ABSTRACT
Among urological tumors, renal cell carcinoma (RCC) is the third-highest mortality rate tumor, and 20%–30% of RCC patients present with metastases at the time of diagnosis. While the treatment of RCC has been improved over the last few years, its mortality stays high. Y-box binding protein 1 (YBX1) is a well-known oncoprotein that has tumor-promoting functions. YBX1 is widely considered to be an attractive therapeutic target in cancer. To develop novel therapeutics to target YBX1, it is of great importance to understand how YBX1 is finely regulated in cancer. Our previous studies showed that YBX1 in RCC cells significantly promoted cell adhesion, migration, and invasion. However, the role of YBX1 in RCC cells apoptosis has not been reported. In this study, we investigated the effect of YBX1 on cell apoptosis and elucidated the mechanisms involved. Results showed that YBX1 regulated RCC cells apoptosis and reactive oxygen species (ROS) generation via Kindlin-2. These findings indicated that YBX1 inhibited RCC cells apoptosis and may serve as a candidate RCC prognostic marker and a potential therapeutic target. Abbreviations: RCC: Renal cell carcinoma; YBX1: Y-box binding protein 1; ROS: Reactive oxygen species; ccRCC: Clear cell renal cell carcinoma; mccRCC: Metastatic clear cell renal cell carcinoma; G3BP1: Ras-GTPase activating protein SH3 domain-binding proteins 1; SPP1: Secreted phosphoprotein 1; NF-κB: Nuclear factor kappa beta; ECM: Extracellular matrix; EMT: Epithelial-mesenchymal transition; PYCR1: Pyrroline-5-carboxylate reductase 1; MEM: Eagle’s Minimum Essential Medium; DMEM: Dulbecco’s modified Eagle medium; FBS: Fetal bovine serum; PCR: Polymerase chain reaction; shRNA: Short hairpin RNA; siRNA: Small interfering RNA; BSA: Bovine serum albumin; DCFH-DA: 2,7-Dichlorodihydrofluorescein diacetate; FITC: Fluorescein isothiocyanate; PI: Propidium iodide;
Introduction
Renal cell carcinoma (RCC) has become one of the most common malignancies in the genitourinary system and accounts for approximately 3% of adult malignant tumors and 2% of all cancer deaths [Citation1]. RCC is classified into four major histological cell types, and clear cell renal cell carcinoma (ccRCC) accounts for approximately 80% of RCC cases. Gender difference, genetic factors, and lifestyle are common risk factors for ccRCC [Citation2,Citation3]. It is estimated that 25%–30% of RCC patients have approximately metastases at the time of diagnosis. Even after resection of the primary tumor by radical or partial nephrectomy, relapse occurs in 20%–30% of RCC patients [Citation4]. The worldwide morbidity and mortality rates of RCC are growing approximately 2%–3% per decade [Citation5]. Therefore, it is crucial to explore novel molecules involved in the progression of RCC to identify new therapeutic targets for RCC treatment.
YBX1 is a multifunctional protein that functions as a transcription factor and RNA-binding factor in the nucleus and cytoplasm, respectively [Citation6]. YBX1 interacts with DNA and RNA and regulates many DNA- and mRNA-dependent processes, including DNA transcription, replication, repair, environmental stress, chromatin remodeling, pre-mRNA splicing, etc [Citation7]. YBX1 regulates tumor cell proliferation, apoptosis, invasion, migration, and chemoresistance through the related pathways [Citation8]. Besides, YBX1 binds the consensus sequence, 5′‐CTGATTGG‐3′, to mediate transcriptional activation of genes involved in epithelial-mesenchymal transition (EMT) and drug resistance, likely thereby promoting cancer progression [Citation9]. YBX1 has been extensively studied in cancer, and its overexpression is associated with many hallmarks of the diseases. YBX1 is suggested to be a prognostic clinical biomarker and correlated with poor prognoses in different cancer, such as breast cancer [Citation10,Citation11], lung cancer [Citation12,Citation13], multiple myeloma [Citation14], osteosarcoma [Citation15], synovial sarcoma [Citation16], prostate cancer [Citation17], and ovarian cancer [Citation18]. Although our previous findings indicate that YBX1 interacts with Ras-GTPase activating protein SH3 domain-binding proteins 1 (G3BP1) to promote migration and invasion of RCC cells via activating the SPP1/NF-κB signaling pathway [Citation19], the relationship and molecular mechanism between YBX1 and apoptosis in RCC have not been well elucidated.
Kindlins consist of three members, Kindlin-1, Kindlin-2, and Kindlin-3, which are a family of adapter proteins that mediate cell-cell and cell-matrix adhesions [Citation20–22]. Among them, Kindlin-2, encoded by the FERMT2 gene (chromosome 14q22.1), is the most broadly distributed [Citation23]. It is well documented that Kindlin-2 localizes to focal adhesions [Citation24], sites where the extracellular matrix (ECM) is connected to the actin cytoskeleton [Citation25]. Kindlin-2 is found to trigger EMT by activating Wnt signaling in vitro [Citation26], resulting in increased adhesion, migration, and proliferation [Citation27]. Recent studies have linked Kindlin-2 to multiple malignancies including gastric cancer, bladder cancer, large cell lung carcinoma, esophageal squamous cell carcinoma, pancreatic ductal adenocarcinoma, ccRCC, malignant mesothelioma, and glioma [Citation28–36]. It has been demonstrated that Kindlin-2 is involved in the growth of primary breast cancer by regulating the interaction between the tumor and its microenvironment [Citation37]. ECM stiffening promotes Kindlin-2 translocation into mitochondria and its interactions with pyrroline-5-carboxylate reductase 1 (PYCR1), resulting in elevation of PYCR1 level and the consequent increase of proline synthesis and cell proliferation. Depletion of Kindlin-2 reduces PYCR1 level, increases ROS production and apoptosis, and abolishes ECM stiffening-induced increasing of proline synthesis and cell proliferation [Citation38]. However, the specific role of Kindlin-2 in RCC progression remains unknown until now.
The present study investigated the role of YBX1 in RCC cells apoptosis. Our findings indicate that YBX1 is over-expression and inversely correlated with prognosis in RCC. Meanwhile, YBX1 interacts with Kindlin-2 to regulate ROS production and apoptosis in RCC cells.
Materials and methods
Cell culture and transduction
The human renal cancer cell lines ACHN and 786-O and the human embryonic kidney 293 T cells were purchased from American Type Culture Collection (ATCC, USA). The ACHN cells were cultured in Eagle’s Minimum Essential Medium (MEM) (Biological Industries, Israel) while the 786-O and 293 T cells were cultured in Dulbecco’s modified Eagle medium (DMEM) (Biological Industries, Israel) supplemented with 10% fetal bovine serum (FBS) (Biological Industries, Israel) at 37 °C under 5% CO2.
To generate YBX1 knockdown or overexpression stable cell lines, 293 T cells were transfected with lentiviral vectors (pLKO.1-Scr, pLKO.1-shYBX1, pWPI-Vec, and pWPI-YBX1) together with lentivirus packaging plasmids (psPAX2 and pMD2.G) using Lipofectamine 2000 (Invitrogen, USA) according to the instructions of the manufacturer. The supernatant containing the lentivirus was collected at 48 h post-transfection. The lentivirus supernatant was used to infected ACHN and 786-O cells for 48 h and selected for 10 days with 2 µg/ml puromycin (Sangon Biotech, China) or 400 ug/ml G418 (MDBio Inc, China), subsequently maintained with 0.5 µg/ml puromycin or 100 ug/ml G418. qRT-PCR and Western blot were used to verify the stable expression of the target genes. The siRNA against Kindlin-2 and non-targeting siRNA were designed and synthesized by RiboBio (Guangzhou RiboBio, China), and the sequences were listed in (Table S1). In all the siRNA transfection experiments, ACHN and 786-O cells were seeded in six-well plates one day before and transfected with 75 nM of the indicated siRNA using Lipofectamine 2000.
RNA extraction and quantitative real-time PCR
Total RNA was isolated with Trizol reagent (Ambion, USA) and cDNA was generated with a FastQuant RT Kit (TIANGEN, China) according to the manufacturer’s recommendation. The sequences of all primers used are listed in (Table S2), and GAPDH was used as the internal control.
Western blot
The cells were washed in ice-cold PBS and lysed in SDS lysis buffer supplemented with 1 × protease inhibitor cocktail (Roche Applied Science, Germany) for 10 min on ice. Protein concentration was quantified using the BCA method (BCA Protein Assay Kit, Thermo Fisher Scientific, USA). The protein samples were separated by SDS-PAGE and blotted onto polyvinylidene difluoride membranes (Millipore, USA). Each membrane was blocked with 5% skim milk for 1.5 h at room temperature and incubated overnight with a specific primary antibody at 4 °C. Then, membranes were incubated with HRP-conjugated secondary antibody for 1 h, and protein expression was detected using ECL (Affinity Biosciences, USA). The primary antibodies used are listed as follows: β-actin (Affinity Biosciences, USA, T0022; 1:3,000), YBX1 (Abcam, USA, ab76149; 1:4,000), Kindlin-2 (Abcam, USA, ab74030; 1:3,000), Bax (Abcam, USA, ab32503; 1:2,000), Bcl-2 (Abcam, USA, ab182858; 1:2,000) and caspase-3 (Abcam, USA, ab184787; 1:2,000).
Microarray
Triplicate samples of YBX1 shRNA knockdown and respective control transfected 786-O cells were extracted for RNA and prepared for microarray profiling. Jingtai Biotech company (Shanghai, China) performed the microarray hybridization and collection of data. Total RNAs were isolated by RNeasy Mini Kit (Qiagen, GER) and cDNA was prepared with SuperScript II (Invitrogen, USA). Labeled with biotin and hybridized at 45 °C for 16 h to Affymetrix GeneChip Human Gene 1.0 ST arrays (Affymetrix, USA). All arrays were washed and scanned using a GeneChip Scanner 3000 (Affymetrix, USA) at the correct pixel value (3 µm) and wavelength (570 nm), and data were collected and analyzed. Genes with more than 2-fold change and P < 0.05 were considered as differentially expressed.
Clinical specimens
In this study, we collected 20 pairs of fresh ccRCC tissues, 42 pairs of formalin-fixed, paraffin-embedded ccRCC tissues, and paired adjacent normal tissues from patients at The Second Hospital of Tianjin Medical University from 2014 to 2018 to confirm the expression of YBX1 and Kindlin-2. All these patients were treated with radical nephrectomy or partial nephrectomy and no other therapy before surgery. Clinicopathological data including age, gender, tumor size, tumor stage, and Fuhrman grade were collected. Patient informed consent was obtained, and the study was approved by the institutional ethics committee.
Immunohistochemical analysis
Formalin-fixed tissues were embedded in paraffin and 5 µm sections were cut from each paraffin block. The tissue sections were deparaffinized with xylene and dehydrated in gradually decreasing concentrations of ethanol. Then, the sections were deparaffinized and treated with citrate antigen repair buffer to antigen repair, with 3% hydrogen peroxide to block endogenous peroxidase activity, with 5% bovine serum albumin (BSA) for serum blocking. The tissue sections were incubated with primary antibody YBX1 (Santa Cruz Biotechnology, USA, sc-398,340; 1:40) or Kindlin-2 (Affinity, USA, CAT#1 DF12078; 1:150) overnight at 4 °C. After washing with PBS, sections were incubated with secondary antibody (ZSGB-BIO, China) for 1 h at room temperature. The reaction was visualized by the HRP DAB Detection Kit (ZSGB-BIO, China) and counterstained with hematoxylin. After the sections were sealed with neutral balsam, the histopathological changes of the different tissues were observed under an optical microscope. The staining scores were evaluated by both the staining intensity and the proportion of positively stained tumor cells. Scores representing the proportion of positively stained tumor cells were as follows: 0, 0–1%; 1, 1%–5%; 2, 6%–10%; 3, 11%–20%; 4, 21%–50% and 5, 51%–100%. The staining intensity was determined as follows: 0, negative staining; 1, moderate staining (yellow-brown color); 2, strong staining (brown color). The final score was the sum of the intensity and percentage scores, being classified as negative (0–3) and positive (4–7). The results were evaluated by two independent pathologists.
Immunoprecipitation
The ACHN cells were collected and lysed with cell lysis buffer (40 mM Tris, 120 mM NaCl, 1% Triton X-100, 1 mM NaF, 1 mM Na3VO4) supplemented with protease inhibitor cocktail (Roche, Switzerland). Cells were rotated for 30 min at 4 °C. Then the cells were pelleted by centrifugation at 12,000 rpm for 20 min at 4 °C and the cellular lysate was obtained as a supernatant. The primary antibody was incubated with protein lysate overnight at 4 °C, followed by incubation with Protein A/G Magnetic Beads (Invitrogen, USA) for 6 h at 4 °C. After washing the immune complexes six times with lysis buffer, proteins were eluted with 2×SDS sample buffer. The western blotting analysis was used to detect both the input and immunoprecipitation protein lysis.
Immunofluorescence
ACHN and 786-O cells were plated on poly-d-lysine-coated coverslips in 12-well culture plates and incubated for 24 h at 37 °C. Then, cells were fixed with methanol for 10 min at − 20 °C, permeabilized with 0.2% Triton X-100 in PBS for 10 min and blocked with 3% BSA in PBS. Following primary antibody incubation overnight at 4 °C (YBX1, Santa Cruz Biotechnology, USA, sc-101,198; 1:5) or (Kindlin-2, Abcam, USA, ab74030; 1:50), and the cells were stained with Alexa-Fluor 488 and 546 conjugated secondary antibodies for 1 h at room temperature. Fluorescent signals were captured by a confocal microscope (Olympus FV1000).
Measurement of intracellular ROS levels
The cells were detected with ROS Assay Kit (Beyotime Biotechnology, China) according to the manufacture’s instruction. DCFH-DA was diluted as 1: 1,000 in serum-free culture medium (final concentration was 10 μM). The growth medium was removed, and the cells were washed three times with medium without serum. Then, the appropriate volume of DCFH-DA solution was added to cells, and incubate at 37 °C in the dark for 30 min. After the removal of the medium containing DCFH-DA, the cells were rinsed with PBS three times to remove the residual extracellular DCFH-DA. The cells were then washed with PBS once, and the intracellular ROS levels were then immediately examined by measuring the fluorescence intensity of DCF using flow cytometry.
Apoptosis assay
Cell apoptosis assay was performed using an Annexin V-FITC/PI kit (Absin, Shanghai, China). Deionized water was used to dilute 10 × binding buffer into 1 × binding buffer. RCC cells were digested with pancreatic enzymes without EDTA and collected by centrifugation at 2,000 rpm for 5 min. Then, cells were carefully washed once with prechilled PBS and centrifuged at 2,000 rpm at 4 °C for 8 min, and the supernatant was discarded. Next, 300 µl of 1× Binding buffer and 5 μl of Annexin V-FITC binding solution were added, and the samples were incubated for 15 min in the dark. 5 µl of PI was added just before analysis. Cell apoptosis rate was calculated by flow cytometry assay.
Statistical analysis
Statistical analysis was performed using the SPSS version 20.0 program (IBM, USA) or GraphPad Prism 7.0 software. All data were expressed as mean ± SD. Analysis of variance tests or t-test were used for statistical analyses. The correlations between clinicopathological characteristics and protein expression were assessed using chi-square tests. Correlations between YBX1 and Kindlin-2 expression were analyzed using the Spearman rank correlation test. Differences of P < 0.05 were considered statistically significant.
Results
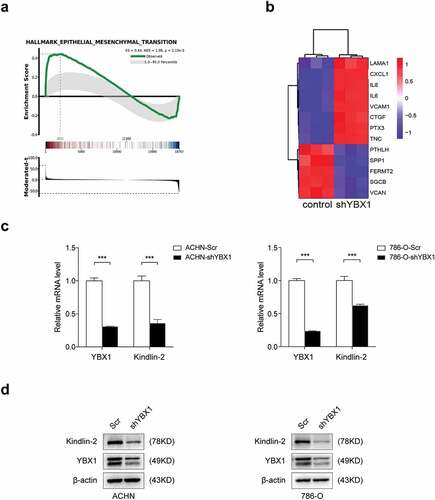
1. YBX1 regulates the expression of Kindlin-2 in RCC cells
Our previous studies indicate that YBX1 plays a critical role in the development and progression of RCC [Citation19]. YBX1 stable knockdown was established in RCC cell lines ACHN and 786-O via lentiviral infection. To investigate the functions of YBX1 in RCC cells, we screened differentially expressed genes in the microarray data. Pathway enrichment analysis revealed that Kindlin-2 is involved in the YBX1-regulated EMT ( and b). Indeed, loss of YBX1 induced a significant decrease in both mRNA and protein levels of Kindlin-2 (. and d).
Figure 1. YBX1 regulates the expression of Kindlin-2 in renal cancer cells. (a) GSEA analyzes genes positively or negatively regulated by YBX1, and the enrichment map shows that the differentially expressed genes are significantly related to EMT. (b) The heat map shows differently expressed genes after YBX1 knockdown during the EMT process. (c) The mRNA levels of Kindlin-2 were examined by qRT-PCR in YBX1 knockdown ACHN (left panel) and 786-O (right-panel) cells. (d) Kindlin-2 protein expressions in YBX1 knockdown ACHN (left panel) and 786-O (right panel) cells were detected by western blot assay. Scr: down-expression empty plasmid control; shYBX1: YBX1 knockdown; Statistically significant differences were indicated: ***, P < 0.001
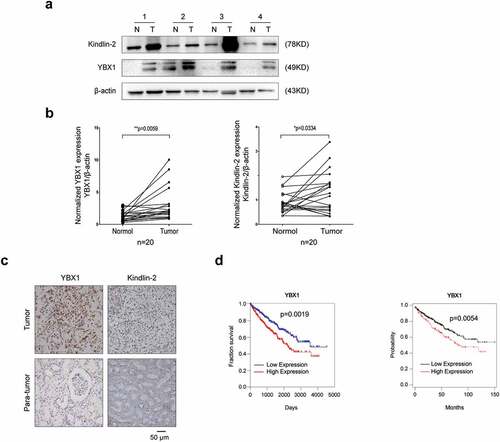
YBX1 and Kindlin-2 expression elevates in RCC
To explore the relationship between YBX1 and Kindlin-2 of RCC, we first evaluated YBX1 and Kindlin-2 expressions in 20 freshly collected clinical RCC and paired adjacent normal kidney tissues. Western blot revealed that the expressions of YBX1 and Kindlin-2 were significantly (P < 0.05) higher in RCC tissues than in matched para-cancerous tissues ( and b). In addition, YBX1 expression positively correlated with Kindlin-2 (r = 0.572, P = 0.008) expression in RCC (). Then, we examined the expression of YBX1 and Kindlin-2 in RCC and matched para-cancerous specimens by immunohistochemical (IHC) analysis. The IHC staining images were randomly selected and demonstrated in (). We analyzed the association between YBX1 and Kindlin-2 expression and clinicopathologic parameters in RCC, including gender, age, tumor size, TNM stage, and Fuhrman grade. The results revealed that the expression levels of YBX1 in RCC patients were significantly associated with tumor size and TNM stages (). Furthermore, survival analysis revealed higher expression of YBX1 was significantly associated with a poorer prognosis in RCC (). Altogether, these findings suggest that the expression of YBX1 and Kindlin-2 is elevated, and high YBX1 expression is correlated with poor prognosis in RCC.
Table 1. Clinical relevance of YBX1 and Kindlin-2 in RCC by western blot
Table 2. Expression of YBX1 and Kindlin-2 in RCC tissues
Figure 2. YBX1 and Kindlin-2 are elevated in RCC and high YBX1 expression correlates with poor prognosis in renal cancer. (a) Relative expression of YBX1 and Kindlin-2 proteins in 20 pairs of RCC tissues (T) and adjacent normal tissues (N) using western blot assay. Upper panel: representative four pairs of western blot images. Lower panel: the expressions of YBX1 and Kindlin-2 was quantified by normalizing with β-actin. The analysis was used paired t-test, P = 0.0059, P = 0.0334, respectively, n = 20. Representative images of immunohistochemical staining for YBX1 and Kindlin-2 are shown in (b). Scale bar = 50 μm. (c) High YBX1 expression correlates with poor prognosis in RCC patients. (http://www.oncolnc.org/, https://kmplot.com/analysis/) Statistically significant differences were indicated: *, P < 0.05, **, P < 0.01
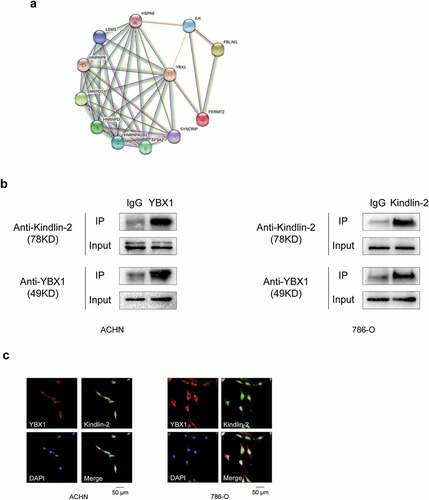
YBX1 physically interacts with Kindlin-2 in RCC
We determined that YBX1 protein was predicted to interact with Kindlin-2 protein using the STRING database (). To validate this interaction, total proteins from ACHN cells were extracted and co-immunoprecipitated with antibodies to detect the endogenous proteins. The presence of Kindlin-2 in the endogenous YBX1 complex confirmed the interaction between YBX1 and Kindlin-2 (). We next sought to clarify the cellular distribution of the two proteins. Immunofluorescent staining showed the colocalization of endogenous YBX1 (red) and Kindlin-2 (green) in RCC ACHN and 786-O cells (). Collectively, these findings confirmed that YBX1 physically interacts with Kindlin-2.
Figure 3. YBX1 physically interacts with Kindlin-2 in RCC (a) An interaction network of YBX1 protein with other proteins was generated using the STRING online database (http://string-db.org/) (b) ACHN and 786-O cell lysates were mixed with an anti-YBX1 antibody or anti-Kindlin-2 antibody or IgG antibody. YBX1, Kindlin-2 and control immunoprecipitates were analyzed by western blot with anti-Kindlin-2 and anti-YBX1 antibodies. IP, immunoprecipitation. (c) ACHN and 786-O cells were subjected to immunofluorescence assays. The cellular localization of YBX1 and Kindlin-2 was assessed by confocal laser scanning microscopy. Scale bar = 50 μm
4. YBX1 regulates intracellular ROS to modulate apoptosis in RCC
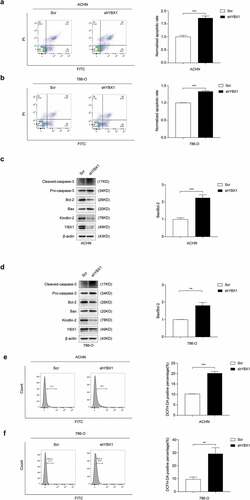
Mitochondrial damage is tightly linked to mitochondrial apoptosis that occurs, at least in part, through mitochondrial ROS release, mitochondrial pro-apoptotic factor leakage, and caspase family activation [Citation39]. Previous studies indicate that Kindlin-2 is partially localized in mitochondria and plays an essential role in apoptosis and ROS production [Citation38]. We used flow cytometry assay to test the function of YBX1 in RCC cell apoptosis regulation. Results revealed that knockdown of YBX1 increased the number of apoptotic cells in ACHN and 786-O cell lines ( and b). Bax, Bcl-2, and caspase-3 are reported to be mainly involved in the mitochondrial apoptotic pathway. Bcl-2 is an anti-apoptotic factor, while Bax enhances apoptosis, so the increased ratio of Bax/Bcl-2 promotes apoptosis. We found that knockdown of YBX1 increased Bax/Bcl-2 ratio and promoted caspase-3 activity in RCC cells ( and d). To investigate whether ROS plays a pivotal role in YBX1-regulated apoptosis, DCFH-DA, a fluorescent probe, was used to detect cellular ROS levels. The levels of intracellular ROS markedly increased after the knockdown of YBX1 ( and f). These results indicate that YBX1 modulates ROS production and apoptosis of RCC cells.
Figure 4. YBX1 regulates intracellular ROS to affect apoptosis in RCC. In ACHN and 786-O cells, apoptotic cells were labeled by Annexin V/PI staining and quantitatively assessed by flow cytometer (a and b). The expression levels of YBX1, Kindlin-2, Bax, Bcl-2, Pro-caspase-3, and cleaved caspase-3 were detected by western blot assay in ACHN and 786-O cells with controls and YBX1 knockdown (c and d). In ACHN and 786-O cells, ROS levels were detected with DCFH-DA and flow cytometry (e and f). Data were presented as mean ± SD by t-test. Statistically significant differences were indicated: **, P < 0.01, ***, P < 0.001
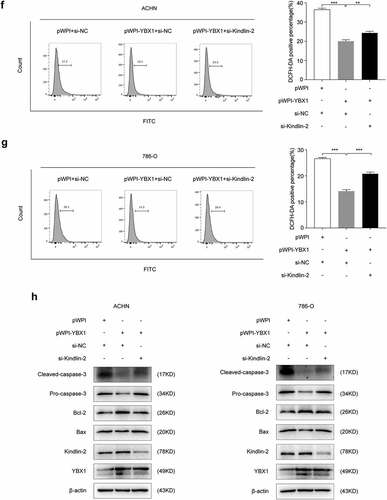
5. YBX1 regulates apoptosis and ROS generation via Kindlin-2 in RCC
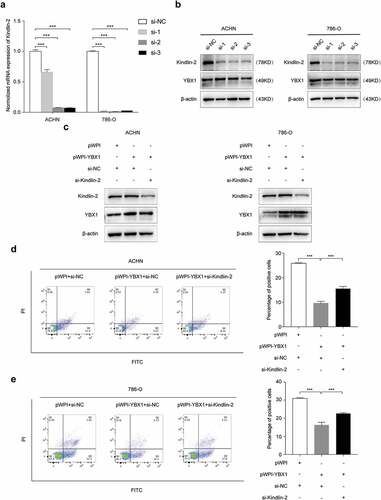
We verified the role of Kindlin-2 on YBX1 regulated cell apoptosis and ROS generation in ACHN and 786-O cells. As showed in ( and b), we selected the most effective siRNAs (si-Kindlin-2-1, si-Kindlin-2-2, si-Kindlin-2-3) against Kindlin-2 expression in RCC cells (). Our results showed that YBX1 overexpression significantly decreaseed cell apoptosis and ROS generation in RCC ACHN and 786-O cells. In meanwhile, Kindlin-2 depletion strongly attenuated the effect of YBX1 induced cell apoptosis and ROS generation (, e, f, and g). We also determined the expressions of Bax, Bcl-2, and caspase-3 proteins were associated with cell apoptosis (). These data showed that YBX1 promoted RCC cells apoptosis, while Kindlin-2 partially restored the effect of YBX1 on apoptosis.
Figure 5. YBX1 regulates apoptosis and ROS in RCC cells via Kindlin-2. The interference efficiency of Kindlin-2 siRNAs (si-Kindlin-2-1, si-Kindlin-2-2, si-Kindlin-2-3) were detected by both quantitative real time-PCR (a) and western blot analysis (b). ACHN and 786-O cells were transfected with pWPI + si-NC, pWPI-YBX1 + si-NC, pWPI-YBX1 + si-Kindlin-2 (c), and then cell apoptosis (d and e) and ROS (f and g) were examined by flow cytometry, and the expression levels of YBX1, Kindlin-2, Bax, Bcl2, Pro-caspase-3, and cleaved caspase-3 were detected by western blot assay (h). β-actin was used as an internal control. Data were presented as mean ± SD by t-test. Statistically significant differences were indicated: **, P < 0.01, ***, P < 0.001
Discussion
At present, RCC is one of the most malignant cancers due to a lack of effecient clinical treatment and diagnostic biomarkers [Citation40]. Approximately 25%–30% of RCC patients present with advanced-stage disease at first diagnosis and 30% of localized RCC patients will develop recurrence and metastasis after surgical operation [Citation41]. Although current treatments benefit RCC patients a lot, the survival rate of metastatic RCC is still low. Thus, the need to find causal molecular events to help develop precise treatment in RCC is urgent. In the present study, it demonstrated that YBX1 was upregulated and associated with tumor size, TNM stages, and poor prognosis in RCC patients. Specifically, depletion of YBX1 markedly reduced the Kindlin-2 expression and increased ROS production and apoptosis. Depletion of Kindlin-2 reversed the decreased ROS production and apoptosis caused by YBX1 overexpression, illustrating the importance of Kindlin-2 in YBX1-mediated regulation of ROS production and apoptosis. So, YBX1 interacts with Kindlin-2 to regulate ROS generation and apoptosis and subsequently promoting RCC development.
YBX1 is a transcription factor that is primarily cytoplasmic, but it shuttles to the nucleus during noxious stimuli such as UV irradiation, hyperthermia, and chemotherapeutic drugs [Citation17,Citation42]. YBX1 impact “hallmarks of cancer” including tumorigenesis, cell proliferation, replicative immortality, angiogenesis, invasion, and metastasis [Citation43,Citation44]. The overexpression of YBX1 is related to the progression and prognosis of tumors, including stromal tumors, pancreatic cancer, colon cancer, and so on [Citation45,Citation46]. Previously published studies established that YBX1 was increased and correlated with metastasis, and poor prognosis in RCC patients [Citation47]. YBX1 suppression inhibited RCC cells’ invasion ability in vitro. Moreover, YBX1 is also associated with chemoresistence [Citation48]. YBX1 increased expression in sunitinib-resistant metastatic clear cell renal cell carcinoma (mccRCC). Our previous studies on RCC have shown that the elevated nuclear YBX1 expression is closely related to the tumor growth and aggressive cancer phenotype, leading to poor prognosis of RCC patients [Citation49–51]. YBX1 exerts its function through ITGB8/TGF-β signaling and eventually contributes to RCC cell adhesion [Citation52]. We have demonstrated that knockdown of YBX1 significantly inhibited the adhesion, migration, and invasion abilities of RCC cells. Specifically, the interaction of YBX1 with G3BP1 promotes RCC metastasis through YBX1/G3BP1-SPP1-NF-κB signaling axis [Citation19]. And the nuclear expression of YBX1 could be used as independent prognostic makers for cancer progression in the RCC patients. In this study, pathway enrichment analysis revealed that Kindlin-2 is involved in the YBX1-regulated EMT. The balance between apoptosis and EMT is critical to embryonic development, organogenesis, tissue repair, and regenerative processes. From the view of tumor transformation, the apoptotic effect has often been termed a tumor-suppressing factor, while EMT has been known as a tumor-promoting factor [Citation53]. In the present study, we discover that YBX1 regulates RCC cells ROS generation and cell apoptosis through Kindlin-2. So, YBX1 plays a vital role in RCC cells apoptosis and EMT and is critical for RCC progression.
Kindlin-2, a member of the focal adhesion protein family, is involved in the activation of the integrin signaling pathway that plays an important role in regulating cancer cell invasion. Kindlin-2 has been found to play a role in embryonic development, cardiac development, and even cancer [Citation54]. And Kindlin-2 is involved in multiple diseases, including kidney fibrosis and tumor progression [Citation55]. Previous studies have shown that patients at a late stage of ccRCC (stages III or IV) were more likely to have high Kindlin-2 expression levels than those at an early stage (stages I or II). Furthermore, patients with high Kindlin-2 expression levels had a higher risk of hematogenous metastasis than those with low Kindlin-2 expression levels. A large proportion of ccRCC patients had high levels of Kindlin-2 expression, which was associated with advanced tumor stages and hematogenous metastasis [Citation56]. However, in this study no correlation was seen between Kindlin-2 expression and tumor size or TMN stage. The relatively small sample size may limit the statistical relevance of the conclusions. Kindlin-2 is present not only in focal adhesion but also in the mitochondria, where it forms a complex with PYCR1 and regulates the level and function of PYCR1. Specifically, Kindlin-2 depletion significantly increases ROS production and apoptosis and inhibits cell proliferation [Citation38]. One of the hallmarks of cancer is the ability of tumor cells to resist cell death, and cancer cells engage various mechanisms to evade apoptosis. The investigation demonstrates that the intracellular redox state is a central determinant of cell fate [Citation57]. Mitochondria is crucial for the maintenance of the redox cellular state and apoptosis. Intracellular redox balance depends on the cellular ROS levels and antioxidant ability, and that ROS levels are strictly controlled in cells to achieve cellular redox homeostasis [Citation58]. ROS plays a significant function in the regulation of apoptosis by inducing caspase activation. Among all the caspase members, caspase-3 is an essential apoptotic effector leading to cytoskeletal breakdown, nuclear demise, and other cell changes associated with apoptosis [Citation59]. Bax and Bcl-2 drives the mitochondrial apoptotic pathway and locates on the outer membrane of mitochondria and belongs to the pro-apoptotic Bcl-2 family and anti-apoptotic Bcl-2 family, separately [Citation60–62]. In our study, we found that YBX1 regulates ROS generation, Bax/Bcl-2 expression, and caspase-3 activity in RCC cells via Kindlin-2, subsequently modulates cell apoptosis and RCC progression. YBX1 has vital roles in both cytoplasm and nucleus. A variety of stimuli are able to induce YBX1 cytoplasm-to-nucleus translocation. Our immunofluorescence staining results demonstrated that YBX1 and Kindlin-2 were expressed both in the cytoplasm and nucleus. Positive staining of Kindlin-2 was in the nucleus, which may be associated with its role in the TGFB1 and integrin signaling pathways [Citation56]. Therefore, we hypothesized that the YBX1/Kindlin-2 interaction occured in both the nucleus and the cytoplasm. On the other hand, there is a possibility part of the YBX1/Kindlin-2 complex translocates to the nucleus. More in-depth explorations to address these notions by determining where and how YBX1 and Kindlin-2 interact (in the cytoplasm, nucleus, and mitochondria) are warranted. We will explore the exact mechanism including the mechanism YBX1/Kindlin-2 regulation of cell apoptosis in future studies.
In summary, our study indicates that YBX1 overexpression is associated with poor patient survival in RCC and YBX1 regulates apoptosis and ROS in RCC cells via Kindlin-2. Future studies are needed to find the specific mechanisms by which YBX1 modulates the RCC cells apoptosis.
Financial support:
This work was supported by the National Natural Science Foundation of China [grant numbers 81,772,945, 81,872,078, and 21,974,094], the Natural Science Foundation of Tianjin [grant number 18JCYBJC26700 and 18JCYBJC25200], the Young Elite Scientists Sponsorship Program [grant number TJSQNTJ-2017-10], and National Training Program of innovation and Entrepreneurship for undergraduates [grant number 202110062007].
Supplemental Material
Download Zip (30.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
Notes on contributors
Dan Yue
Yong Wang, and Dan Yue conceived the study and designed the experiments. Qiqi Cui, and Chao Wang performed in vitro experiments; Qiqi Cui analyzed data; Qiqi Cui, Chao Wang, Shuang Liu, Runxuan Du, and Shaoping Tian performed immunohistochemical staining; Qiqi Cui, and Chao Wang organized clinical samples; Qiqi Cui, Yong Wang, and Dan Yue wrote the manuscript. Dan Yue, Yong Wang, Ruibing Chen, Hua Geng, Saravanan Subramanian and Yuanjie Niu modified the manuscript.
References
- Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30.
- Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol. 2018;JCO2018791905. DOI:10.1200/JCO.2018.79.1905
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257.
- Whelan P. The medical treatment of metastatic renal cell cancer. EAU Update Series. 2003;1(4):237–246.
- Gupta K, Miller JD, Li JZ, et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205.
- Prabhu L, Hartley, AV, Martin, M, et al. Role of post-translational modification of the Y box binding protein 1 in human cancers. Genes Dis. 2015;2(3)(:240–246
- Eliseeva IA, Kim ER, Guryanov SG, et al. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry (Mosc). 2011;76:1402–1433.
- El-Naggar AM, Veinotte C, Cheng H, et al. Translational activation of HIF1alpha by YB-1 promotes sarcoma metastasis. Cancer Cell. 2015;27(5):682–697.
- Kuwano M, Shibata T, Watari K, et al. Y-box binding protein-1 as an effective therapeutic target in drug-resistant cancer. Cancer Sci. 2019;110(5):1536–1543.
- Dahl E, En-Nia A, Wiesmann F, et al. Nuclear detection of Y-box protein-1 (YB-1) closely associates with progesterone receptor negativity and is a strong adverse survival factor in human breast cancer. BMC Cancer. 2009;9(410). DOI:10.1186/1471-2407-9-410
- Fujii T, Kawahara A, Basaki Y, et al. Expression of HER2 and estrogen receptor alpha depends upon nuclear localization of Y-box binding protein-1 in human breast cancers. Cancer Res. 2008;68(1504–1512):1504–1512.
- Shibahara K, Sugio, K, Osaki, T, et al. Nuclear expression of the Y-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin Cancer Res. 2001;7:3151–3155.
- Gessner C, Woischwill C, Schumacher A, et al. Nuclear YB-1 expression as a negative prognostic marker in nonsmall cell lung cancer. Eur Respir J. 2004;23(1):14–19.
- Chatterjee M, Rancso C, Stühmer T, et al. The Y-box binding protein YB-1 is associated with progressive disease and mediates survival and drug resistance in multiple myeloma. Blood. 2008;111(7):3714–3722.
- Oda Y, Sakamoto, A, Shinohara, N, et al. Nuclear expression of YB-1 protein correlates with P-glycoprotein expression in human osteosarcoma. Clin Cancer Res. 1998;4:2273–2277.
- Oda Y, Ohishi Y, Saito T, et al. Nuclear expression of Y-box-binding protein-1 correlates with P-glycoprotein and topoisomerase II alpha expression, and with poor prognosis in synovial sarcoma. J Pathol. 2003;199(2):251–258.
- Gimenez-Bonafe P, Fedoruk MN, Whitmore TG, et al. YB-1 is upregulated during prostate cancer tumor progression and increases P-glycoprotein activity. Prostate. 2004;59(3):337–349.
- Basaki Y, Hosoi F, Oda Y, et al. Akt-dependent nuclear localization of Y-box-binding protein 1 in acquisition of malignant characteristics by human ovarian cancer cells. Oncogene. 2007;26(19):2736–2746.
- Wang Y, Su, J, Wang, Y, et al. The interaction of YBX1 with G3BP1 promotes renal cell carcinoma cell metastasis via YBX1/G3BP1-SPP1- NF-kappaB signaling axis. Journal of experimental & clinical cancer research. 2019: 38: 386.
- Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14(8):503–517.
- Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9(12):1203–1208.
- Rognoni E, Ruppert R, Fassler R. The kindlin family: functions, signaling properties and implications for human disease. J Cell Sci. 2016;129(1):17–27.
- Yu J, Hu Y, Gao Y, et al. Kindlin-2 regulates hepatic stellate cells activation and liver fibrogenesis. Cell Death Discov. 2018;4(1). DOI:10.1038/s41420-018-0095-9
- Tu Y, Wu S, Shi X, et al. Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113(1):37–47.
- Burridge K. Focal adhesions: a personal perspective on a half century of progress. FEBS J. 2017;284(20):3355–3361.
- Yu Y, Wu J, Wang Y, et al. Kindlin 2 forms a transcriptional complex with beta-catenin and TCF4 to enhance Wnt signalling. EMBO Rep. 2012;13(8):750–758.
- Yu Y, Wu J, Guan L, et al. Kindlin 2 promotes breast cancer invasion via epigenetic silencing of the microRNA200 gene family. Int J Cancer. 2013;133(6):1368–1379.
- Zhang H-F, Zhang K, Liao L-D, et al. miR-200b suppresses invasiveness and modulates the cytoskeletal and adhesive machinery in esophageal squamous cell carcinoma cells via targeting Kindlin-2. Carcinogenesis. 2014;35(2):292–301.
- Zhan J, Song J, Wang P, et al. Kindlin-2 induced by TGF-beta signaling promotes pancreatic ductal adenocarcinoma progression through downregulation of transcriptional factor HOXB9. Cancer Lett. 2015;361(1):75–85.
- An Z, Dobra K, Lock JG, et al. Kindlin-2 is expressed in malignant mesothelioma and is required for tumor cell adhesion and migration. Int J Cancer. 2010;127(9):1999–2008.
- Zhan J, Zhu X, Guo Y, et al. Opposite role of Kindlin-1 and Kindlin-2 in lung cancers. PloS One. 2012;7(11):e50313.
- Talaat S, Somji S, Toni C, et al. Kindlin-2 expression in arsenite- and cadmium-transformed bladder cancer cell lines and in archival specimens of human bladder cancer. Urology. 2011;77(6):e1501–1507.
- Ou Y, Zhao Z, Zhang W, et al. Kindlin-2 interacts with β-catenin and YB-1 to enhance EGFR transcription during glioma progression. Oncotarget. 2016;7(46):74872–74885.
- Yan M, Zhang L, Wu Y, et al. Increased expression of kindlin‐2 is correlated with hematogenous metastasis and poor prognosis in patients with clear cell renal cell carcinoma. FEBS Open Bio. 2016;6(7):660–665.
- Shen Z, Ye Y, KAUTTU T, et al. The novel focal adhesion gene kindlin-2 promotes the invasion of gastric cancer cells mediated by tumor-associated macrophages. Oncol Rep. 2013;29(2):791–797.
- Shen Z, Ye Y, Dong L, et al. Kindlin-2: a novel adhesion protein related to tumor invasion, lymph node metastasis, and patient outcome in gastric cancer. Am J Surg. 2012;203(2):222–229.
- Sossey-Alaoui K, Pluskota E, Bialkowska K, et al. Kindlin-2 Regulates the Growth of Breast Cancer Tumors by Activating CSF-1-Mediated Macrophage Infiltration. Cancer Res. 2017;77(18):5129–5141.
- Guo L, Cui C, Zhang K, et al. Kindlin-2 links mechano-environment to proline synthesis and tumor growth. Nat Commun. 2019;10(1). 10.1038/s41467-019-08772-3
- Hu S, Gao Y, Zhou H, et al. New insight into mitochondrial changes in vascular endothelial cells irradiated by gamma ray. Int J Radiat Biol. 2017;93(5):470–476.
- Rodriguez-Fernandez IA, Rodríguez-Romo L, Hernandez-Barajas D, et al. Adjuvant radiation therapy after radical nephrectomy in patients with localized renal cell carcinoma: a systematic review and meta-analysis. European urology oncology. 2019; 2(4):448–455.
- Bedke J, Gauler T, Grünwald V, et al. Systemic therapy in metastatic renal cell carcinoma. World J Urol. 2017;35(2):179–188.
- Stein U, Jürchott K, Walther W, et al. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J Biol Chem. 2001;276(30):28562–28569.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- Lasham A, Print CG, Woolley AG, et al. YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem J. 2013;449(1):11–23.
- Kohno K, Izumi H, Uchiumi T, et al. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003;25(7):691–698.
- Kuwano M, Oda, Y, Izumi, H, et al. The role of nuclear Y-box binding protein 1 as a global marker in drug resistance. Mol Cancer Ther. 2004;3:1485–1492.
- Zhao X, Zhao Z, Xu W, et al. Circ-SAR1A promotes renal cell carcinoma progression through miR-382/YBX1 axis. Cancer Manag Res. 2020;12:7353–7361.
- D’Costa N, Lowerison MR, Raven PA, et al. Y-box binding protein-1 is crucial in acquired drug resistance development in metastatic clear-cell renal cell carcinoma. J Exp Clin Cancer Res. 2020;39(1):33.
- Yue D, Wang Y, Sun Y et al. C1QBP Regulates YBX1 to Suppress the Androgen Receptor (AR)-Enhanced RCC Cell Invasion. Neoplasia. 2017;19:135–144.
- Wang Y, Yue D, Xiao M, et al. C1QBP negatively regulates the activation of oncoprotein YBX1 in the renal cell carcinoma as revealed by interactomics analysis. J Proteome Res. 2015;14(2):804–813.
- Wang Y, Chen Y, Geng H, et al. Overexpression of YB1 and EZH2 are associated with cancer metastasis and poor prognosis in renal cell carcinomas. Tumour Biol. 2015;36(9):7159–7166.
- Wang Y, Su J, Fu D, et al. The role of YB1 in renal cell carcinoma cell adhesion. Int J Med Sci. 2018;15(12):1304–1311.
- Liu Y, He, K, Hu, Y, et al. YAP modulates TGF-β1-induced simultaneous apoptosis and EMT through upregulation of the EGF receptor. Sci Rep-Uk. 2017;7(45523). DOI:10.1038/srep45523
- Dowling J, Gibbs E, Russell M, et al. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res. 2008;102(4):423–431.
- Zhao T, Guan L, Yu Y, et al. Kindlin-2 promotes genome instability in breast cancer cells. Cancer Lett. 2013;330(2):208–216.
- Yan M, Zhang L, Wu Y, et al. Increased expression of kindlin-2 is correlated with hematogenous metastasis and poor prognosis in patients with clear cell renal cell carcinoma. FEBS Open Bio. 2016;6(7):660–665.
- Liu B, Tan X, Liang J, et al. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci Rep. 2014;4(1). DOI:10.1038/srep07041
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990.
- Bratton SB, Cohen GM. Apoptotic death sensor: an organelle’s alter ego? Trends Pharmacol Sci. 2001;22(6):306–315.
- Li C, Li X, Wu L, et al. Elevated AQP1 expression is associated with unfavorable oncologic outcome in patients with hilar cholangiocarcinoma. Technol Cancer Res Treat. 2017;16(4):421–427.
- Shen A, Chen H, Chen Y, et al. Pien Tze huang overcomes multidrug resistance and epithelial-mesenchymal transition in human colorectal carcinoma cells via suppression of TGF- β pathway. Evid Based Complement Alternat Med. 2014;2014(679436):1–10.
- Mallat Z, Tedgui A. Apoptosis in the vasculature: mechanisms and functional importance. Br J Pharmacol. 2000;130(5):947–962.