ABSTRACT
Isoflurane has been studied in ischemia-reperfusion injury, while the regulatory mechanism by which isoflurane regulates microRNA(miR)-9-3p in hepatic ischemia/reperfusion injury (HIRI) via targeting fibronectin type III domain containing 3B (FNDC3B) remains seldom investigated. This study aims to determine the role of miR-9-3p in HIRI progression under the treatment of isoflurane. Rat HIRI models were established and treated with isoflurane. MiR-9-3p was altered to assess its role in inflammation, oxidative stress, transaminases, pathology, and hepatocyte apoptosis in HIRI rat liver tissues. Expression of miR-9-3p and FNDC3B in rat liver tissues was determined, and the targeting relationship between miR-9-3p and FNDC3B was confirmed using bioinformatic prediction and dual luciferase reporter gene assay. MiR-9-3p was downregulated, whereas FNDC3B was upregulated in HIRI rat liver tissues. Isoflurane treatment upregulated miR-9-3p and attenuated pathological changes, inflammation, oxidative stress, transaminases, and hepatocyte apoptosis in HIRI rat liver tissues. MiR-9-3p upregulation further strengthened the effect of isoflurane on HIRI, while miR-9-3p downregulation suppressed the therapeutic role of isoflurane. FNDC3B was confirmed as a target gene of miR-9-3p. Isoflurane upregulates miR-9-3p to protect rats from HIRI by inhibiting FNDC3VB. Our research may provide novel targets for HIRI treatment.
Introduction
Hepatic ischemia-reperfusion injury (HIRI) is a complex process occurring during partial hepatectomy, liver transplant, and other medical conditions. It is considered that the essence of HIRI involves the neurogenic inflammatory response resulted from the reperfusion after organ ischemia [Citation1]. This injury occurs when the blood supply restored to ischemic liver instead of functional recovery ensuing, tissue dysfunction and structural damage occur [Citation2]. The mechanisms of HIRI include mitochondrial dysfunction, increased lipid peroxidation, toxic radical release, endothelial cell hypersensitivity, and increased hepatocyte apoptosis [Citation3]. Although multiple researches have been conducted to reveal the potential mechanisms of HIRI, efficient therapies are still unavailable [Citation4]. Thus, it is urgent to investigate novel targets to attenuate the detrimental physiological effects of HIRI.
Isoflurane is one of the inhalation anesthetics broadly used in clinical practice and exerts a neuroprotective effect [Citation5]. It has been revealed that isoflurane anesthesia protected rats against HIRI [Citation6], and emulsified isoflurane preconditioning reduced lung injury triggered by HIRI in rats [Citation7]. Therefore, the interest in isoflurane as an effective therapeutic strategy has been increasing. MicroRNAs (miRNAs) are short non-coding RNAs that post-transcriptionally modulate gene expression by binding to mRNAs [Citation8]. As one of the miRNAs, miR-9-3p has been identified as a functional biomarker in hepatocellular carcinoma (HCC) [Citation9], and it has also been confirmed that sevoflurane-induced miR-9-5p upregulation could ameliorate HIRI [Citation10]. Nevertheless, the role of miR-9-3p, especially isoflurane-regulated miR-9-3p in HIRI progression, remains largely unknown. Fibronectin type III (FNIII) domain containing 3B (FNDC3B), also known as factor for adipocyte differentiation 104, belongs to the fibronectin family and includes nine FNIII domains and a transmembrane domain [Citation11]. FNDC3B has been identified to participate in the metastasis of hepatocarcinoma [Citation12]. Moreover, a study has demonstrated that FNDC3B was able to promote the migration and invasion of HCC cells [Citation13]. However, the impact of FNDC3B on HIRI, as well as the binding relationship between miR-9-3p and FNDC3B, has been seldom studied. Therefore, the molecular mechanisms remain to be further explored.
We conducted this study to verify the role of isoflurane treatment regulating miR-9-3p in HIRI in a rat model with the involvement of FNDC3B. We hypothesized that isoflurane may upregulate miR-9-3p to protect the rats against HIRI via inhibiting FNDC3B.
Materials and methods
Ethics statement
Animal experiments were strictly in accordance with the Guide to the Management and Use of Laboratory Animals issued by the National Institutes of Health. The protocol of animal experiments was approved by the Institutional Animal Care and Use Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University.
Experimental animals
Male Wistar rats (weighed 200–240 g, aged 6–8 w, obtained from SLAC Laboratory Animal Co., Ltd., Shanghai, China) were fed under standard environment with 12 h day/night cycle.
Establishment of HIRI rat models
Anesthetized mice were conducted with a midline laparotomy and the hepatic lobes were isolated by separating the surrounding ligaments. The vasculature that supplied the left and middle lobes of the liver was suffered 70% hepatic thermal ischemia by a vascular clamp, and the incision was covered with gauze and plastic film. Throughout the modeling, mice were placed on a heating pad at 37°C. After 60-min ischemia, mice were performed with hepatic reperfusion and the abdominal incision was sutured. Mice in the sham group underwent the same operation, except for vessel clamping [Citation14]. Then, mice were euthanized with the serum and liver samples collected.
Animal grouping
Rats were separated into seven groups (n = 12): the sham group, the IR group (modeled rats), the Isoflurane group (rats were anesthetized by 1.5% isoflurane for 6 h and then the IR model was established), the agomir-negative control (NC) group (2 nmol miR-9-3p agomir-NC was injected intravenously into the rat tail. Thirty minutes later, the rats were anesthetized with 1.5% isoflurane for 6 h, and then the IR model was established), agomir-miR-9-3p (2 nmol miR-9-3p agomir was injected intravenously into the rat tail. Thirty minutes later, the rats were anesthetized with 1.5% isoflurane for 6 h, and then the IR model was established), antagomir-NC group (2 nmol miR-9-3p antagomir-NC was injected intravenously into the rat tail. Thirty minutes later, the rats were anaesthetized with 1.5% isoflurane for 6 h, and then the IR model was established) and antagomir-miR-9-3p group (2 nmol antagomir-miR-9-3p was injected intravenously into the rat tail. Thirty minutes later, the rats were anesthetized with 1.5% isoflurane for 6 h, and then the IR model was established) [Citation15].
Enzyme-linked immunosorbent assay (ELISA)
Rat postcava blood (5 μL) was centrifuged at 2,000 r/min for 20 min with the supernatant collected, and the serum levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-1β were evaluated based on the manufacturer’s information of ELISA kits (Westang Bio-tech Co., Ltd., Shanghai, China) [Citation16].
Serum enzyme analysis
Rat venous blood was centrifuged and the serum was collected and appropriately diluted. The levels of hepatocellular injury markers alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) after HIRI were detected [Citation17].
Hematoxylin-eosin (HE) staining
Liver tissues were fixed, embedded, and sectioned (4 μm), and then were performed with HE staining. The histopathological analysis was performed under a light microscope. HIRI was graded according to sinusoidal hyperemia, vacuolization in cytoplasm of hepatocytes, and parenchymal necrosis: 0 point = no injury, 1 point = very mild injury, 2 points = mild injury, 3 points = moderate injury, and 4 points = severe injury.
Liver function examination
Malondialdehyde (MDA) concentration and superoxide dismutase (SOD) activity were respectively determined using thiobarbituric acid method and nitrogen blue tetrazole method as previously described [Citation18].
Immunohistochemical staining
Sections were reacted with 3% hydrogen peroxide solution for 15 min, blocked with 1% bovine serum albumin for 30 min and incubated with primary antibodies caspase-3 (1:1000, 9661, Cell Signaling Technology, MA, USA) and FNDC3B (1:300, HPA007859, Millipore Inc., MA, USA) at 4°C overnight. Subsequently, the sections were incubated with secondary antibody goat anti-rabbit immunoglobulin G (1:200, ab125900, Cell Signaling Technology) and immunoperoxidase (VECTASTAIN Elite ABC kits, Vector Labs, CA, USA) for 1 h. Developed by diaminobenzidine and stained with hematoxylin for 1 min, the sections were dehydrated and sealed with neutral gum. A microscope (Nikon Corporation., Tokyo, Japan) was used for observation and the positive signals were counted [Citation19].
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) staining
Paraffin sections were fixed in 10% formalin for 15 min and performed with TUNEL staining referring to a publication [Citation20]. Results were observed under the OlympusIX51 fluorescent microscope (Olympus, Tokyo, Japan) and the apoptosis index (AI) was calculated: (number of apoptotic hepatocytes/number of total hepatocytes) × 100%.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Trizol kits (Invitrogen Inc., CA, USA) were used to extract total RNA in liver tissues and the reverse transcription was performed with HiScript QRT SuperMix kits (Vazyme, Nanjing, China). The PCR was conducted with SYBR RT-PCR kits (Vazyme) and the LightCycler 480II system (Roche, CA, USA). Primers are shown in , and data were analyzed using 2−ΔΔCt method [Citation21]. U6 and glyceraldehyde phosphate dehydrogenase (GAPDH) were respectively taken as the loading control of miR-9-3p and FNDC3B.
Table 1. Primer sequence
Western blot analysis
Total proteins were extracted from cells and tissues by radio-immunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China), and then were conducted with 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto membranes. Blocked with 5% skim milk, the membranes were incubated with primary antibodies rabbit anti-FNDC3B (1:300, HPA007859, Millipore), anti-Bax (1:1000, 2772, Cell Signaling Technology) and anti-GAPDH (1:200, ab6728, Abcam Inc., MA, USA) at 4°C overnight. Afterward, the membranes were incubated with relative secondary antibody (1:200, ab6728, Abcam) for 1–2 h. Enhanced chemiluminescent reagent (Pierce, IL, USA) was used for development [Citation22].
Dual luciferase reporter gene assay
The bioinformatic prediction website Targetscan was used to verify whether FNDC3B was the target gene of miR-9-3p. After digestion by restriction endonuclease, target fragment was inserted into pMIR reporter gene plasmid by T4DNA ligase. The wild type (WT) and mutant type (MUT) plasmids were co-transfected into HEK293T cells together with agomir-miR-9-3p and its NC. The luciferase activity was determined by the luminometer LB960 (Berthold technologies, Germany) [Citation23].
Statistical analysis
All data analyses were conducted using SPSS 21.0 software (IBM Corp. Armonk, NY, USA). The measurement data conforming to the normal distribution were expressed as mean ± standard deviation. The unpaired t-test was performed for comparisons between two groups, one-way analysis of variance (ANOVA) was used for comparisons among multiple groups and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA. P value < 0.05 was indicative of statistically significant difference.
Results
Isoflurane alleviates HIRI in rat liver tissues
The occurrence of HIRI might partly ascribe to the injury during liver ischemia, and partly to a series of injuries that caused by the reperfusion of the ischemic liver. It has been shown that ischemia-reperfusion causes interaction among hepatocytes, Kupffer cells, neutrophils, sinusoidal endothelial cells, and lipid storage cells [Citation24]. Activated cells release a large number of proinflammatory factors, lipid inflammatory factors, thus leading to the responses of inflammatory and cell apoptosis. The oxygen-free radicals that play an crucial role in HIRI are mainly produced from Kupffer cells, neutrophils, including superoxide radical, hydrogen oxygen ion, etc. The oxygen-free radicals can oxidate the lipids in phospholipid biomolecular structure of cell membrane, and changes membrane permeability and fluidity, thus further to directly damage liver cells. The oxidation end product is the cytotoxic MDA, which leads to the cross-linked polymer of proteins, nucleic acids, and other biomacromolecules. Therefore, the detection of MDA can reflect the degree of lipid peroxidation and indirectly reveal the degree of cell damage. SOD is a kind of antioxidant metalloenzyme that can catalyze the disproportionation of superoxide anion radical to generate oxygen and hydrogen peroxide. SOD plays a crucial role in the balance of oxidation and oxidation.
ALT and AST are mainly distributed in hepatocytes, and a small portion are found in muscle cells. If the liver is damaged, the transaminases in the liver cells will enter the bloodstream. The increased levels of ALT and AST in the bloodstream are the indicators of liver disease signals. In most cases, the elevations of ALT and AST are consistent with the degree of hepatocyte injury, which are the most commonly used indices of liver function test. LDH is a lactic dehydrogenase, which mainly exists in animal tissues. The liver diseases can upregulate the level of LDH.
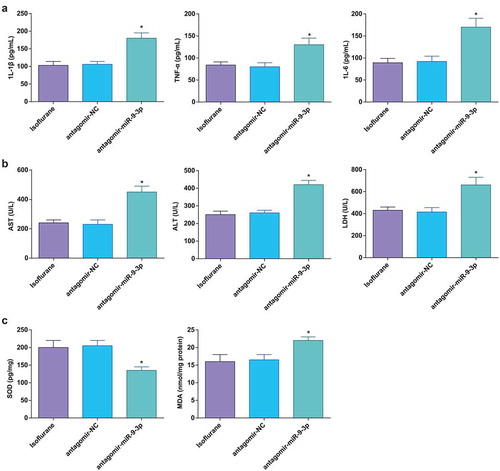
Therefore, in order to investigate the effect of isoflurane on HIRI in rats, rats were injected with isoflurane through the tail vein, and 6 h later, the IR rat model was established and then the expression of inflammatory factors, oxidative stress factors and transaminases was determined. We found that levels of IL-1β, IL-6, TNF-α, and MDA, and activities of AST, ALT, and LDH were elevated and SOD activity was reduced in modeled mice, while these alterations could be ameliorated by isoflurane treatment (–c)).
Figure 1. Isoflurane alleviates HIRI in rat liver tissues. (a) Levels of 1 L-1β, IL-6, and TNF-α were determined by ELISA (n = 12); (b) activities of AST, ALT, and LDH were evaluated using a biochemical analyzer (n = 12); (c) activities of SOD and MDA in rat liver tissues (n = 12); in (a–c) * P < 0.05 vs. the sham group, # P < 0.05 vs. the IR group; the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, one-way ANOVA was used for comparisons among multiple groups, and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA
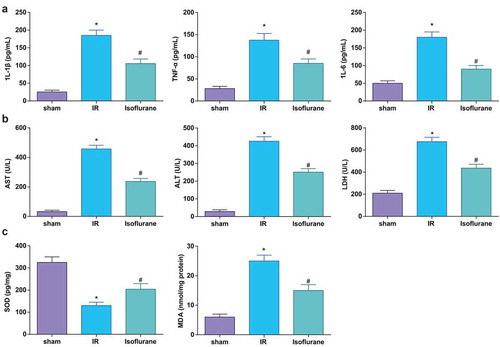
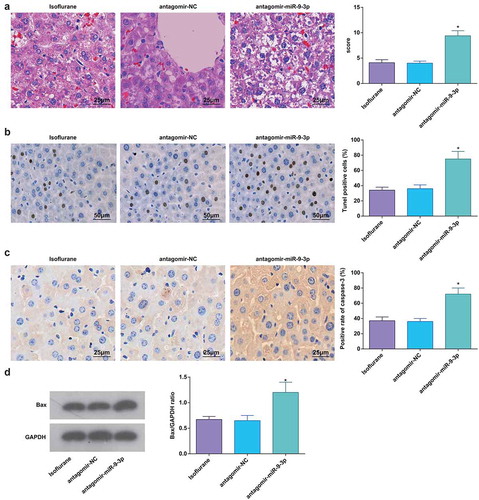
It was found in HE staining that ()) in the sham group, there were complete and clear hepatic lobule structure, hepatocytes radially arranged around the central veins, and the inflammatory cell infiltration could not be found (0 point). In the IR group, the structure of liver lobule was indistinct, the arrangement of hepatocytes was disordered, there appeared dilation and congestion in central vein and hepatic sinusoid, without inflammatory cell infiltration, and the pathological grade was increased significantly. In the Isoflurane group, the structure of liver lobule was more complete, the arrangement of hepatocytes was more regular than that in model group, the swelling and degeneration of hepatocytes, and the congestion of central vein and hepatic sinuses were all relieved, and the pathological grade was significantly decreased (P < 0.05).
Figure 2. Isoflurane alleviates HIRI in rat liver tissues. (a) Pathological changes in rat liver tissue observed using HE staining (n = 12); (b) apoptosis in rat liver tissue detected by TUNEL staining; (c) Caspase-3 expression in rat liver tissues was assessed by immunohistochemical staining (n = 12); (d) Bax protein expression was detected by Western blot analysis (n = 12); in (a–d), * P < 0.05 vs. the sham group, # P < 0.05 vs. the IR group; the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, one-way ANOVA was used for comparisons among multiple groups, and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA
Results of TUNEL staining indicated that there were no TUNEL-positive cells in the sham group, which were increased in the IR group; isoflurane treatment decreased the TUNEL-positive cells ()).
Expression of Bax and caspase-3 in rat liver tissues was determined, and it was found that the IR mice had higher Bax and caspase-3 expression; isoflurane decreased the expression of Bax and caspase-3 in rat liver tissues (). The above results showed that isoflurane could alleviate HIRI in rats.
Elevated miR-9-3p ameliorates HIRI-induced changes in inflammation, oxidative stress, and transaminases in HIRI rat liver tissues
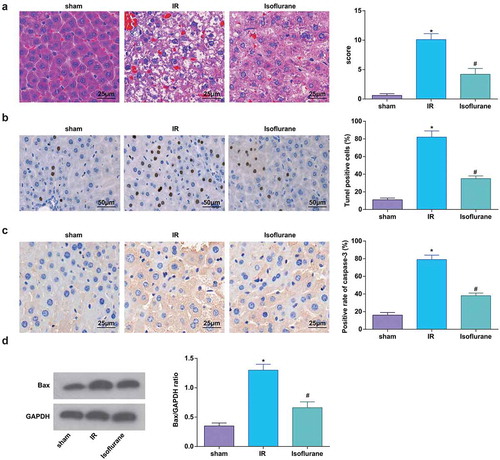
MiR-9-3p was elevated to assess its role in the related indicators in HIRI rat liver tissues. The outcomes reflected that miR-9-3p upregulation decreased IL-1β, IL-6, and TNF-α levels and AST, ALT, LDH, and MDA activities, while increased SOD activity (–c)), indicating that miR-9-3p elevation relieved HIRI in rats.
Figure 3. Elevated miR-9-3p ameliorates HIRI-induced changes in inflammation, oxidative stress, and transaminases in HIRI rat liver tissues. (a) Levels of 1 L-1β, IL-6, and TNF-α were determined by ELISA (n = 12); (b) activities of AST, ALT, and LDH were evaluated using a biochemical analyzer (n = 12); (c) activities of SOD and MDA in rat liver tissues (n = 12); in (a–c) * P < 0.05 vs. the agomir-NC group; the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, one-way ANOVA was used for comparisons among multiple groups, and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA
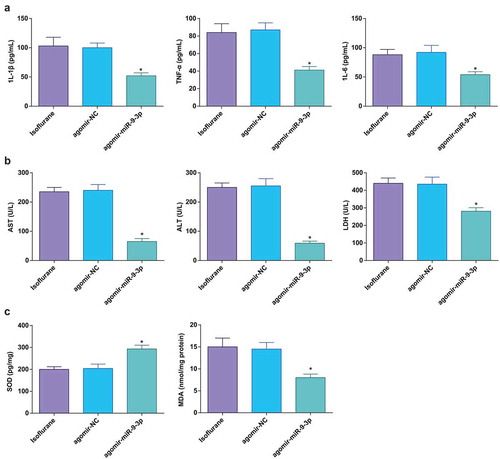
Elevated miR-9-3p relives pathological changes and hepatocyte apoptosis in HIRI rat liver tissues
According to the pathological assessment, miR-9-3p elevation resulted in more complete hepatic lobule structure, regular hepatocyte arrangement, ameliorated hepatocyte swelling, attenuated dilation and congestion in central veins and hepatic sinusoid, and decreased focal necrosis. The pathological score was significantly reduced ()).
Figure 4. Elevated miR-9-3p relives pathological changes and hepatocyte apoptosis in HIRI rat liver tissues. (a) Pathological changes in rat liver tissue observed using HE staining (n = 12); (b) apoptosis in rat liver tissue detected by TUNEL staining (n = 12); (c) Caspase-3 expression in rat liver tissues was assessed by immunohistochemical staining (n = 12); (d) Bax protein expression was detected by Western blot analysis (n = 12); in (a–d), * P < 0.05 vs. the agomir-NC group; the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, one-way ANOVA was used for comparisons among multiple groups, and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA
Results of TUNEL staining, immunohistochemical staining, and Western blot analysis revealed that miR-9-3p upregulation suppressed hepatocyte apoptosis and expression of Bax and caspase-3 (–d)).
MiR-9-3p inhibition aggravates HIRI-induced changes in inflammation, oxidative stress and transaminases in HIRI rat liver tissues
MiR-9-3p was downregulated in HIRI rat liver tissues to evaluate its role and we found that miR-9-3p downregulation promoted inflammatory factor levels, oxidative stress and transaminase activity, showing that downregulated miR-9-3p aggravated the HIRI in rats (–c)).
Figure 5. MiR-9-3p inhibition aggravates HIRI-induced changes in inflammation, oxidative stress, and transaminases in HIRI rat liver tissues. (a) Levels of 1 L-1β, IL-6, and TNF-α were determined by ELISA (n = 12); (b) activities of AST, ALT, and LDH were evaluated using a biochemical analyzer (n = 12); (c) activities of SOD and MDA in rat liver tissues (n = 12); in (a–c), * P < 0.05 vs. the antagomir-NC group; the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, one-way ANOVA was used for comparisons among multiple groups, and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA
MiR-9-3p inhibition promotes pathological changes and hepatocyte apoptosis in HIRI rat liver tissues
Outcomes of HE staining suggested that miR-9-3p inhibition resulted in vague hepatic lobule structure, disorderly arranged hepatocytes, dilation and congestion in central veins and hepatic sinusoid, inflammatory cell infiltration, swelled and transformed hepatocytes, and increased pathological score ()).
Figure 6. MiR-9-3p inhibition promotes pathological changes and hepatocyte apoptosis in HIRI rat liver tissues. (a) Pathological changes in rat liver tissue observed using HE staining (n = 12); (b) apoptosis in rat liver tissue detected by TUNEL staining (n = 12); (c) Caspase-3 expression in rat liver tissues was assessed by immunohistochemical staining (n = 12); (d) Bax protein expression was detected by Western blot analysis (n = 12); in (a–d) * P < 0.05 vs. the antagomir-NC group; the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, one-way ANOVA was used for comparisons among multiple groups, and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA
Outcomes of TUNEL staining, immunohistochemical staining, and Western blot analysis verified that miR-9-3p downregulation promoted hepatocyte apoptosis and expression of Bax and caspase-3 (–d)).
Isoflurane upregulates miR-9-3p and FNDC3B is targeted by miR-9-3p
Results of RT-qPCR, Western blot analysis, and immunohistochemical staining showed that the Isoflurane could elevate miR-9-3p expression in HIRI rats; miR-9-3p agomir downregulated FNDC3B while miR-9-3p antagomir had an opposite effect on FNDC3B expression (–h)).
Figure 7. Isoflurane upregulates miR-9-3p and FNDC3B is targeted by miR-9-3p. (a–d) MiR-9-3p and FNDC3B expression was assessed by RT-qPCR (n = 12); (e/f) Protein expression of FNDC3B in rat liver tissues was determined by Western blot analysis (n = 12); (g/h), FNDC3B level in rat liver tissues was detected by immunohistochemical staining (n = 12); (i) binding site of miR-9-3p and FNDC3B was predicted at Targetscan (N = 3); (j) regulatory relationship between miR-9-3p and FNDC3B was confirmed using dual luciferase reporter gene assay; in (a, c, e, g), * P < 0.05 vs. the sham group, # P < 0.05 vs. the IR group; in (b, d, f, h), * P < 0.05 vs. the agomir-NC group, # P < 0.05 vs. the antagomir-NC group; the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, unpaired t-test was performed for comparisons between two groups, one-way ANOVA was used for comparisons among multiple groups, and Tukey’s post hoc test was used for pairwise comparisons after one-way ANOVA
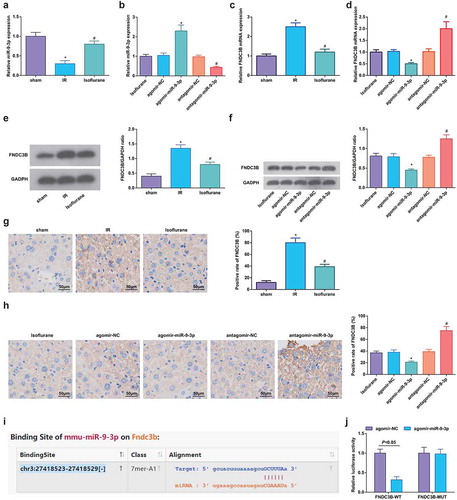
It was predicted by Targetscan that there existed binding site between miR-9-3p and FNDC3B ()), and it was further confirmed that co-transfection of WT-FNDC3B and agomir-miR-9-3p suppressed the luciferase activity, while co-transfection of MUT-FNDC3B and agomir-miR-9-3p did not affect the luciferase activity ()). Thus, it could be inferred that isoflurane upregulated miR-9-3p and miR-9-3p targeted FNDC3B.
Discussion
HIRI is a clinical condition that may result in cellular injury and organ dysfunction, mainly mediated by the production of reactive oxygen species and inflammatory cytokines [Citation25]. This research was designed to explore the role of isoflurane regulating miR-9-3p expression in HIRI, and results of our experiments indicated that isoflurane treatment could upregulate miR-9-3p to protect against HIRI through inhibiting FNDC3B.
Results of our research showed that the isoflurane treatment attenuated pathological changes, inflammation, oxidative stress, transaminases, and hepatocyte apoptosis in liver tissues from HIRI rats. Similarly, a recent study has suggested that management of ISO reduced cardiomyocyte apoptosis and damage in a rat model of middle cerebral artery occlusion [Citation26]. Ran et al. have revealed that rabbits with myocardial IRI had increased expression level of myocardial caspase-3, which was decreased by isoflurane [Citation27]. Additionally, it has been elucidated that after isoflurane administration in a rat myocardial IRI model, the pathological damage in myocardial tissues was alleviated, the MDA content was decreased, and the SOD activity was increased [Citation28]. Qin et al. have found that emulsified isoflurane reduced MDA content, histological scores and TNF-α and IL-6 levels, and increased SOD activity in rats with renal IRI [Citation29]. Moreover, our results indicated that isoflurane upregulated miR-9-3p in HIRI rat liver tissues. Consistently, it has been reported that isoflurane promoted miR-9 expression during embryonic stem cell self-renewal and neural differentiation [Citation30]. Nevertheless, the regulatory role of isoflurane in miR-9-3p expression has been scarcely investigated.
Expression of miR-9-3p and FNDC3B in HIRI models was determined, and we found that miR-9-3p was downregulated whereas FNDC3B was upregulated in HIRI rat liver tissues. Similarly, miR-9-3p has been verified to be downregulated in HCC tissues [Citation31], and Wang et al. have unearthed that miR-9-3p expression was decreased in coronary artery disease samples versus normal arterial tissues, and it was also downregulated by oxidized low-density lipoprotein-treated endothelial cells [Citation32]. As for the abnormal expression of FNDC3B, it has been reported that FNDC3B was high-expressed in hepatocarcinoma cells [Citation12], and Kong et al. have revealed that FNDC3B expression was elevated in HCC cells [Citation13]. We up- or downregulated miR-9-3p in HIRI rats to assess its role in progression of HIRI, and it was observed that miR-9-3p upregulation reduced pathological changes, inflammation, oxidative stress, transaminases, and hepatocyte apoptosis in HIRI rat liver tissues, and miR-9-3p downregulation had opposite effects on HIRI rats. In line with our findings, Yue et al. have demonstrated that miR-9-5p elevation suppressed the inflammatory response in mice with experimental autoimmune encephalomyelitis [Citation33], and it has been validated that elevated miR-9-5p protected against inflammatory response in rats with deep vein thrombosis [Citation34]. In addition, exosomal miR-9-5p has been uncovered to downregulate expression of inflammatory factors and reduce oxidative stress injury in osteoarthritis [Citation35], and Chi et al. have identified that miR-9 elevation repressed LDH activity and cell apoptosis in oxygen-glucose deprivation-treated neurons [Citation36]. Furthermore, miRNAs are known to regulate gene expression by binding to the 3ʹ untranslated region of target mRNAs [Citation37]. We assessed the targeting relationship between miR-9-3p and FNDC3B using bioinformatic prediction and dual luciferase reporter gene assay, and it was found that miR-9-3p could target FNDC3B in HIRI rats. MiR-9-3p has been clarified to target BLCAP in medullary thyroid carcinoma [Citation38] and target HBGF-5 in HCC [Citation9], while the binding relation between miR-9-3p and FNDC3B remains to be further studied.
To sum up, we demonstrated that isoflurane treatment could upregulate miR-9-3p to protect against HIRI through inhibiting FNDC3B. This research may be helpful for investigating therapeutic strategies for HIRI. However, the study samples are not so sufficient and still necessitate further enrichment to get more precise and representative data for a convincing conclusion.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Disclosure statement
The authors declare that they have no conflicts of interest.
References
- Wang J, Yu S, Li J, et al. Protective role of N-acetyl-l-tryptophan against hepatic ischemia-reperfusion injury via the RIP2/caspase-1/IL-1beta signaling pathway. Pharm Biol. 2019;57(1):385–391.
- Tang H, Qu E-Z, Li K, et al. Effect of hepatic artery spasm on a rat model of hepatic ischemia-reperfusion injury. J Ultrasound Med. 2019;38(3):597–604.
- Sikalias N, Karatzas T, Alexiou K, et al. Intermittent ischemic preconditioning protects against hepatic ischemia-reperfusion injury and extensive hepatectomy in steatotic rat liver. J Invest Surg. 2018;31(5):366–377.
- Zhang Y, Shen Q, Liu Y, et al. Hepatic ischemic preconditioning alleviates ischemia-reperfusion injury by decreasing TIM4 expression. Int J Biol Sci. 2018;14(10):1186–1195.
- Yuan M, Ge M, Yin J, et al. Isoflurane post-conditioning down-regulates expression of aquaporin 4 in rats with cerebral ischemia/reperfusion injury and is possibly related to bone morphogenetic protein 4/Smad1/5/8 signaling pathway. Biomed Pharmacother. 2018;97:429–438.
- Ko JS, Gwak MS, Kim GS, et al. The protective effect of ischemic preconditioning against hepatic ischemic-reperfusion injury under isoflurane anesthesia in rats. Transplant Proc. 2013;45(5):1704–1707.
- Lv X, Wang Z-M, Huang S-D, et al. Emulsified isoflurane preconditioning reduces lung injury induced by hepatic ischemia/reperfusion in rats. Int J Med Sci. 2011;8(5):353–361.
- Jiang S, Miao D, Wang M, et al. MiR-30-5p suppresses cell chemoresistance and stemness in colorectal cancer through USP22/Wnt/beta-catenin signaling axis. J Cell Mol Med. 2019;23(1):630–640.
- Tang J, Li Y, Liu K, et al. Exosomal miR-9-3p suppresses HBGF-5 expression and is a functional biomarker in hepatocellular carcinoma. Minerva Med. 2018;109(1):15–23.
- Liao X, Zhou S, Zong J, et al. Sevoflurane exerts protective effects on liver ischemia/reperfusion injury by regulating NFKB3 expression via miR-9-5p. Exp Ther Med. 2019;17(4):2632–2640.
- Bian T, Zheng L, Jiang D, et al. Overexpression of fibronectin type III domain containing 3B is correlated with epithelial-mesenchymal transition and predicts poor prognosis in lung adenocarcinoma. Exp Ther Med. 2019;17(5):3317–3326.
- Zhang X, Liu S, Hu T, et al. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology. 2009;50(2):490–499.
- Kong D, Ma W, Zhang D, et al. EYA1 promotes cell migration and tumor metastasis in hepatocellular carcinoma. Am J Transl Res. 2019;11(4):2328–2338.
- Cai C, Shi X, Korff S, et al. CD14 contributes to warm hepatic ischemia-reperfusion injury in mice. Shock. 2013;40(2):115–121.
- Yang W, Guo Q, Li J, et al. microRNA-124 attenuates isoflurane-induced neurological deficits in neonatal rats via binding to EGR1. J Cell Physiol. 2019;234(12):23017–23032.
- Nong K, Wang W, Niu X, et al. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18(12):1548–1559.
- Li X, Wu Y, Zhang W, et al. Pre-conditioning with tanshinone IIA attenuates the ischemia/reperfusion injury caused by liver grafts via regulation of HMGB1 in rat Kupffer cells. Biomed Pharmacother. 2017;89:1392–1400.
- Yucel A, Aydogan MS, Ucar M, et al. Effects of apocynin on liver ischemia-reperfusion injury in rats. Transplant Proc. 2019;51(4):1180–1183.
- Liu Y, Ding Y, Nie Y, et al. EMP1 promotes the proliferation and invasion of ovarian cancer cells through activating the MAPK pathway. Onco Targets Ther. 2020;13:2047–2055.
- Yu S, Zheng J, Jiang Z, et al. Protective effect of N-acetylserotonin against acute hepatic ischemia-reperfusion injury in mice. Int J Mol Sci. 2013;14(9):17680–17693.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408.
- Zhou CZ, Wang RF, Cheng DL, et al. FLT3/FLT3L-mediated CD103(+) dendritic cells alleviates hepatic ischemia-reperfusion injury in mice via activation of treg cells. Biomed Pharmacother. 2019;118:109031.
- Li Y, Gao M, Xu L-N, et al. MicroRNA-142-3p attenuates hepatic ischemia/reperfusion injury via targeting of myristoylated alanine-rich C-kinase substrate. Pharmacol Res. 2020;156:104783.
- Zeng Z, Huang HF, Chen MQ, et al. Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells. World J Gastroenterol. 2010;16(10):1285–1292.
- Zhang YQ, Ding N, Zeng Y-F, et al. New progress in roles of nitric oxide during hepatic ischemia reperfusion injury. World J Gastroenterol. 2017;23(14):2505–2510.
- Peng L, Yin J, Wang S, et al. TGF-beta2/Smad3 signaling pathway activation through enhancing VEGF and CD34 ameliorates cerebral ischemia/reperfusion injury after isoflurane post-conditioning in rats. Neurochem Res. 2019;44(11):2606–2618.
- Ran K, Zou DQ, Xiao YY, et al. Effects of isoflurane preconditioning in the delayed phase on myocardial tumor necrosis factor alpha levels and caspase-3 protein expression in a rabbit model of ischemia-reperfusion injury. Genet Mol Res. 2015;14(3):7267–7273.
- Zhou Y, Peng DD, Chong H, et al. Effect of isoflurane on myocardial ischemia-reperfusion injury through the p38 MAPK signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(3):1342–1349.
- Qin Z, Lv E, Zhan L, et al. Intravenous pretreatment with emulsified isoflurane preconditioning protects kidneys against ischemia/reperfusion injury in rats. BMC Anesthesiol. 2014;14:28.
- Zhang L, Zhang Y, Hu R, et al. Isoflurane inhibits embryonic stem cell self-renewal and neural differentiation through miR-9/E-cadherin signaling. Stem Cells Dev. 2015;24(16):1912–1922.
- Yang S, Cai H, Hu B, et al. LncRNA SAMMSON negatively regulates miR-9-3p in hepatocellular carcinoma cells and has prognostic values. Biosci Rep. 2019;39(7). DOI:10.1042/BSR20190615
- Wang X, Zhao Z, Zhang W, et al. Long noncoding RNA LINC00968 promotes endothelial cell proliferation and migration via regulating miR-9-3p expression. J Cell Biochem. 2018. doi:10.1002/jcb.28103.
- Yue P, Jing L, Zhao X, et al. Down-regulation of taurine-up-regulated gene 1 attenuates inflammation by sponging miR-9-5p via targeting NF-kappaB1/p50 in multiple sclerosis. Life Sci. 2019;233:116731.
- Ou M, Zhang Y, Cui S, et al. Upregulated MiR-9-5p protects against inflammatory response in rats with deep vein thrombosis via inhibition of NF-kappaB p50. Inflammation. 2019;42(6):1925–1938.
- Jin Z, Ren J, Qi S. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 2020;381(1):99–114.
- Chi L, Jiao D, Nan G, et al. miR-9-5p attenuates ischemic stroke through targeting ERMP1-mediated endoplasmic reticulum stress. Acta Histochem. 2019;121(8):151438.
- Yin H, He H, Shen X, et al. miR-9-5p inhibits skeletal muscle satellite cell proliferation and differentiation by targeting IGF2BP3 through the IGF2-PI3K/Akt signaling pathway. Int J Mol Sci. 2020;21(5):1655.
- Chen Y, Zhang S, Zhao R, et al. Upregulated miR-9-3p promotes cell growth and inhibits apoptosis in medullary thyroid carcinoma by targeting BLCAP. Oncol Res. 2017;25(8):1215–1222.
