ABSTRACT
The metastasis of tumor cells is a challenge for the clinical treatment of glioma. Epithelial-mesenchymal transition (EMT) contributes to glioma cell invasiveness. Our previous study confirmed that the expression of miRNA-451, which inhibits the PI3K/Akt signaling pathway by directly targeting CAB39 and plays a repressive role in glioma, is downregulated in glioma. However, the specific mechanism of miRNA-451 regulation in glioma is unclear. In this study, we investigated whether miRNA-451 blocks the processes of EMT and metastasis in glioma cells in vivo and in vitro. By targeting CAB39, miRNA-451 likely triggers the PI3K/Akt/Snail signaling pathway to reduce glioma proliferation, invasion, migration and EMT. We used Western blotting experiments to demonstrate that overexpression of miRNA-451 significantly reduced p-AKT(Ser473), N-cadherin, Vimentin, Twist, Snail and Cyclin D1 expression and increased E-cadherin expression. We demonstrated that overexpression of miR-451 suppressed glioma cell proliferation, invasion, migration and EMT by MTT and colony formation assays, Transwell assays, wound healing assays and animal experiments. Taken together, these results suggest that miRNA-451 can reduce EMT and metastasis in glioma cells through the suppression of the PI3K/Akt/Snail signaling pathway by targeting CAB39 in vitro and in vivo. miR-451 may be a new target for glioma treatment.
1. Introduction
Glioblastoma multiforme (GBM) is one of the most common types of primary brain tumors in adults, but despite multimodal treatment that combines surgery, radiation, and chemotherapy, the median survival time of GBM patients remains poor[Citation1]. Tumor cell invasiveness is a critical challenge in the clinical management of glioma patients[Citation2]. Additionally, the complex molecular features of multiple genetic mutations that occur during glioma formation and progression make the identification of critical targets that are involved in essential pathophysiological processes in glioma highly challenging. Clinically, it is urgent to further understand the molecular basis by which glioma cells may be identified and to identify new targets for the development of approaches for malignant glioma treatment [Citation2,Citation3].
In recent decades, an increasing amount of evidence has shown that microRNAs (miRNAs), which are approximately 22-nucleotide short noncoding RNAs, play critical regulatory roles in a wide range of biological and pathological processes and act as oncogenes or tumor suppressor genes in glioma, depending on their target genes [Citation4,Citation5]. miRNAs have been shown to play important roles in various cellular processes, including proliferation, differentiation and apoptosis. [Citation6,Citation7]
The molecular mechanism involved in metastasis has been shown to dysregulate the signaling pathways that control epithelial-mesenchymal transition (EMT). EMT is closely related to the transformation and infiltration of tumor cells. Substantial research on the relationship between miRNAs and tumor development and metastasis is focused on the expression of EMT-related proteins. Transcription factors (TFs), such as Snail, Zeb, and Twist, are crucial for EMT [Citation8,Citation9]. Therefore, it is important to block metastasis by inhibiting EMT in the treatment of glioma [Citation8–10]. PI3K signaling plays an important role in cell cycle progression, motility, metabolism, and survival. Aberrations of PI3K signaling induce the pathogenesis of numerous cancers by altering cell growth and apoptosis[Citation11]. A previous study confirmed that miRNA-451 can inhibit cell proliferation and induce cell apoptosis through the PI3K/Akt signaling pathway by directly targeting CAB39 in glioma [Citation11–13].
Given that the PI3K/Akt and Snail-mediated signaling pathways activate the processes of EMT and tumor metastasis [Citation14,Citation15], we hypothesized that overexpression of miR-451 can reduce the EMT process and metastasis in glioma through the suppression of the PI3K/Akt/Snail signaling pathway. Therefore, based upon the relevant literature and preliminary experiments, we evaluated whether overexpression of miR-451 in glioma cells resulted in lower expression of CAB39 and reduced EMT and metastasis through the suppression of the PI3K/Akt/Snail signaling pathway in vitro and in vivo.
2. Materials and methods
2.1. Cell culture
The human glioblastoma cell lines U251 and U87 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science (Shanghai, China). All the cells were cultured in high-glucose DMEM (Dulbecco’s modified Eagle’s medium Dulbecco) (Corning, New York, USA) supplemented with 10% FBS (fetal bovine serum) (Thermo Fisher Scientific, Massachusetts, USA) at 37°C in 5% CO2.
2.2. Lentiviral infection and cell transfection
Lentiviruses containing hsa-miRNA-negative control (miRNA-NC) or hsa-miRNA-451 (miRNA-451) were obtained from GenePharma (Shanghai, China). The coding sequences were miRNA-NC, 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ and miRNA-451, 5ʹAAACCGUUACCAUUACUGAGUU-3ʹ. For transfection, the human glioma cell lines U251 and U87 were grown to 50–80% confluence and were transfected with viral suspensions under normal conditions. Stable cell lines were established with lentiviruses and were selected with puromycin according to the manufacturer’s instructions. The cells were harvested 48 h after transfection, and the RNA and protein were extracted. Three groups were used throughout our experiments: the human glioma cell group without transfection (Control), the lentivirus-miRNA-negative control group (LV-miRNA-NC) and the lentivirus-hsa-miR-451-overexpression group (LV-miRNA-451).
2.3. RNA extraction and real-time PCR
Total RNA was extracted from glioma cells using TRIzol reagent (Invitrogen) after transfection for 48 h. RNA (1 μg) was reverse-transcribed using a Promega kit (Promega, USA) and converted to cDNA. PCR was performed using Taq hot start polymerase (Promega, USA) in a Bio-Rad instrument; U6 was used as a control to compare the relative RNA expression between samples. The oligonucleotide primers (GenePharma, Shanghai, China) used for quantitative PCR were as follows: miRNA-451 forward, 5ʹ-GCGGCGCAAAGAATTCTCCT-3ʹ and reverse, 5ʹ-GTGCAGGGTCCGAGGT-ʹ; U6 forward, 5ʹ-ATTGGAACGATACAGAGAAGATT-3ʹ and reverse, 5ʹ-GGAACGCTTCACGAATTTG-3ʹ. The PCR cycling parameters were 95°C for 3 min, 40 cycles of amplification (95°C for 12 s, 62°C for 1 min, and 72°C for 1 min), and extension (72°C for 2 min). Quantitative PCR of all the mRNAs was performed according to the manufacturer’s protocol (Promega, USA).
2.4. MTT and colony formation assays
Glioma cells in the logarithmic phase of growth were seeded into 96-well plates at 2 × 103 cells per well. Five wells from each group were tested on days 1, 2, 3, 4, 5, and 6 after transfection by using the MTT assay. The optical density (OD) was measured at 570 nm in an automated microplate reader, and the cell proliferation of each group was analyzed using GraphPad software. For the cell colony formation assay, cells were seeded in a 6-well plate (0.5 × 103 cells per well) and cultured for 2 weeks. Then, the cells were fixed in 4% phosphate-buffered paraformaldehyde (Solarbio, China) and stained dyed with 0.1% crystal violet (Solarbio, China). ImageJ software (ImageJ, NIH) was used to determine the colony formation rate.
2.5. Transwell invasion and wound healing assays
The cell invasion assay was conducted using 24-well BD Matrigel invasion chambers (BD, USA) according to the manufacturer’s instructions. Glioma cells (5 × 104) were seeded in the upper well of the invasion chamber in DMEM without serum. The lower well of the chamber contained DMEM supplemented with 10% FBS to stimulate cell invasion. After incubation for 24 h, the glioma cells remaining in the top well were removed with a cotton swab, and the invading cells in the bottom well were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Five random fields (magnification: 100×) in each well in the different treatment groups were counted under a microscope, and the results were analyzed. For the wound healing assay, glioma cells were seeded in 6-well plates at 3 × 105 cells/well and cultured for 48 h after transfection. The glioma cells were grown to 70~90% confluence, and the cell layers from each group were scratched using a 200-μL tip to form wound gaps. The cells were incubated in DMEM containing 5% FBS after washing three times with PBS. An inverted microscope was used to observe the migration of the cells at the wound site, and images of the wound site were taken at 0 and 24 h. ImageJ (version 1.45 software) was used to analyze the percentage of wound closure.
2.6. Western blotting assay
RIPA lysis buffer (Solarbio, R0010, China) containing protease inhibitors (Solarbio, A8260, China) was used to isolate proteins from U251 and U87 glioma cells, and the protein concentrations were determined by a BCA kit (Solarbio, PC0020, China). Then, the denatured proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, USA). The membranes were blocked in TBST with 5% skim milk for 1 h and with mouse antibodies against GAPDH, CAB39, AKT, p-AKT(Ser-473), Twist, Snail, Cyclin D1, N-cadherin, E-cadherin and Vimentin (1:1000; CST, USA) at 4°C overnight. The next day, the membranes were washed with phosphate-buffered saline/Tween three times for 5 min each and incubated with goat anti-mouse HRP-conjugated secondary antibodies (1:2000; CST, USA) for 1 h. The protein blots were developed using the ECL Protein Detection kit (Pierce, USA) and analyzed in the gel imaging analyzer (Syngene, UK).
2.7. Animal experiments
Four-week-old BALB/c-A nude mice were purchased from the Animal Center of the Cancer Institute of the Chinese Academy of Medical Sciences. The mice were randomly assigned into three groups and intracranially implanted with 5 × 105 U87 cells (U87 cells pretreated with lentivirus carrying miRNA-451, U87 cells pretreated with lentivirus carrying negative control sequences or U87 cells without transfection) using a stereotactic instrument. Intracranial tumor growth was detected by using bioluminescence imaging. After the mice were anesthetized, they were intraperitoneally injected with 50 mg/mL D-luciferin (Promega, Wisconsin). An IVIS imaging system (Caliper Life Sciences) was used for imaging for 10~120 s. During the experimental period, the overall survival of the mice was monitored. All the protocols involving animals were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee.
2.8. Immunohistochemistry
Paraffin-embedded sections (5 μm) of brain specimens were stained. Brain tissue sections embedded in paraffin blocks were deparaffinized in xylene, and the antigens were retrieved in citrate buffer for 20 min. After blocking, the samples were incubated with primary antibodies (1:100 dilution) against CAB39, p-AKT(Ser-473), E-cadherin, N-cadherin, Vimentin, Twist, Snail and Cyclin D1 overnight at 4°C. The samples were brought to room temperature for 1 h, washed with PBS three times, and incubated with a biotinylated secondary antibody (1:200 dilution) at room temperature for 2 h. After washing with PBS, the sections were incubated with ABC-peroxidase reagent for 40 min. The sections were stained with diaminobenzidine (DAB) (Peroxidase Envision kit; Dako Corp, Carpinteria, CA, USA) for 5 min, washed in water, and counterstained with hematoxylin. The expression of CAB39, p-AKT(Ser473), E-cadherin, N-cadherin, Vimentin, Twist, Snail and Cyclin D1 in the tumor tissue samples from the three groups was compared.
2.10. Statistical analyses
Student’s t test was performed to compare differences among the different groups with respect to parametric variables. Overall survival was evaluated using the Kaplan-Meier method and log rank test. All the data are presented as the mean ± standard error. P value <0.05 was regarded as significant.
3. Results
3.1. miRNA-451 expression was upregulated after transfection and Overexpression of miR-451 reduces the proliferation, invasion, and migration abilities of glioma cells
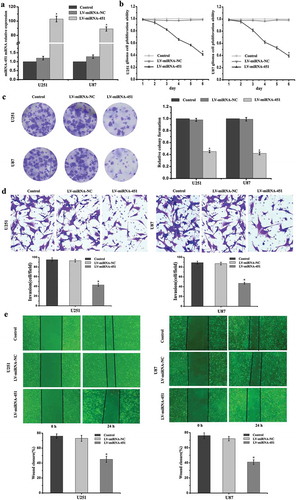
To investigate miRNA-451 mRNA expression after transfection, we introduced lentiviruses expressing miRNA-451 or miRNA-NC into human U251 and U87 glioma cells. We performed RT-PCR to verify the transfection efficacy of the lentivirus expressing miRNA-451 in glioma cells. Compared with the Control and LV-miRNA-NC groups, we found that miRNA-451 expression was significantly increased in the U251 and U87 glioma cells in the LV-miRNA-451 group ()). To confirm whether overexpression of miRNA-451 reduces the proliferation, invasion, and migration abilities of U251 and U87 glioma cells, we performed MTT and colony formation assays, Transwell invasion assays and wound healing assays. The MTT and colony formation assays showed that cell viability and proliferation were reduced by miRNA-451 in the LV-miRNA-451 group (–c)). The Transwell invasion and wound healing assays showed that cell invasion and migration were reduced by miRNA-451 in the LV-miRNA-451 group (–e)).
Figure 1. The transfection efficiency, viability, proliferation, invasion and migration abilities of glioma cells were detected after treatment with LV-miRNA-451. (a) Compared with the that in the Control and LV-miRNA-NC groups, miRNA-451 expression was significantly increased in the U251 and U87 glioma cells in the LV-miRNA-451 group, as shown by RT-PCR analysis (*P < 0.05). (b-c) MTT and colony formation assays showed that cell viability and proliferation ability were reduced by miRNA-451 in the LV-miRNA-451 group. (*P < 0.05). (d-e) Cell Transwell invasion and wound healing assays showed that cell invasion and migration abilities were suppressed by miRNA-451 in the LV-miRNA-451 group compared with the Control and LV-miRNA-NC groups. (*P < 0.05)
3.2. Overexpression of miR-451 regulates the expression of EMT-related genes in glioma cells
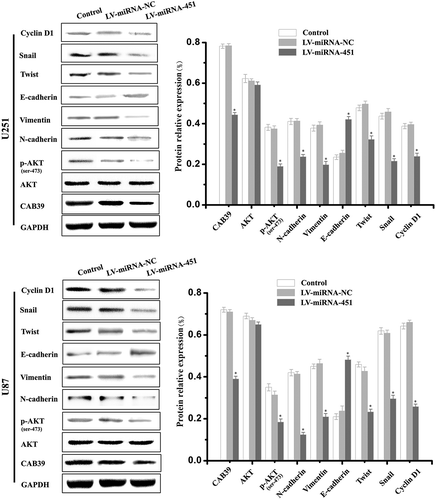
To confirm whether overexpression of miRNA-451 regulates EMT-related genes expression, we performed Western blot experiments. We examined the expression of proteins related to the PI3K/Akt and Snail-mediated signaling pathways by Western blot experiments. The Western blot experiments results showed that the expression of proteins related to the biological behavior of malignant glioma cells, namely, CAB39, p-AKT(Ser-473), N-cadherin, Vimentin, Twist, Snail and Cyclin D1, was decreased in the LV-miRNA-451 group, and the protein expression of E-cadherin was increased in the LV-miRNA-451 group. However, there is no statistically significant difference in the relative expression level of AKT in the three experiments groups (). These results suggested that overexpression of miR-451 can reduce the EMT process and metastasis of glioma cells through the suppression of the PI3K/Akt/Snail signaling pathway.
Figure 2. Western blotting analyses of the protein expression levels of EMT-related genes and proteins related to the PI3K/Akt pathway in glioma cells treated with LV-miRNA-451. Compared with the Control and LV-miRNA-NC groups, Western blotting experiments showed that the protein expression of CAB39, p-AKT(Ser-473), N-cadherin, Vimentin, Twist, Snail and Cyclin D1 was decreased in the LV-miRNA-451 group, and the protein expression of E-cadherin was increased in the LV-miRNA-451 group (*P < 0.05). There was no statistically significant difference in the relative expression of AKT among the three experimental groups (P > 0.05)
3.3. miRNA-451 reduces glioma cell EMT and metastasis in xenograft models
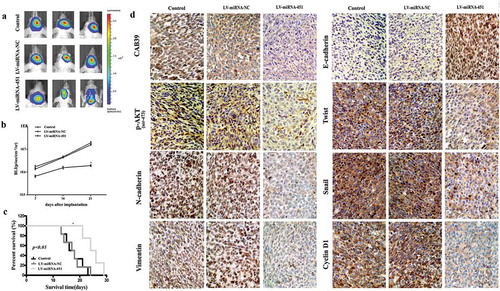
To evaluate the effects of miRNA-451 reduction on the EMT process and metastasis through the suppression of the PI3K/Akt/Snail signaling pathway by targeting CAB39 in vivo, we established intracranial xenograft tumors in nude mice. U87 cells were pretreated with lentiviruses carrying a luciferase reporter. Compared with the Control and LV-miRNA-NC groups, the LV-miRNA-451 group exhibited significantly decreased tumor growth ()). To analyze the survival of the three groups, we generated Kaplan-Meier survival curves and found that the survival of the BALB/c-A nude mice in the LV-miRNA-451 group was significantly prolonged compared with that of the mice in the Control and LV-miRNA-NC groups (–c)). Immunohistochemistry analysis showed that CAB39, p-AKT(Ser-473), N-cadherin, Vimentin, Twist, Snail and Cyclin D1 expression in the sections from the LV-miRNA-451 group were significantly reduced compared with that in the sections from the Control and LV-miRNA-NC groups, and E-cadherin expression in the sections from the LV-miRNA-451 group was significantly increased compared with that in the sections from the Control and LV-miRNA-NC groups ()). Therefore, miRNA-451 significantly reduced the protein expression of CAB39, p-AKT(Ser-473), N-cadherin, Vimentin, Twist, Snail, and Cyclin D1, increased the protein expression of E-cadherin, reduced the glioma EMT process and metastasis and clearly prolonged the survival of nude mice.
Figure 3. Antitumor effect of miRNA-451 on the growth of human U87 glioma cells in xenograft models. (a) Bioluminescence images from Control, LV-miRNA-NC and LV-miRNA-451-treated animals at 21 days after tumor implantation. (b) Tumor growth curves were evaluated at 7, 14, and 21 days after tumor implantation. (c) The survival time was determined in the three groups. (d) After sacrifice, mouse brain tissues were harvested, embedded, and cut into paraffin sections for immunohistochemistry analysis (200x). The results showed that CAB39, p-AKT(Ser-473), N-cadherin, Vimentin, Twist, Snail and Cyclin D1 expression in the sections of the LV-miRNA-451 group was significantly reduced compared with that in the sections of Control and LV-miRNA-NC groups, and E-cadherin expression in the sections of the LV-miRNA-451 group was significantly increased compared with that in the sections of the Control and LV-miRNA-NC groups
4. Discussion
GBM is the most common form of primary malignant brain cancer, and it is one of the most aggressive and fatal human cancers [Citation1,Citation2]. The poor prognosis and mortality associated with this disease are mainly due to the high invasiveness and mobility of glioma cells, which can diffuse and spread extensively to the surrounding brain tissue[Citation2]. To date, the molecular mechanisms underlying glioma invasion and mobility have not been fully understood[Citation2]. One important factor that contributes to the invasiveness of high-grade gliomas is the EMT of glioma cells. EMT is a complex cellular process reflecting a high level of phenotypic plasticity, and this process is characterized by the loss of epithelial markers, namely, E-cadherin, and the enhanced expression of mesenchymal cell markers, including N-cadherin, Vimentin, Snail and Twist. EMT has been recognized as a major contributor to cancer cell migration, invasion, and metastasis [Citation8–10,Citation14,Citation15]. Understanding the mechanisms that drive EMT is therefore important to identify new targets for the prevention of the diffuse infiltration of tumor cells in glioma.
miRNAs are short noncoding RNAs of approximately 22 nucleotides that act as both tumor suppressors and oncogenes by negatively regulating their mRNA targets through degradation or translational repression [Citation5,Citation9,Citation12]. Recently, many studies have suggested that miR-451 expression is frequently downregulated in glioma, and its downregulation has been correlated with poor prognosis in glioma patients [Citation11–13,Citation16]. We conclude that miR-451 represses glioma in vitro and in vivo, likely by directly targeting CAB39 and by indirectly inhibiting the PI3K/Akt pathway[Citation11]. The PI3K/Akt signaling pathway plays an important role in regulating cell proliferation and maintaining the biological characteristics of malignant cells [Citation6,Citation11]. Other studies have shown that overexpression of miR-451 inhibits cell proliferation, migration, and invasion and induces the apoptosis of bladder cancer cells. MiR-451 could maintain the epithelial phenotype of bladder tumor cells and inhibit the EMT process, thereby reducing the invasion and migration of tumor cells[Citation17]. Previous studies reported that the PI3K/Akt signaling pathway can activate the process of EMT and induce tumor metastasis [Citation14,Citation18]. Activated AKT will then phosphorylate a series of substrates, thereby affecting a variety of cellular and physiological processes, including the cell cycle, cellular growth, differentiation, survival, angiogenesis, metabolism and migration [Citation8,Citation18]. These changes may induce the EMT process. The PI3K/Akt- and Snail-mediated signaling pathways mediate the process of EMT and have attracted widespread attention as potential targets for the prevention and treatment of metastatic tumors [Citation8,Citation18,Citation19]. The EMT process is regulated by a variety of factors within tumor cells. After stimulation by these inducible factors, a series of chain reactions occur, which results in enhanced cellular mobility and invasion [Citation20–22]. Thus, suppression of the EMT process is crucial to block metastasis in cancer therapies. However, it is unclear whether miR-451 can affect the glioma EMT process and metastasis through the suppression of the PI3K/Akt- and Snail-mediated signaling pathways by targeting CAB39.
In the present study, to verify the transfection efficacy of the lentivirus expressing miRNA-451 in glioma cells, we performed RT-PCR and found that miRNA-451 expression was significantly increased in the U251 and U87 glioma cells in the LV-miRNA-451 group. The MTT and colony formation assays showed that cell viability and proliferation were reduced by miRNA-451 in the LV-miRNA-451 group. Transwell invasion and wound healing assays showed that cell invasion and migration were suppressed by miRNA-451 in the LV-miRNA-451 group. The Western blotting experiment results showed that the expression of proteins related to the biological behavior of malignant glioma cells, namely, CAB39, p-AKT(Ser-473), N-cadherin, Vimentin, Twist, Snail and Cyclin D1, was decreased in the LV-miRNA-451 group, and the protein expression of E-cadherin was increased in the LV-miRNA-451 group. Compared with the control and LV-miRNA-NC groups, the LV-miRNA-451 group exhibited significantly decreased tumor growth. The survival of the BALB/c-A nude mice in the LV-miRNA-451 group was significantly prolonged, as shown by Kaplan-Meier survival curves. Immunohistochemical analysis showed that the protein expression of CAB39, p-AKT(Ser-473), N-cadherin, Vimentin, Twist, Snail and Cyclin D1 was decreased in the LV-miRNA-451 group, and the protein expression of E-cadherin was increased the in LV-miRNA-451 group. These results, both in vitro and in vivo, suggest that overexpression of miR-451 can reduce the glioma EMT process and metastasis through the suppression of the PI3K/Akt/Snail signaling pathway by targeting CAB39.
In conclusion, we demonstrate that miR-451 can reduce the EMT process and metastasis of glioma through the suppression of the PI3K/Akt/Snail signaling pathway in vitro and in vivo. miR-451 acts as a tumor suppressor gene that can suppress glioma growth, metastasis, and EMT by directly targeting CAB39. Our results suggest that overexpression of miR-451 may be a useful therapeutic strategy for treating glioma in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hersh DS, Harder BG, Roos A, et al. TNF receptor family member Fn14 is highly expressed in recurrent glioblastoma and in GBM patient-derived xenografts with acquired temozolomide resistance. Neuro Oncol. 2018;20:1321–1330.
- Delgado-Martín B, Medina MÁ. Advances in the knowledge of the molecular biology of glioblastoma and its impact in patient diagnosis, stratification, and treatment. Adv Sci (Weinh). 2020;7:1902971.
- Le Rhun E, Preusser M, Roth P, et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896.
- Lucero R, Zappulli V, Sammarco A, et al. Glioma-derived miRNA-containing extracellular vesicles induce angiogenesis by reprogramming brain endothelial cells. Cell Rep. 2020;30:2065–2074.e4.
- Jiang XH, Liu YY. LINC00467 promotes proliferation and invasion in glioma via interacting with miRNA-485-5p. Eur Rev Med Pharmacol Sci. 2020;24:766–772.
- Jiang L, Wang C, Lei F, et al. miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway. Oncotarget. 2015;6:8286–8299.
- Tan Y, Huang N, Zhang X, et al. KIAA0247 suppresses the proliferation, angiogenesis and promote apoptosis of human glioma through inactivation of the AKT and Stat3 signaling pathway. Oncotarget. 2016;7:87100–87113.
- Cai Z, Cao Y, Luo Y, et al. Signalling mechanism(s) of epithelial-mesenchymal transition and cancer stem cells in tumor therapeutic resistance. Clin Chim Acta. 2018;483:156–163.
- Pang H, Zheng Y, Zhao Y, et al. miR-590-3p suppresses cancer cell migration, invasion and epithelial-mesenchymal transition in glioblastoma multiforme by targeting ZEB1 and ZEB2. Biochem Biophys Res Commun. 2015;468:739–745.
- Zhang X, Wei C, Li J, et al. MicroRNA-194 represses glioma cell epithelial-to-mesenchymal transition by targeting Bmi1. Oncol Rep. 2017;37:1593–1600.
- Tian Y, Nan Y, Han L, et al. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma. Int J Oncol. 2012;40:1105–1112.
- Nan Y, Han L, Zhang A, et al. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21.
- Nan Y, Guo H, Guo L, et al. MiRNA-451 inhibits glioma cell proliferation and invasion through the mTOR/HIF-1α/VEGF signaling pathway by targeting CAB39. Hum Gene Ther Clin Dev. 2018;29:156–166.
- Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317–324.
- Liu L, Hu J, Yu T, et al. miR-27b-3p/MARCH7 regulates invasion and metastasis of endometrial cancer cells through Snail-mediated pathway. Acta Biochim Biophys Sin (Shanghai). 2019;51:492–500.
- Zhao K, Wang L, Li T, et al. The role of miR-451 in the switching between proliferation and migration in malignant glioma cells: AMPK signaling, mTOR modulation and Rac1 activation required. Int J Oncol. 2017;50:1989–1999.
- Zeng T, Peng L, Chao C, et al. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int J Clin Exp Pathol. 2014;7:7653–7662.
- Liu T, Wu X, Li Y, et al. RBFOX3 regulates the chemosensitivity of cancer cells to 5-fluorouracil via the PI3K/AKT, EMT and cytochrome-C/caspase pathways. Cell Physiol Biochem. 2018;46:1365–1380.
- Li C, Ao H, Chen G, et al. The Interaction of CDH20 with β-Catenin inhibits cervical cancer cell migration and invasion via TGF-β/Smad/SNAIL mediated EMT. Front Oncol. 2020;9:1481.
- Zhu H, Chen Z, Shen L, et al. Long noncoding RNA LINC-PINT suppresses cell proliferation, invasion, and EMT by blocking Wnt/β-Catenin signaling in glioblastoma. Front Pharmacol. 2021;11:586653.
- Zhang Q, Yang L, Guan G, et al. LOXL2 upregulation in gliomas drives tumorigenicity by activating autophagy to promote TMZ resistance and trigger EMT. Front Oncol. 2020;10:569584.
- Srivastava C, Irshad K, Dikshit B, et al. FAT1 modulates EMT and stemness genes expression in hypoxic glioblastoma. Int J Cancer. 2018;142:805–812.
