-time PCR was conducted with the SYBR Green PCR kit (Thermo, USA). All the reactions were performed on the ABI-7300 Real-Time PCR System (ABI, USA). The expression of each gene was normalized to the expression of GAPDH and calculated using the 2−ΔΔCT method.
Western blot
Cells and tissues were lysed with the RIPA lysis buffer. The protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). The PVDF membranes were incubated overnight at 4°C with the following primary antibodies: anti-RELT (Ab96220, Abcam), anti-P27 (#2552,CST), anti-Caspase3 (Ab2302, Abcam), anti-survivin (#2802,CST), anti-NF-κB (#8242, CST), anti-H3 (#4499,CST), or anti-β-actin (#4970,CST). After three washes with Tris-Buffered Saline, 0.1% Tween® 20 Detergent (TBST buffer), the PVDF membranes were incubated with a secondary goat anti-rabbit antibody. Signals were then visualized using the Tanon-5200 ECL system (Tanon, China). The results were quantified with ImageJ and normalized to that of β-actin.
Immunohistochemical analysis
Tissues were fixed with formaldehyde, embedded with paraffin, and cut into 5-μm thick sections. Slides were dewaxed with xylene, rehydrated, and unmasked according to standard immunohistochemical (IHC) methods. The antibody against RELT (Ab96220, Abcam) was diluted (1:500). The slides were then stained with 3,3‘- diaminobiphenylamine and re-stained with hematoxylin. Images were taken with a Leica Camera.
RELT knock-down and overexpression
Three small hairpin RNAs (shRELT1–shRELT3) targeting RELT and the scramble shRNA (shNC) were designed by RiboBio (China) and the sequences were as follows: 5'- CCTGACATCAACAACCCTT −3' for shRELT-1; 5'- GGGTACTCATGGCTGTGAT −3' for shRELT-2; 5'- GCCGTACAAAGTGGCTGAA −3' for shRELT-3. shRNAs and scramble shRNA were cloned into pLKO.1 (Addgene, USA). A full-length RELT was inserted into pLVX-Puro (Clontech, USA). For lentiviral particle preparation, the 293 T cells were transfected with targeted viral plasmids, psPAX2, and pMD2G. The ESCC cell lines were then infected with the indicated lentivirus, and the RELT level was determined by qRT-PCR and western blotting.
Cell growth assay
The cell viability value was accessed by the cell counting kit-8 assay (CCK-8) (Beyotime, China). Cells (3 × 103 cells/well) were seeded into the 96-well plates overnight. After 0 h, 12 h, 24 h, 48 h, and 72 h, cells were incubated with 10 μL of the CCK-8 reagent for 2 h at 37°C. The absorbance was detected at 450 nm.
Cell apoptosis assay
Cell apoptosis was detected with the Annexin V-FITC/PI Apoptosis Kit (Beyotime, China). Cells were collected and incubated with 5 μL of annexin V-FITC and 5 μL of propidium iodide (PI) in the dark for 15 min at 25°C after re-suspending. Next, the samples were analyzed by flow cytometry (BD Biosciences).
Cell cycle assay
Cells were harvested, fixed with 75% ethanol, stored at 4°C overnight, and subsequently re-suspended in 500 μL of phosphate-buffered saline. Next, the cells were stained with PI and incubated in the dark for 30 min. The cell cycle analysis was assessed using the BD Accuri C6 instrument (BD Biosciences, USA).
Gene set enrichment analysis
Gene Set Enrichment Analysis (GSEA v2.2.2, http://www.broad.mit.edu/gsea/) was performed to identify the RELT-associated KEGG pathways (MSigDB, v4.0). Data from the TCGA were divided into high- or low-RELT expression groups, with the median as the cutoff. The default weighted enrichment method was used for enrichment analyses. Significance thresholds were determined by the permutation analysis (1000 permutations).
Xenograft experiments
Four-week-old BALB/c nude mice were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Animal experiments were approved by the Animal Experiments Ethics Committee of the Henan Provincial People’s Hospital. Mice were randomly divided into two groups: the control group (shNC) and the transfected group with shRELT-2 (shRELT), which were subcutaneously injected with a total of 5 × 106 cells. The tumor length and tumor width were recorded every 3 days and the tumor volume was calculated using the formula: volume (mm3) = 1/2 length × width [Citation2]. The mice were culled 33 days later, the tumors were excised and weighed. The dissected tumors were prepared for western blotting, qRT-PCR, and hematoxylin-eosin (HE) staining.
Statistical analysis
Quantitative data have been expressed as the mean ± standard error of the mean (SEM) from at least three independent replicates. The comparisons among groups were analyzed by the Student’s t-test or one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
RELT is highly expressed in the ESCC tissues and cell lines
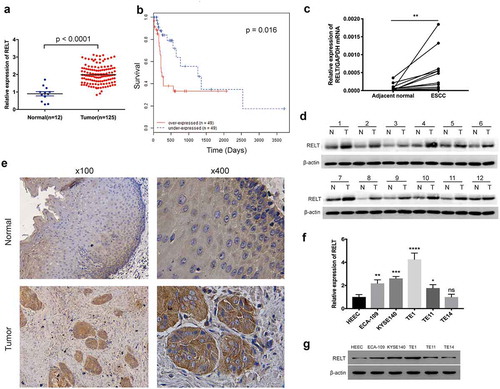
TCGA cohorts revealed that RELT expression was found to be markedly higher in the ESCC tissues (n = 125), as compared with the expression in the normal esophageal samples (n = 12) ()). The survival analysis indicated that patients with high RELT levels had poorer overall survival rates than those with a low RELT level (P = 0.016, )). The RELT levels in the twelve paired ESCC and the corresponding non-cancerous tissues were evaluated to further verify the results. The qRT-PCR and the western blot results both revealed that the RELT expression was significantly increased in the ESCC samples compared to the corresponding non-cancerous samples () and )). Indeed, images of the IHC stained-specimens showed that RELT expression was higher in the ESCC tissue than that in the normal esophageal sample ()). To investigate the function of RELT in ESCC, we determined the RELT expression in five ESCC cell lines and HEEC cell lines by qRT-PCR and western blot. The data showed that the mRNA and protein expression of RELT was higher in the ESCC cells compared to the HEEC cells () and )).
Figure 1. Expression of RELT in the ESCC tissues. (a) Expression levels of RELT in the normal esophageal tissues (n = 12) and the ESCC samples (n = 123) from the TCGA database were analyzed. (b) Survival analysis for patients with low- or high- expression levels of RELT, by the Kaplan-Meier method and the log-rank test. (c) The mRNA and (d) protein expressions of RELT in the paired ESCC and the corresponding normal tissues as determined by qRT-PCR and western blot. (e) Representative images of the IHC stained-normal esophageal and the IHC stained-ESCC tissues. (f) The mRNA and (g) protein expressions of RELT in the HEEC, ECA-109, KYSE140, TE-1, TE11, and TE-14 cell lines as detected by qRT-PCR and western blot
RELT promotes viability of the ESCC cells
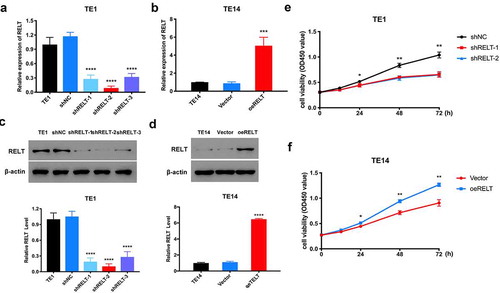
To determine the role of RELT in ESCC cells, we used shRNAs to knock-down the expression of RELT in the TE1 cells. The shRELT1 and shRELT2 with better silencing efficiencies were selected for demonstrating a loss of function in the in vitro experiments () and )). Meanwhile, the TE14 cells with relatively low RELT levels were used to generate a stable RELT-overexpressing cell line by transfection with pLVX-Puro-RELT (oeRELT) () and )). The CCK-8 results showed that the RELT knock-down significantly inhibited the proliferation of the TE1 cells ()). In contrast, cell proliferation was found to be markedly increased in the oeRELT group compared to that in the control group (vector) ()). These data suggest that RELT may play a key role in promoting ESCC cell proliferation.
Figure 2. RELT enhanced cell viability of the ESCC cells. (a) RELT knock-down efficiency in the TE1 cells after the transfection with negative control (shNC) or with shRNAs (shRELT1–shRELT3) and (b) RELT overexpression efficiency in the TE14 cells after transfection with the negative control (vector) or pLVX-Puro-RELT (oeRELT) as evaluated by qRT-PCR. (c) RELT knock-down efficiency and (d) RELT overexpression efficiency as evaluated by western blotting. (e) The viability of the TE1 cells of shNC, shRELT-1, and shRELT-2 groups as examined by the CCK-8 assay. (f) The TE14 cells viability of vector group and the oeRELT group were examined by the CCK-8 assay
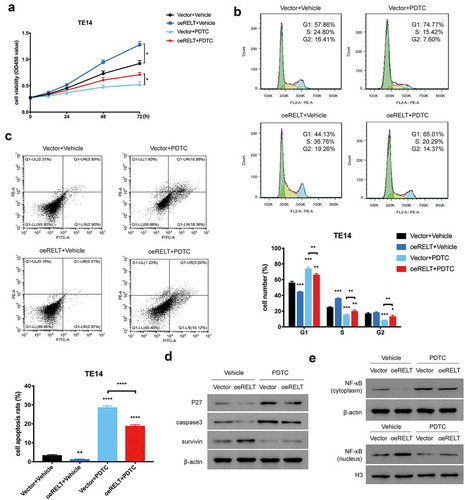
RELT accelerates cell cycle progression and inhibits apoptosis of the ESCC cells
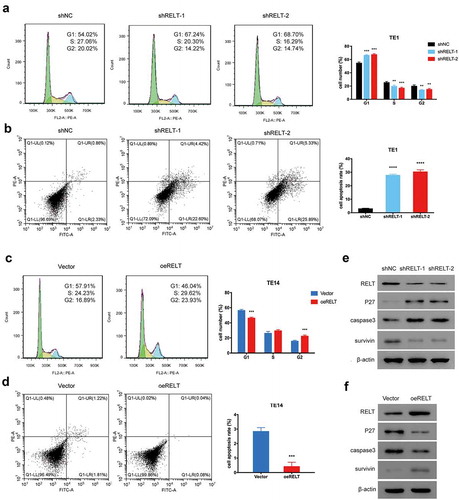
We studied the effects of RELT on cell cycle progression and cell apoptosis to further explore the cell proliferation-promoting function of RELT. The cell cycle analyses revealed that the proportion of cells in the G1 phase in the shRELT1 and shRELT2 groups was markedly increased compared with that in the shNC group, whereas the proportions of cells in the S and the G2 phases were found to be markedly decreased ()). Indeed, the flow-cytometric analyses revealed that the percentage of the apoptotic cells of the shNC, shRELT, and shRELT2 groups was 3.19%, 27.02%, and 29.22%, respectively ()). Conversely, the proportion of cells in the G1 phase of the oeRELT group was markedly decreased compared with that in the vector groups, whereas the sum of cells in the S and the G2 phases were markedly increased ()). The percentage of apoptotic cells in the vector and oeRELT groups was 3.03% and 0.12%, respectively ()). Furthermore, the protein expression of p27, which is a critical biomarker for cell cycle, was significantly increased when silenced by RELT. The protein levels of caspase 3 and survivin, which are key regulators for cell apoptosis, were significantly increased and decreased, respectively, with the RELT knock-down ()). On the contrary, overexpression of RELT led to an opposite effect ()). Altogether, these results suggest that RELT may have promoted the growth of ESCC cells by accelerating the cell cycle transition and suppressing cell apoptosis.
Figure 3. Effects of RELT on the cell cycle and cell apoptosis. (a, b) Cell cycle distribution and the effect of RELT on cell apoptosis in the T1 cells as evaluated with flow cytometry. (c, d) Cell cycle distribution and the effect of RELT on cell apoptosis in the T14 cells as determined by flow cytometry. (e, f) The levels of RELT, p27, caspase 3, and survivin as evaluated by western blotting
Overexpression of RELT induces activation of the NF-κB pathway
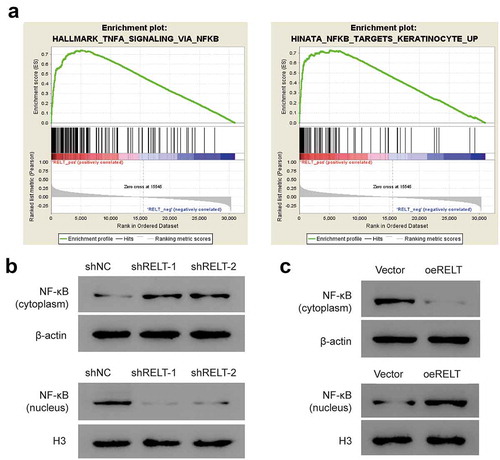
To further investigate the potential mechanism of RELT in the oncogenesis of ESCC, GSEA was performed to identify the key pathways altered in the patients with high- or low-RELT expression, based on the TCGA dataset. The GSEA analysis revealed that a high level of RELT was significantly enriched in the NF-κB pathway ()). Hence, we performed western blotting to determine whether RELT was involved in the pathogenesis of ESCC through the NF-κB pathway. The western blotting results showed that the protein level of the cytosolic NF-κB was markedly higher in the shRELT1 and shRELT2 group, whereas the nuclear NF-κB level was significantly lower ()). In contrast, the protein level of NF-κB was markedly decreased in the cytoplasm and increased in the nucleus, when RELT was overexpressed ()). These data indicated that RELT promoted the nuclear NF-κB expression and activated the NF-κB pathway in vitro.
Figure 4. RELT up-regulation activated the NF-κB signaling pathway in the ESCC cells. (a) GSEA showed positive association between high expression of RELT and the NF-κB pathway. (b, c) Western blotting was performed to detect the level of NF-κB in the cytoplasm and the nucleus under the influence of RELT. PDTC, pyrrolidine dithiocarbamate. *, P < 0.05; **, P < 0.01; ***, P < 0.001;***, P< 0.0001
The NF-κB-pathway inhibitor reduced the RELT-mediated NF-κB-pathway activation and malignancy in the ESCC cells
In accordance with our study that demonstrated that RELT activated the NF-κB pathway, it was necessary to further investigate if the NF-κB pathway was sufficient to explain the effects of an RELT overexpression on the ESCC cells. To determine this, the NF-κB inhibitor PDTC and the vehicle were used. First, the increased cell proliferation of the RELT-overexpressing T14 cells was significantly reversed when PDTC was added ()). Meanwhile, the promotion of the cell cycle process and the inhibition of cell apoptosis of the RELT-overexpressing T14 cells was also abolished by PDTC () and )). Moreover, PDTC treatment reversed the change in p27, caspase 3, survivin, the cytosolic NF-κB, and the nuclear NF-κB expressions led by the up-regulation of RELT (). Collectively, these results suggested that the NF-κB pathway may have mediated the functions of RELT in cell growth, the cell cycle, and cell apoptosis in ESCC.
Figure 5. The NF-κB-pathway inhibitor PDTC reduced the RELT-mediated NF-κB-pathway activation and malignancy in the ESCC cells. (a) Cell viability, (b) cell cycle, and (c) cell apoptosis of the vector and the oeRELT group with or without PDTC treatment. (d) Western blotting was performed to detect the level of p27, caspase 3, survivin, and NF-κB in the vector and the oeRELT group with or without PDTC treatment
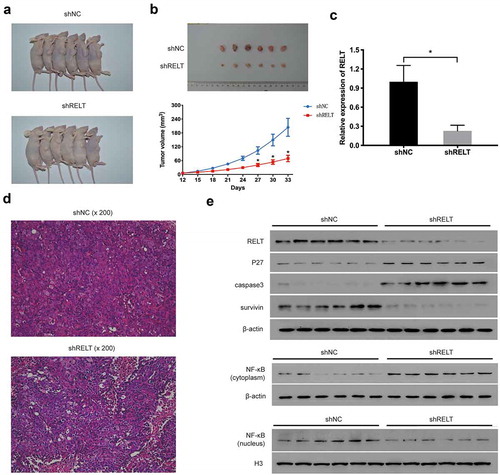
RELT knock-down suppressed ESCC tumorigenesis in vivo
In order to verify the effect of RELT on the growth of ESCC, the TE1 cells transfected with the lenti-shNC or the lenti-shRELT were subcutaneously injected into mice. All nude mice developed xenogeneic tumors at the injection site ()). The mice of the shRELT group had smaller tumors compared with that of the shNC group ()). HE staining confirmed that the malignancy of tumors was attenuated by the RELT knock-down ()). Additionally, qRT-PCR and western blot analyses indicated that the levels of RELT, survivin, and the nuclear NF-κB were significantly reduced in the tumor tissues of the shRELT group ()). Conversely, the levels of p27, caspase3, and the cytosolic NF-κB were markedly up-regulated ()). Altogether, these data revealed that the RELT shRNA suppressed the tumorigenesis of ESCC in vivo.
Figure 6. RELT knock-down inhibits tumor growth of ESCC in the xenograft tumor model. (a) Representative images of tumors after 33 days of implantation. (b) Tumor volume significantly decreased after 33 days of implantation in the shRELT group. (c) qRT-PCR performed to evaluate the levels of p27, caspase 3, survivin, the cytosolic NF-κB, and the nuclear NF-κB in two groups of mice. (d) Representative images of HE staining of xenograft tumors. (e) Western blotting was performed to evaluate the levels of p27, caspase 3, survivin, the cytosolic NF-κB, and the nuclear NF-κB in two groups of mice
Discussion
Many researches have proven that members of the TNFR superfamily play important roles in various cancers, including ESCC, and biologics targeting the TNFR have been studied for the treatment of cancer [Citation9,Citation16]. However, RELT, a member of the TNFR superfamily, has rarely been studied for its function in the pathogenesis of various cancers. To our knowledge, this study is the first to investigate the function of RELT in the oncogenesis of ESCC.
In this study, we determined the level of RELT expression in the ESCC tissues via the TCGA database and found that RELT was overexpressed in the ESCC tissues. Overall, survival analysis indicated that a higher RELT level was accompanied with a poorer prognosis in the ESCC patients. The paired ESCC and the corresponding normal sample analysis further validated that the mRNA and protein levels of RELT were increased in ESCC. We then explored the effect of RELT on ESCC cell growth, cell cycle, and cell apoptosis. The results revealed that the overexpression of RELT led to the acceleration of the growth and cell cycle process, and the suppression of cell apoptosis in the ESCC cells, suggesting that RELT may play a role as an oncogene in ESCC. Notably, we further explored the potential mechanism of the involvement of RELT in the tumorigenesis of ESCC and suggest that RELT may function via activation of the NF-κB pathway. Inhibiting the NF-κB pathway with PDTC reversed the effects of RELT on cell viability, cell cycle, and cell apoptosis; conversely, it also reversed the levels of p27, caspase 3, survivin, the cytosolic NF-κB, and the nuclear NF-κB commonly mediated by an RELT overexpression. Collectively, these outcomes indicated that RELT promoted the development of ESCC through the NF-κB pathway.
The NF-κB pathway plays a critical role in the development of various human cancers. Studies have demonstrated that activated NF-κB induces the proliferation, angiogenesis, invasion, and metastasis of cancer cells by up-regulating the expression of pro-inflammatory cytokines, chemokines, and growth factors [Citation17–21]. The TNFR superfamily is closely associated with the NF-κB pathway, and many members have been proven to activate the NF-κB pathway [Citation22]. However, the effect of RELT on the NF-κB pathway remains controversial. As we have mentioned above, when RELT was first identified by John KC et al [Citation11], it demonstrated the ability to activate NF-κB, but a previous study by Tara CP et al [Citation10] reported that up-regulation of RELT in the 293 cells resulted in the activation of p38 and JNK, but not NF-κB. In our study, we demonstrated that overexpression of RELT led to the activation of NF-κB in the ESCC cells by up-regulating the expression of the nuclear NF-κB, which was found to be consistent with the results reported by John KC et al. However, further investigation will be needed to explore the functions of RELT in the NF-κB pathway.
In conclusion, RELT was found to be expressed at higher levels in the ESCC tissues than that in the normal esophageal tissues and was correlated with the prognosis of ESCC patients. Besides, RELT demonstrated the activity of an oncogene in ESCC by promoting cell proliferation, accelerating the cell cycle process, and inhibiting cell apoptosis. Moreover, we also provided evidence that RELT was involved in the tumorigenesis of ESCC through the NF-κB pathway. These outcomes highlight the biological function of RELT and suggest that RELT may be a vital target for the prognosis and therapy of ESCC. However, extensive studies should be conducted to explore and establish the role of RELT in ESCC.
Acknowledgments
This study was supported by “23456 Talent Project” of Henan Provincial People’s Hospital & Medical Service Capacity Improvement Project in Henan Provincial Medical Institutions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412.
- Ohashi S, Miyamoto S, Kikuchi O, et al. Recent advances from basic and clinical studies of Esophageal Squamous cell carcinoma. Gastroenterology. 2015;149(7):1700–1715.
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756.
- Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27(1):19–26.
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501.
- Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115(1):1–20.
- Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–665.
- Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12(2):147–168.
- Polek TC, Talpaz M, Spivak-Kroizman T. The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochem Biophys Res Commun. 2006;343(1):125–134.
- Sica GL, Zhu G, Tamada K, et al. RELT, a new member of the tumor necrosis factor receptor superfamily, is selectively expressed in hematopoietic tissues and activates transcription factor NF-kappaB. Blood. 2001;97(9):2702–2707.
- Cusick JK, Xu LG, Bin LH, et al. Identification of RELT homologues that associate with RELT and are phosphorylated by OSR1. Biochem Biophys Res Commun. 2006;340(2):535–543.
- Zhong L, Ge K, Zu JC, et al. Autoantibodies as potential biomarkers for breast cancer. Breast Cancer Res. 2008;10(3):R40.
- Johansson J, Tabor V, Wikell A, et al. TGF-beta1-Induced Epithelial-Mesenchymal Transition Promotes Monocyte/Macrophage properties in breast cancer cells. Front Oncol. 2015;5:3.
- Sun Y, Pan J, Zhang N, et al. Knockdown of long non-coding RNA H19 inhibits multiple myeloma cell growth via NF-kappaB pathway. Sci Rep. 2017;7(1):18079.
- Liu Z, Ma C, Ma G, et al. High expression of tumor necrosis factor receptor 2 in tissue is associated with progression and prognosis of esophageal squamous cell carcinoma. Hum Pathol. 2018;80:179–185.
- Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005;26(6):318–325.
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18(1):19–26.
- DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400.
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–723.
- Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer. 2012;12(2):121–132.
- Wu Z, Bruggeman LA. Assaying NF-kappaB activation and signaling from TNF receptors. Methods Mol Biol. 2014;1155:1–14.
