ABSTRACT
Human amniotic epithelial cells (hAECs), derived from an epithelial cell layer of the human amniotic membrane, possess embryonic stem-like properties and are known to maintain multilineage differentiation potential. Unfortunately, an inability to expand hAECs without significantly compromising their stem cell potency has precluded their widespread use for regenerative therapies. This article critically evaluates the methods used for isolation, expansion, and cryopreservation of hAECs. We assessed the impact of these methods on ex-vivo expansion and stem cell phenotype of hAECs. Moreover, the progress and challenges to optimize clinically suitable culture conditions for an efficient ex-vivo expansion and storage of these cells are highlighted. Additionally, we also reviewed the currently used hAECs isolation and characterization methods employed in clinical trials. Despite the developments made in the last decade, significant challenges still exist to overcome limitations of ex-vivo expansion and retention of stemness of hAECs in both xenogeneic and xenofree culture conditions. Therefore, optimization and standardization of culture conditions for robust ex-vivo maintenance of hAECs without affecting tissue regenerative properties is an absolute requirement for their successful therapeutic manipulation. This review may help the researchers to optimize the methods that support ex-vivo survival, proliferation, and self-renewal properties of the hAECs.
Abbreviations: AM: Human amniotic membrane; CM-HBSS: Ca++ and Mg++ free HBSS; DMEM: Dulbecco’s Modified Eagle Medium; DMEM-HG: DMEM-high glucose; EMEM: Eagle’s Modified Essential Medium; EMT: Epithelial-to-mesenchymal transition; EpM: Epi-life complete media; ESC: Embryonic stem cells; ESCM: Epithelial cell surface markers; hAECs: Human amniotic epithelial cells; HLA: Human leukocyte antigen; IM: Immunogenicity markers; iPSC: Induced pluripotent stem cells; KOSR; KSR: Knockout serum replacement; KSI: Key success indicators; CHM: Cell heterogeneity markers; Nanog: NANOG homeobox; Oct-4: Octamer binding transcription factor 4; OR: Operation room; P: Passage; PM: Pluripotency markers; SCM: Stem cell markers for non-differentiated cells; Sox-2: Sry-related HMG box gene 2; SSEA-4: Stage-specific embryonic antigen; TRA: Tumor rejection antigen; UC: Ultra-culture; XF: Xenogeneic free
Background
Stem cell therapy is a promising regenerative medicine approach with the potential to treat a myriad of human diseases. Many well-established types of stem cells including embryonic stem cells (ESCs), fetal stem cells (FSCs), adult stem cells (ASCs), and induced pluripotent stem cells (iPSCs) exhibit remarkable tissue regeneration properties, but major impediments exist to widespread their clinical uses [Citation1,Citation2]. The main challenges include ethical consideration in the case of ESCs, the tumorigenic potential of iPSCs [Citation3], and the limited ex-vivo expansion of ASCs [Citation1]. To overcome these limitations, the therapeutic uses of stem cells isolated from the human amniotic membrane (AM) of discarded placenta have emerged in the last decade.
The AM is a readily available and rich reservoir of different types of stem cells. Stem cells isolated from the postpartum placenta offer many advantages including indefinite tissue supply, minimal ethical consideration, and the ease and noninvasive mode of sample collection [Citation4,Citation5]. The two major stem cell types, amniotic epithelial cells (hAECs) and amniotic mesenchymal stem cells (hAMSCs) can be isolated from the epithelial cell layer of the basement membrane and an underlying avascular mesenchymal matrix of AM, respectively [Citation6]. Both hAECs and hAMSCs differ significantly in their marker expression profile but possess exciting stem cell-like capabilities and multilineage differentiation potential [Citation1]. The hAECs are flat, cuboidal, and columnar cells formed from pluripotent placental epiblasts on the 8th day after fertilization [Citation1,Citation7]. Immunohistochemical and genetic analysis performed by Miki and colleagues show that hAECs were capable of differentiation into cells of all three germ layers, are non-tumorigenic and non-immunogenic [Citation6]. In addition, hAECs lack the expression of the major HLA molecules due to their embryonic origin and can be implanted into an immune-competent host without rejection by the host immune system [Citation8]. Unlike iPSC, hAECs do not form tumors or teratomas and possess immunomodulatory and anti-inflammatory properties offering significant advantages over other stem cell sources [Citation6]. The therapeutic potential of hAECs in the field of tissue regeneration was clinically established decades ago and is discussed in detail elsewhere [Citation4].
In addition to these advantages, the use of these cells is still restricted due to an inefficient ex-vivo expansion and the propensity of hAECs to transdifferentiate into mesenchymal stem cells (EMT) while in culture [Citation8]. In addition, carefully designed methods that meet the international criteria of good clinical lab practices (GCLP) are a definite prerequisite for their success in clinical applications. Therefore, the isolation and expansion protocols for hAECs are continuously evolving to identify optimized conditions that may address these challenges.
This review summarizes issues and solutions associated with current protocols of hAECs isolation, expansion, and cryopreservation. The technical details of the methods published until recently are compared and evaluated critically. The preclinical development of methods that meet the standards of GCLP and address the current challenges in culturing hAECs are discussed in detail. Moreover, we focused on the factors that may predefine the optimized conditions for efficient isolation and ex-vivo expansion of hAECs while retaining their stem cell phenotype.
An extensive electronic search was conducted by 2 independent reviewers using PubMed, Web of Science, Scopus database, and Google Scholar, to identify the isolation, culture, and cryopreservation methods for hAECs. The original protocols, well-documented modifications based on original protocols (based on citation score analysis), and studies that have provided cell growth kinetics and detailed characterization on freshly isolated or cultured hAECs were included in the study for the comparison. All the commercially available hAEC cell lines are listed in this document. Furthermore, we also evaluated the currently used hAECs isolation and characterization methods employed in clinical trials registered on clinicaltrials.gov and anzctr.org.au (ANZCTR). We summarized the information on techniques used to collect and harvest hAECs as well as the number of hAECs required per medical intervention obtained from these clinical trials.
Critical parameters for the isolation of hAECs
Isolation of hAECs is technically challenging due to various factors that may impact the quantity, quality, and regenerative properties of isolated cells for downstream experiments. These factors mainly include sample collection parameters, handling of the tissue, and the protocols to isolate hAECs.
Sample collection
The yield and regenerative properties of hAECs can be impacted by several sample collection parameters such as patient-to-patient variability [Citation7,Citation9], the size and quality of the placenta [Citation10,Citation11], the regional area of the placental disk [Citation12,Citation13], and the stage of pregnancy [Citation14–17]. Placenta from cesarean section is recommended due to sterility purposes but theoretically, hAECs can be isolated from any placenta [Citation7,Citation10,Citation18]. The effect of these parameters on cell phenotype and marker expression profile has been discussed in detail recently [Citation19].
Few reports have highlighted the variation in morphology and stemness of the hAECs due to sub-anatomical placental regions of the AM [Citation13,Citation19]. In utero, the AM is divided into the placental amnion area (includes the central area closer to the umbilical cord, the intermediate area, the peripheral area), the reflected amnion area (linked to the extra-embryonic fetal chorion leave), and the umbilical amnion (covers umbilical cord). The morphology and functional properties of hAECs differ significantly among the placental areas due to variations in mitochondrial activity and levels of reactive oxygen species (ROS) [Citation12]. Significantly higher levels of potentially damaging ROS were found in the reflected amnion while higher mitochondrial activity was noted in the placental region [Citation12]. Concomitant with the higher mitochondrial activity [Citation20,Citation21], the proliferation capacity of hAECs isolated from the placental region was found to be higher than that of the reflected region [Citation22]. Among stem cell markers, relatively homogenous expression of embryonic cell surface markers was noted in the placental amnion area and the reflected amnion area [Citation19,Citation23,Citation24]. And the expression of stem cell markers (Oct-4 and Sox-2) was highest in the reflected and peripheral area (which is part of placental amnion) as compared to other regions [Citation23]. These observations suggest the influence of regional differences on the ex-vivo expansion and regenerative potential of hAECs [Citation13]. However, further studies are required to standardize the selection of regions of the placental disk for the isolation of hAECs.
Tissue handling
As with all clinical samples, the proper collection, transportation, and handling of the placental tissue play an important role in the efficient processing of AM. Mode of cell collection (e.g. type of buffer), waiting time between delivery of the placenta and cell isolation [Citation7]: personal observation), cold ischemic time (time in refrigerator/storage after collection) [Citation11], total processing time, and contamination of other cells, all contribute significantly to the yield and quality of the isolated cells. Placenta or amnion can be stored from 3 to 24 hrs. in the refrigerator before isolation in PBS or HBSS; though, immediate processing is highly recommended to avoid compromised cell viability [Citation11,Citation25].
The timely collection of placentae (approximately 60 minutes post-delivery) and an immediate shift to a proper storage condition (e.g. storage buffer, cold ischemic time) may significantly improve the cell yield. For example, in our study following the protocol of Gramignoli et al. [Citation10], the total yield and percent cell viability increased when the waiting time in the operation room (OR) before processing was significantly reduced (unpublished results). This suggests that the quick transfer of the tissue to proper storage conditions ensures high cell yield and cell viability, as documented in other studies as well [Citation10,Citation11,Citation25].
Isolation of hAECs
Various isolation protocols are proposed in the literature and are continuously evolving to optimize xenogeneic free (XF) conditions for the derivation of cells from the AM. Herein, we compared 15 isolation protocols to assess the factors that may lead to the variations in the key success indicators (KSI) of a protocol i.e. yield, purity, viability, and molecular characterization of the isolated cells (). Standard steps of the isolation method are presented in .
Figure 1. A comparison of hAECs isolation protocols. AM, amniotic membrane; Cells (m)/g, cells (in million) per gram of tissue; cells (M)/amnion, cells (in million) per amnion; Cg, collagenase; CF, calcium-free; CHM, cell heterogeneity markers; CMF, calcium magnesium-free; CM, clinical method; ESCM, epithelial cell surface markers; GS, gentle shaking; IM, immunogenicity markers; NA, not available; P/S, penicillin/ streptomycin; PM, pluripotency markers; SCM, stem cell markers for non-differentiated cells; SW, shaking water bath; TM, traditional method; VS, vigorous shaking. *Original protocols.
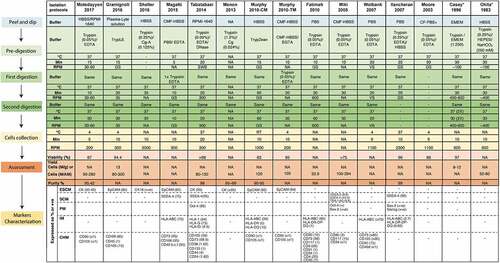
1) Peel and dip: After collection, the AM is mechanically peeled off from the placenta tissue. The AM is manually stripped from the underlying chorionic membranes starting from the center of the amnion and moving toward the umbilical cord. The peeling process should be performed with the umbilical cord facing up. The placental excision must be X-shaped, and separation should be initiated from the center of the placenta [Citation7,Citation25]. It is crucial to remove all the pieces of the chorionic membrane from the amnionic layer to avoid contamination by other cells [Citation26]. After peeling, the membrane is washed repeatedly in a pre-digestion buffer to remove blood or any other contamination and minced or cut into small pieces. Proper washing of the tissue ensures the complete removal of the blood clots and increases the efficiency of trypsin in digestions steps [Citation7]. The matte, translucent appearance of the AM assures the absence of contamination with extra connective tissue and blood, which may result in reduced yield and purity if not removed completely [Citation7]. Most studies used HBSS with antibiotics [Citation7,Citation26–29], in some cases HBSS without antibiotics [Citation9,Citation25], and occasionally only PBS [Citation30,Citation31] as pre-digestion buffers. Moore et al. [Citation31] used Ca++ free PBS plus gentamicin sulfate (known as PBS+) to maintain the intra and extracellular osmotic balance. In later studies, Ca++ and Mg++ free HBSS (CMF-HBSS) was recommended [Citation7,Citation25,Citation27,Citation32] due to the membrane adhesive properties of Ca++ and Mg++ that may hinder cellular dissociation during the isolation of cells at later stages. Some authors recommended the use of tissue culture media (such as RPMI or EMEM) in washing steps [Citation9,Citation18]. A more physiologically balanced plasma-like solution, Plasma-Lyte (Baxter), was suggested in a recent protocol [Citation10].
2) Tissue digestion: After mechanical processing of the AM, the tissue undergoes enzymatic digestions for dissociation followed by the subsequent release of epithelial cells. A significant variation exists in the enzymatic digestion process among protocols, for example, the composition of digestion buffers, the number of digestion steps, type and concentration of the trypsin, and use of other reagents (e.g. collagenases) (). In general, most protocols used 0.05–0.25% of trypsin in digestion steps leading to 80–300 M cells [Citation25,Citation31,Citation33]. The notably high yield, purity, and viability were attained by adding DNase in the digestion step in addition to 0.05% trypsin/EDTA () [Citation34]. Some recent protocols recommended a higher concentration of trypsin (1.2%) along with the addition of collagenase yielding highly positive cytokeratin (CK) cells; however, the purity of cells was highly compromised in these studies [Citation29,Citation35,Citation36]. Gramignoli et al. [Citation10] also reported that the use of collagenase results in increased cell heterogeneity due to the release of hAMSCs in the early digestion stage ().
Due to the concerns in the use of animal-based products in procuring hAECs, few studies have assessed the animal product-free Trypsin, TrypLE (recombinant trypsin obtained from bacteria) [Citation10] and TrypZean (recombinant trypsin obtained from corn) [Citation33] for the isolation of hAECs. Gramignoli et al. [Citation10] tested the efficacy of 10X vs 1X TrypLE to isolate hAECs from five amnions. The authors found that despite increasing the number of digestion steps, 1X TrypLE failed to recover any cells in two cases. Overall efficacy of 10X TrypLE in cell recovery was almost twice as compared to 1X TrypLE. Murphy et al. [Citation26] compared the effect of TrypZean (clinical method (CM)) with the traditional method (TM) [Citation7] and reported that yield was 15% higher in CM which used XF trypsin (). The XF modifications introduced in these isolation protocols did not compromise the viability, yield, and purity of the hAECs, suggesting that a comparatively good balance of yield and purity can be effectively achieved in XF isolation conditions.
The viability and phenotype of the cells can be profoundly affected by the total time of incubation in the enzymatic digestion process. Multiple or single digestion incubations varying significantly in total incubation time were performed in different protocols (). Casey et al. [Citation18] performed four successive trypsin treatments (30 min each) to ensure the complete dissociation of the epithelial cells from the tissue. The supernatant was collected from each incubation step to harvest hAECs. In contrast, single 90-min incubation was performed by Moore et al. [Citation31] lead to 10% less viability as compared to the protocol adopted by Casey et al. [Citation18]. According to Tabatabaei et al. [Citation34], more than two digestion steps had an unfavorable effect on the cell viability and purity and did not result in an increased cell yield. Magatti et al. [Citation28] modified the protocol by Miki et al. [Citation25] and reduced the incubation time from 40 min to 5 and 10 min in first and second digestion, respectively, resulting in high contamination with mesenchymal cells. Conversely, Garcia-Castro et al. [Citation37] increased the incubation time from 30 to 40 min in the first and second digestion steps yielding 80 M cells with high purity. In another comparative study [Citation26], an increase in incubation time of enzymatic digestion from 30 to 60 min did not affect viability but may have contributed to higher cell yield with 60 min incubation. Taken together, increasing the digestion incubation period may increase the cell yield but reduce cell viability and purity to some extent.
3) Cells collection: Finally, the supernatant from the different digestion steps is collected, passed through a 40–70 µm filter, and centrifuged to collect cells. Centrifugation speeds of 300–400 RPM resulted in yield and viability above 95%, incurring less damage to cells during the centrifugation process [Citation10,Citation18,Citation28]. Lower centrifugation speeds, e.g. 200 RPM also resulted in favorable cell yields [Citation7,Citation9,Citation27]. Higher speeds of more than 1000–3000 RPM are also used in several protocols [Citation26] ().
Most of the methods analyzed in this study resulted in a good balance of cell yield (8–30 x107 cells), purity (95%), and viability (>90%). Variation among methods and criteria for the assessment of these variables may also account for some of the differences seen between the protocols. It is important to standardize methods as well as criteria to assure the high quality of hAECs for the delivery of safe and effective hAEC-based tools to the medical community.
Ex-vivo expansion of hAECs
hAECs were first isolated and cultured using RPMI 1640 medium supplemented with 20% calf serum by British biologists C.K. Akle in 1981 [Citation38]. In this study, sterile placental membranes were washed, cut into strips, and cultured overnight in containing RMPI 1640 culture medium with 10% FBS and antibiotics. The monolayers of hAECs were implanted in volunteers and resulted in no identifiable tissue rejection [Citation38]. This study marked the first successful use of hAECs and laid the groundwork for the clinical use of freshly isolated, short-term cultured, and cryopreserved hAECs for many clinical and non-clinical studies [Citation18,Citation31,Citation39]. While hAECs are abundantly present in the placenta (over 100 M cells per placenta), the interest in long-term ex-vivo cell expansion and preservation has been heightened over the years to meet the demand for therapeutic interventions [Citation4,Citation6,Citation40]. However, culturing methods significantly influence not only proliferative capacity and ex-vivo survival of hAECs but also stem cells characteristics. Therefore, it is crucial to determine the physiologically relevant and clinically applicable culture conditions for uniform and reproducible expansion of hAECs without the loss of multi-differentiation potential. As identified by many studies [Citation1,Citation18,Citation25,Citation39], major cell expansion problems include a low rate of initial attachment, low colony-forming efficiency, low cell survival, early senescence, EMT, loss of stem cell markers, and differentiation potential. We present the successive improvement in the standard culture protocols to overcome these challenges (). The variations in cell culture components such as basic media, serum concentration, growth factors, cell seeding density, and other supplements were assessed in this study (). A particular emphasis was given to the specific modifications and standardization of protocols that meet the GCLP criteria.
Table 1. A comparison of hAECs culture protocols.
Initially, hAECs were cultured in standard cell culture conditions using RPMI 1640, DMEM, EMEM, or 1:1 mixture of DMEM and Ham’s F-12 (). Some authors recommended the use of high-glucose (HG) DMEM or Knock out (KO) DMEM, a basal medium optimized for the growth of undifferentiated stem cells (). Miki et al. [Citation25] was the first group to publish a detailed culture protocol and introduced the standard culture medium containing DMEM, 2 mmol/l L-glutamine,10% FBS, 1% non-essential amino acids, 1 mmol/L sodium pyruvate, antibiotics, 10 ng/mL epidermal growth factor (EGF), and 55 μm β-mercaptoethanol (BME) to culture hAECs, which is subsequently used by many researchers.
Serum concentration is one of many factors that was optimized to promote the attachment efficiency and ex-vivo survival of freshly isolated cells. Although many studies reported that the standard FBS concentration (10%) along with EGF supplementation (discussed below) is optimal for the initial attachment of hAECs [Citation18,Citation31,Citation39] but the higher FBS concentrations were tested in a few studies as well (). Both 15% [Citation29] and 20% [Citation35,Citation41] FBS produced viable hAECs that were positive for epithelial cell surface markers. No observation on cell adherence and proliferation was reported in these studies. On the other extreme, recently Xu et al. [Citation42] used 5% FBS while supplementing the media with high glucose. A monolayer of adherent cells was established within five days of culture with the expected cell growth rate at P0. However, the cell proliferation was slowed down by P4 along with the change in cell morphology from cuboidal and columnar to expanded cell bodies with large nuclei and cytoplasmic fat droplets, suggestive of cell differentiation [Citation42]. Pratama et al. [Citation43] reported a complete lack of cell growth and proliferation in absence of serum supplementation.
Concerns surrounding the use of non-human animal serum in cell culture have led to the development of XF culture systems [Citation44,Citation45]. Recent studies, therefore, have demonstrated the use of S7 supplements in Epilife media [Citation33,Citation46,Citation47], serum substitute supplement (SSS, constituted with high human serum albumin) [Citation41], or human serum (HS) [Citation46] to study the growth kinetics of hAECs. Murphy et al. [Citation33] showed that the hAECs maintained their epithelial morphology in complete Epi-life growth media (Epilife basic media plus S7); however, the growth rate was compromised significantly (). Pratama et al. [Citation46] reported a lower growth potential of hAECs in complete Epi-life media and very limited growth in HS as shown by cell number, population doubling time (PDT), and cumulative population doubling (CPD) (). In the presence of heat-inactivated HS, cells failed to grow beyond P2 () [Citation43]. Similarly, Gottipamula et al. [Citation47] also reported the cell growth in Epi-life media was significantly (p< 0.0001) slower than other serum-containing and serum-free media (). While ultra-culture (UC) media supplemented with 10% FBS and 2 mM Glutamax was found to be superior to other media as demonstrated by the highest CDP and a comparatively lower PDT [Citation47]. Evron et al. [Citation41] reported that the cells were expanded to P5 in the presence of SSS; however, a significant change in cell adherence and morphology was observed (). Large loosely attached and floating cells were found in the presence of SSS [Citation41].
It is worth noting that EGF supplementation was inevitable to successfully culture and expand hAECs. Many studies noticed a lack of cell survival and proliferation without EGF supplementation [Citation9,Citation34,Citation48] suggesting that EGF might be imperative for the ex-vivo survival and proliferation of hAECs. EGF, either in serum-containing or serum-free complete media, significantly increased the attachment efficiency, growth, and proliferation of hAECs [Citation34,Citation43,Citation49]. In initial studies of media optimization, complete adherence, and time to reach confluency of cells were reduced from 7–10 days [Citation31,Citation39] to 48 hours [Citation25,Citation43] with the supplementation of EGF (). Moreover, Ilancheran et al. [Citation48] reported that the hAEC colony formation was promoted significantly in the presence of both EGF and basic FGF than EGF alone [Citation48]. Motedayyen et al. [Citation9] successfully cultured hAECs up to passages (P) 5–6 in the presence of 10ug/ml EGF. Multiple studies show that despite the addition of EGF, hAEC transformed from cuboidal to fibroblast-like cells pointing toward EMT while in culture without EGF [Citation43,Citation47,Citation50–52].
Besides EGF, initial plating efficiency is an equally important critical factor for in-vivo survival of hAECs (). The time to reach confluency was determined by both seeding density and plating efficiency. Miki et al. [Citation7,Citation25] observed that plating efficiency rather than purity is a better indicator of successful epithelial cell culture. Furthermore, the authors found that maintaining 75% plating efficiency is more critical than EGF supplementation, therefore different cell seeding densities have been investigated in various protocols (). With a plating efficiency of 80%, cells reached confluency with 24–48 hours [Citation9,Citation25]. Additionally, collagen coating of the culture dishes is recommended by many studies to increase the cell attachment efficiency ([Citation7,Citation9,Citation10,Citation26]. Murphy et al. [Citation33] utilized a human recombinant collagen coating matrix that resulted in an improved plating efficiency.
Table 2. Commercially available human amniotic epithelial cell lines.
Overall, the culture methods published to date show promising outcomes in the ex-vivo expansion of hAECs at least until P4. However, significant challenges still exist to propagate hAECs in xenogeneic-free culture conditions. Therefore, further optimization is required to develop a standard protocol for the successful expansion of cells that comply with GCLP regulatory standards for cell manufacturing for human clinical trials.
We have recently used an alternative method for ex-vivo expansion of hAECs, which is based on the well-established and published methodology called the conditional reprogramming (CR) methodology, developed at Georgetown [Citation53–56]. This robust culture technique allows the epithelial cells to bypass replicative senescence and become “conditionally” immortal without detectable cell crisis. Our method uses a Rho kinase inhibitor (Y-7632) and J1 mouse fibroblast feeder cells to drive the indefinite proliferation of all known epithelial cell types. To humanize the hAEC-CR culturing method, we have cultured hAECs in CR medium supplemented with HS. We have already established that hAECs thrive under the CR conditions; having grown them out to over 6 passages while still expressing pluripotent stem markers and non-immunogenic markers suggesting that even late-passage hAEC-CRs retain their privileged phenotype (manuscript in preparation).
We have further looked into the availability of commercial hAEC cell lines. According to our search, only six commercially available human amniotic epithelial cell lines are available with the subculture potential of one to two passages as described in their protocol description (). However, we were unable to find any article based on research using any of the listed commercial cell lines, as searched in one of the searchable fields in large databases such as PubMed, Scopus, and Google Scholar.
Characterization of markers
Besides cell survival and proliferation, maintenance of the stem cell features of hAECs is a key element that defines the successful ex-vivo expansion of hAECs. Many studies demonstrated the transformation of hAECs from round and strongly adherent cells to flattened, fibroblast-like cells along with the loss of stem cell markers as time in culture increases, imposing the greatest challenge of ex-vivo expansion of hAECs [Citation9,Citation29,Citation34,Citation37]. Typically, the hAECs are characterized by the presence of epithelial cell surface markers (ESCM), markers of undifferentiated cells (SCM), pluripotency makers (PM), immunogenicity markers (IM), and absence of cell heterogeneity markers (CHM) such as mesenchymal and hematopoietic cell markers. Extensive literature exists describing the complete list and the expression pattern of the markers in hAECs [Citation19,Citation34]. In this review, we focused on the impact of cell isolation and culture conditions on the expression of markers in hAECs (, ).
Epithelial Cell Surface Markers (ESCM): Studies evaluated in this article mostly assessed the expression of cytokeratin (CK-18, CK-19, CK-7), E-Cadherin (E-Cad), EpCAM, and CD49f (integrin α-6). In general, freshly isolated hAECs appears to sustain the expression of ESCM such as CK and EpCAM in many protocols (). Standard culture conditions as defined by Miki et al. [Citation25] maintained the expression of the ESCM at early passages (). However, a decrease in the expression of cell surface markers in late passages was documented in many studies (). For example, expression of E-Cad and CK was much lower in P5 than P0 while EpCAM expression increased significantly upon culturing [Citation43]. Takashima et al. [Citation39] showed that CK-18 expression of freshly isolated hAECs was comparable to that of hAECs culture at days 7 and 14 in the standard culture conditions. Fatimah et al. [Citation11] reported that the sustained expression of CK-18 up to P4, while expression patterns of CK-3, CK-19, CK-1, and CK-14 varied during passaging. The varying concentration of EGF or FBS did not seem to impact the expression of cell surface markers. However, serum-free conditions impacted the expression of ESCM at later passages. At P1, there was no difference in ESCM marker expression of cells cultured in five different media () [Citation50]. Only one study compared the expression of ESCM in cells at P5 cultured in Epilife (serum-free) vs. standard culture conditions and reported a significantly lower expression of ESCM (E-Cad, EpCAM, CK, CD49f) in P5 cells maintained in Epilife media [Citation43] (). It is worth mentioning that CK-18 expression was assessed to examine the purity of cells in many studies but according to Parolini et al. [Citation1], CK-18 expression is not specific for cultured hAECs.
Pluripotency Markers (PM): The expression of the PMs; Oct-4 (octamer-binding transcription factor 4), Nanog (Nanog Homeobox protein), and Sox-2 (sex-determining region Y-box 2), is critical for self-renewal and were evaluated in different studies (). In general, PM expression was not consistent across different passages in standard culture conditions [Citation25,Citation37]. Miki et al. [Citation25] reported that Nanog and Oct-4 expression was increased over the first 12 to 15 days in high-density culture while the relative expression of Nanog was reduced by day 18, suggesting that long term culture may result in the loss of some markers. Notably in this study, Nanog and Oct-4 expression were higher in middle-layer cells than the adherent cells [Citation25]. These results indicate that spheroid cell culture conditions may be more conducive for maintaining stemness-related genes as compared to adherent cultures [Citation27,Citation57]. Yang et al. [Citation58] reported Oct-4 and Nanog positive cells in serum-free culture conditions; however, no comparison with cell cultures maintained in standard culture medium was shown in the study. Uchida et al. [Citation57] found a decrease in the expression of Nanog and Oct-4 by day 10 of 2D-culture in placental epithelial cell basal medium in the absence of any growth factors while Sox-2 expression was maintained.
Stem Cell Markers (SCM): In addition, specific cell surface markers of undifferentiated cells (TRA-1-60, TRA-1-81, SSEA-3, SSEA-4) are predominantly expressed in hAECs [Citation6]. Only a few studies reported the higher expression of these markers in freshly isolated hAECs [Citation25,Citation28,Citation34,Citation48] (). Surprisingly, a very low percentage of cells expressing SSEA-3/4 and TRA-1-60 were found in a protocol by Miki et al. [Citation25]. Overall, standard culture conditions maintained the expression of these markers across the passages as reported by various studies (). Similar to ESCM, Miki et al. [Citation25] noticed a higher expression of SSEA‐4, TRA-1‐60, and TRA-1‐81 in middle-layer cells (loosely attached cells) vs. adherent hAECs. Cells cultured in serum-free conditions were shown to express SSEA-4 at levels comparable to cells cultured in standard culture conditions; however, the expression of TRA-1-60 was low [Citation58]. The decrease in expression of SSEA-3/4 and TRA-1-60 from P0 to P2 in cells cultured in SSS supplemented medium was noted by Evron et al. [Citation41]. In contrast, the expression of SSEA-3 and TRA-1-60 were maintained across passages in standard culture conditions while SSEA-4 expression was reduced by almost 50%. SSEA-4 expression was maintained in P1-3 in cells cultured in DMEM/F12 supplemented with 10% KSR but the percentage of cells expressing TRA-1-60 was very low [Citation58]. Interestingly, high expression of SSEA-3 and SSEA-4 was also noted in hAMSCs as well [Citation2,Citation59] highlighting the need to perform a comprehensive characterization of the cells. Cell cultured in HG-DMEM with EGF stably expressed SSEA-4, Oct-4, and Nanog until p5 [Citation43].
Immunogenicity Markers (IM): The lack of expression of class I and class II antigens on hAECs make them particularly attractive for clinical applications. After the first indication of the non-immunogenic nature of these cells in human transplantation due to lack of expression of MHC-1 and beta 2-microglobulin [Citation38], various studies have investigated IM expression immediately after isolation and/or following cell expansion of hAECs (, ). Generally, Class I antigens HLA-ABC antigens are expressed at higher levels in freshly isolated hAECs except in the report by Ilancheran et al. [Citation48] (). Overall, a very low expression of class II antigens HLA-DR, HLA-DQ, and HLA-DP was seen in freshly isolated cells (, ). In a comparison of P0 vs. P5 cells that were isolated and cultured in XF conditions, the expression of HLA-ABC decreased, HLA-DQ increased while HLA-A2 and HLA-DR remained negligible [Citation33]. Yang et al. [Citation58] also reported that HLA-DR and HLA-DQ were expressed at very low levels from P0-3 in serum-free conditions. Fatimah et al. [Citation11] found the negative expression of HLA-DR and HLA-DQ was sustained up to P4 while HLA-ABC was expressed by a high proportion of freshly isolated as well as cultured cells. Interestingly, cells cultured in HG-DMEM showed negative expression of both class I and II antigens from P0 to P15 [Citation43].
Cell Heterogeneity Markers (CHM): To assess the purity of the hAECs populations, many studies used positive expression of ESCM or absence of CHM and hematopoietic cell markers (). Based on these criteria, only a few isolation protocols attained a comparatively pure population of hAECs i.e. with more than 90% of ESCM positive cells and roughly 1% contamination of CD90 or CD105 positive cells [Citation7,Citation9,Citation33]. Gramignoli et al. [Citation10] reported 85% EpCAM positive cells with less than 15% stromal cell population in hAECs. A high percentage of CHM-expressing cells were reported in other isolation protocols [Citation11,Citation30,Citation34,Citation60] (). Many studies reported a gain of CHM (CD90, CD73, CD105, VIM) upon subculturing, indicating mesenchymal transdifferentiating cells (). Under XF conditions, serial passaging up to P5 led to altered MSC markers expression, early EMT, and/or senescence [Citation33]. Notably, a higher number of CD73 expressing cells than the CD90 or CD105 expressing cells were found in freshly isolated cells [Citation33,Citation43]. Liu et al. [Citation43] found a very high expression of CD29, CD73, and CD105 (but not CD90), along with the transition to fibroblast morphology from P0 to P5 (). Interestingly, Murphy et al. [Citation33] noticed the variable expression pattern of CD90, CD73, and CD29 at different passages. No expression of the CD90 was observed at P0 and P5 while CD73 expression was decreased from 98% to 14% in cells from P0 to P5, respectively. The expression of CD29 remained constant between passages [Citation33].
Various studies reported the non-tumorigenicity of hAECs after transplantation due to the lack of telomerase expression [Citation61]. Miki et al. [Citation7] reported the absence of tumorigenicity even after 7 months of hAECs transplants in different regions of immunodeficient or Rag 2 knockdown mice. Similarly, Yang et al. [Citation58] did not observe any tumor formation at 4 weeks after transplantation of hAECs, while the mice injected with melanoma cells died.
Despite the intense efforts over the last two decades, significant challenges still exist to sustain the expression of stem cell-associated markers in hAECs, especially in XF culture conditions. Unfortunately, many reports evaluating the marker expression are not performed rigorously, and often lack sufficient validation tests to allow the generalizability of study results. In addition, inconsistencies in markers selection as well as approaches to quantifying and reporting the expression of the markers further necessitate not only the standardization of protocols but also the marker selection and reporting methods.
Cryopreservation of hAECs
A standardized long-term storage of stem cells, maintaining up to 90% post-thaw viability is of specific interest for stem cell biologists. In addition, effective hAECs preservation must maintain stem cell properties. In an effort to develop a reliable and clinically applicable media, many cryopreservation media have been investigated ().
Table 3. Comparison of cryopreservation media.
Contradictory reports exist on the effect of cryopreservation on cell viability and stem cell marker expression [Citation62–64]. In general, the post-thaw viability of amniotic cells is typically expected to be 5–10% lower vs. pre-cryopreservation viability [Citation26]. Also, compared to the freshly isolated and cultured hAECs, freeze-thawed hAECs displayed a lower proliferation rate as well as attachment efficiency [Citation10,Citation26]. In a study [Citation64], the AM was stored at −80°C before processing the tissue, the cell surface marker (CD59) was maintained on viable cells upon thawing and processing even after 6 months of cryopreservation. Contrariwise, other studies reported a lack of viable cells following freezing at −80°C [Citation62,Citation63].
The effect of various cryoprotectant agents including DMSO, FBS, amniotic fluid, glycerol in suspension, or glycerol in the presence of a natural scaffold AM were compared in a study [Citation65]. The hAECs were preserved for 12 months in different media. There were no significant differences in viability or the expression of Oct-4 between FBS or amniotic fluid (). Conversely, preserving cells in the presence of the AM significantly increased post-thaw cell yield as well as the expression of Oct-4 in restored cells [Citation65]. The comparative analysis of all the cryoprotectant agents used in the study is shown in .
Cryopreservation of hAECs in XF media such as Cryostor and Synth-a-Freeze (>90% viability) did not result in significant variations in cell viability than the regular freezing media (i.e. FBS + 10% DMSO) (). However, the 7-day post-thaw cell metabolism was highest in cells preserved in Cryostor CS5 (Cryostor media containing 5% DMSO) [Citation26]. Miki et al. [Citation49] reported no significant differences in post-thaw cell viability of cells preserved in five different commercially available cryopreservation media for two weeks (). In general, Stem Cell Banker media showed promising results in preserving hAECs post-thaw viability as well the expression of PM and SCM [Citation25,Citation58].
Cryopreservation in University of Wisconsin solution (UW; BEL GEN 1000, Lissieu, France) supplemented with 10% DMSO lead to 78% post-thaw viability [Citation66]. A high expression of ESCM and PM and negative expression of TERT and HLA-G were observed in freshly isolated and cryopreserved cells. We have introduced CRC freezing media supplemented with HS [Citation53–56,Citation67] to cryopreserve hAECs and found no effect on epithelial cell markers until P5 (unpublished data). For recovery from freezing, rapid thawing (average freezing rate of 1°C/min) is highly recommended to reduce cell exposure to DMSO [Citation10,Citation26].
hAECs in clinical studies
The first study to advocate the implantation of hAECs in patients with enzymatic deficiencies was performed in 1981 [Citation38], since then hAECs have been used for implantation in patients with lysosomal storage disease [Citation68–70]. No evidence of acute rejection and/or in vitro lymphocyte reaction was found toward the transplanted cells in these studies. However, despite the lack of immune response in all these trials, no objective improvement was observed in one trial [Citation69] while only modest improvement in the clinical and biochemical status of the patients was noted in others [Citation38]. Scaggiante et al. [Citation70] reported a significant increase in enzyme activity after transplantation of cryopreserved hAECs. These studies played a significant role in permitting hAECs research to progress from the laboratory into human clinical trials.
Currently, hAECs have been registered as an intervention for the treatment of various medical conditions (). Dose-escalation clinical studies have been registered for investigating the safety and effectiveness of hAECs for the treatment of hepatic fibrosis (ACTRN1261600043746), ischemic stroke (ACTRN1261800007627), and graft versus host disease (NCT03764228). Only one trial has been completed (ACTRN12614000174684) so far and was registered in 2014 to treat bronchopulmonary dysplasia in infants, no safety concerns have been reported to date. Other trials are investigating the route of administration of hAECs (NCT03207412, NCT03223454). Moreover, another trial (NCT04728906) submitted in 2020 seeks to treat COVID-19 to determine the capability of the hAECs in regenerating cardiomyocytes that are lost due to hypoxia.
Table 4. Clinical trials using hAECs as a method of intervention.
The sources and numbers of hAECs used in the clinical trials are shown in . A standardized source of cells was used in trials being conducted in China, i.e. Shanghai iCELL Biotechnology Co., Ltd, Shanghai, except in NCT03031509 where investigators used the protocol by Miki et al. [Citation25] for hAECs isolation. We were unable to find the isolation and/or culture protocol used by Shanghai iCELL Biotechnology Co., Ltd. Only one trial NCT04676269 in Indonesia isolated and cultured hAECs using collagenase-1 and hyaluronidase and characterized the cells for TRA-1-60, SSEA-4, Oct 3/4, and Nanog expression. In this study, the endometrial cells and hAECs were co-cultured on an amnion bilayer and stained for α-cadherin, estrogen receptor α, and progesterone receptor. The clinical trials registered at ANZCTR used the isolation protocol by Murphy et al. [Citation26,Citation33].
shows the number of hAECs used per intervention in one patient, with a range of 0.5 to 3 × 108 cells. Overall, most of the well-established isolation protocols listed in yield over 2 × 107 cells on average, satisfying the requirement of cells used in clinical trials per treatment. While the number of patients involved in each study is small, there is an optimism that as these trials progress, additional trials will be launched with higher numbers of enrolled patients. Such an advancement in the use of the hAECs technology would justify the creation of a biobanking system of hAECs isolated and cultured using a standardized approach.
Conclusion
Defining optimal isolation, culture, and cryopreservation conditions for stem cell expansion is still extremely challenging. The in vitro expansion of hAECs is one of the current objectives to expand the extensive use of this novel stem cell type in regenerative medicine. While most of the isolation methods selected for the comparison in this study generally represent a good balance of yield and purity, significant challenges exist due to lack of standardization of sample selection parameters such as region of placental disk for cell isolation, marker selection, and markers evaluation methods. Significant progress has been made to improve the culture protocols to meet clinical standards ranging from serum-containing to xenobiotic-free media i.e. serum-free or supplemented with clinical-grade human serum. Unfortunately, reliable procedures for efficient ex-vivo expansion without compromising their privileged nature remain to be established. To date, only freshly isolated cells have been used in clinical studies registered so far, highlighting the need to further standardize culture and cryopreservation methods for successful biobanking of hAECs.
Authors’ contributions
Aisha Naeem, Wanxing Cui, Chris Albanese Conceptualization, Data curation, Data curation, Investigation, Formal Methodology, Project administration, Validation, Writing – review & editing, Supervision.
Nikita Gupta, Natasha Arzoo, Usra Naeem- produced initial drafts of the manuscript, tables, and figures,
Muhammad Jawad Khan, Muhammad Umer. Validation-Editing review.
Acknowledgment(s)
Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Parolini O, Alviano F, Bagnara GP, et al. Concise Review: isolation and Characterization of Cells from Human Term Placenta: outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–311.
- Samsonraj RM, Raghunath M, Nurcombe V, et al. Concise Review: multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173–2185.
- Scheiner ZS, Talib S, Feigal EG. The potential for immunogenicity of autologous induced pluripotent stem cell-derived therapies. J Biol Chem. 2014;289(8):4571–4579.
- Zhang Q, Lai D. Application of human amniotic epithelial cells in regenerative medicine: a systematic review. Stem Cell Res Ther. 2020;11(1):1–16.
- Cui W, Khan KM, Ma X, et al. Human Amniotic Epithelial Cells and Human Amniotic Membrane as a Vehicle for Islet Cell Transplantation. Transplant Proc. 2020;52(3):982–986.
- Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133–141.
- Miki T, Marongiu F, Dorko K, et al. Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol. 2010;Chapter 2:1–10.
- Oliveira MS. Placental-derived stem cells: culture, differentiation and challenges. World J Stem Cells. 2015;7(4):769–775.
- Motedayyen H, Esmaeil N, Tajik N, et al. Method and key points for isolation of human amniotic epithelial cells with high yield, viability and purity. BMC Res Notes. 2017;10(1):552–561.
- Gramignoli R, Srinivasan RC, Kannisto K, et al. Isolation of human amnion epithelial cells according to current good manufacturing procedures. Curr Protoc Stem Cell Biol. 2016;10(10):1–13. Unit 1E
- Fatimah SS, Ng SL, Chua KH, et al. Value of human amniotic epithelial cells in tissue engineering for cornea. Hum Cell. 2010;23(4):141–151.
- Banerjee A, Weidinger A, Hofer M, et al. Different metabolic activity in placental and reflected regions of the human amniotic membrane. Placenta. 2015;36(11):1329–1332.
- Weidinger A, Poženel L, Wolbank S, et al. Sub-Regional Differences of the Human Amniotic Membrane and Their Potential Impact on Tissue Regeneration Application. Front Bioeng Biotechnol. 2021;8:613804.
- Lim R, Chan ST, Tan JL, et al. Preterm human amnion epithelial cells have limited reparative potential. Placenta. 2013;34(6):486–492.
- Izumi M, Pazin BJ, Minervini CF, et al. Quantitative comparison of stem cell marker-positive cells in fetal and term human amnion. J Reprod Immunol. 2009;81(1):39–43.
- Barboni B, Russo V, Curini V, et al. Gestational stage affects amniotic epithelial cells phenotype, methylation status, immunomodulatory and stemness properties. Stem Cell Rev Rep. 2014;10(5):725–741.
- Zhu D, Kusuma GD, Schwab R, et al. Prematurity negatively affects regenerative properties of human amniotic epithelial cells in the context of lung repair. Clin Sci. 2020;134(20):2665–2679.
- Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol Reprod. 1996;55(6):1253–1260.
- Ghamari SH, Abbasi-Kangevari M, Tayebi T, et al. The Bottlenecks in Translating Placenta-Derived Amniotic Epithelial and Mesenchymal Stromal Cells Into the Clinic: current Discrepancies in Marker Reports. Front Bioeng Biotechnol. 2020;8:180.
- Mandal S, Lindgren AG, Srivastava AS, et al. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2011;29(3):486–495.
- Banerjee A, Lindenmair A, Hennerbichler S, et al. Cellular and Site-Specific Mitochondrial Characterization of Vital Human Amniotic Membrane. Cell Transplant. 2018;27(1):3–11.
- Yoon HH, Jae-Il-Ahn LSK, Lee S-K, et al. Amniotic epithelial cells have different in vitro proliferation capacity depending on their anatomical origin. Biotechnol Bioprocess Eng. 2014;10(6):1091–1096.
- Centurione L, Passaretta F, Centurione MA, et al. Mapping of the Human Placenta: experimental Evidence of Amniotic Epithelial Cell Heterogeneity. Cell Transplant. 2018;27(1): 12–2. DOI:10.1177/0963689717725078.
- García-López G, Ávila-González D, García-Castro IL, et al. Pluripotency markers in tissue and cultivated cells in vitro of different regions of human amniotic epithelium. Exp Cell Res. 2019;375(1):31–41.
- Miki T, Lehmann T, Cai H, et al. Stem Cell Characteristics of Amniotic Epithelial Cells. Stem Cells. 2005;23(10):1549–1559.
- Murphy SV, Kidyoor A, Reid T, et al. Isolation, cryopreservation and culture of human amnion epithelial cells for clinical applications. J Visualized Exp. 2014;94:52085.
- Ferdousi F, Sasaki K, Uchida Y, et al. Exploring the potential role of rosmarinic acid in neuronal differentiation of human amnion epithelial cells by microarray gene expression profiling. Front Neurosci. 2019;13:779.
- Magatti M, Caruso M, De Munari S, et al. Human amniotic membrane-derived mesenchymal and epithelial cells exert different effects on monocyte-derived dendritic cell differentiation and function. Cell Transplant. 2015;24(9):1733–1752.
- Sheller S, Papaconstantinou J, Urrabaz-Garza R, et al. Amnion-epithelial-cell-derived exosomes demonstrate physiologic state of cell under oxidative stress. PLoS ONE. 2016;6,11.
- Wolbank S, Peterbauer A, Fahrner M, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13(6):1173–1183.
- Moore RM, Silver RJ, Moore JJ. Physiological apoptotic agents have different effects upon human amnion epithelial and mesenchymal cells. Placenta. 2003;24(2–3):173–180.
- Okita JR, Sagawa N, Casey ML, et al. A comparison of human amnion tissue and amnion cells in primary culture by morphological and biochemical criteria. Vitro. 1983;9(2):117–126.
- Murphy S, Rosli S, Acharya R, et al. Amnion epithelial cell isolation and characterization for clinical use. Curr Protoc Stem Cell Biol. 2010;1:1E.6.1–1E.6.25.
- Tabatabaei M, Mosaffa N, Nikoo S, et al. Isolation and Partial Characterization of Human Amniotic Epithelial Cells: the Effect of Trypsin. Avicenna J Med Biotechnol. 2014;1(1):10–18.
- Menon R, Boldogh I, Urrabaz-Garza R, et al. Senescence of primary amniotic cells via oxidative DNA damage. PLoS ONE. 2013;8(12):e83416.
- Richardson L, Menon R. Proliferative, Migratory, and Transition Properties Reveal Metastate of Human Amnion Cells. Am J Pathol. 2018;188(9):2004–2015.
- García-Castro IL, García-López G, Ávila-González D, et al. Markers of pluripotency in human amniotic epithelial cells and their differentiation to progenitor of cortical neurons. PLoS ONE. 2015;10(12):e0146082.
- Akle CA, Welsh KI, Adinolfi M, et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003–1005.
- Takashima S, Ise H, Zhao P, et al. Human Amniotic Epithelial Cells Possess Hepatocyte-like Characteristics and Functions. Cell Struct Funct. 2004;29(3):73–84.
- Chen Y-T, Li W, Hayashida Y, et al. Human Amniotic Epithelial Cells as Novel Feeder Layers for Promoting Ex Vivo Expansion of Limbal Epithelial Progenitor Cells. Stem Cells. 2007;25(8):1995–2005.
- Evron A, Goldman S, Shalev E. Human Amniotic Epithelial Cells Cultured in Substitute Serum Medium Maintain Their Stem Cell Characteristics for Up to Four Passages. Int J Stem Cells. 2011;4(2):123–132.
- Xu Z, Liu C, Wang R, et al. A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J Nutr Biochem. 2021;88:108558.
- Liu QW, Liu QY, Li JY, et al. Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure. Stem Cell Res Ther. 2018;9(1):321–335.
- Bjare U. Serum-free cell culture. Pharmacol Ther. 1992;53(3):355–374.
- Mannello F, Tonti GA. Concise Review: no Breakthroughs for Human Mesenchymal and Embryonic Stem Cell Culture: conditioned Medium, Feeder Layer, or Feeder-Free; Medium with Fetal Calf Serum, Human Serum, or Enriched Plasma; Serum-Free, Serum Replacement Nonconditioned Medium, or Ad Hoc Formula? All That Glitters Is Not Gold! Stem Cells. 2007;25(7):1603–1609.
- Pratama G, Vaghjiani V, Tee JY, et al. Changes in Culture Expanded Human Amniotic Epithelial Cells: implications for Potential Therapeutic Applications. Connon CJ, editor. PLoS ONE. 2011;6(11):e26136.
- Gottipamula S, Sridhar KN. Large-scale isolation, expansion and characterization of human amniotic epithelial cells. Int J Stem Cells. 2018;11(1):87–95.
- Ilancheran S, Michalska A, Peh G, et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577–588.
- Miki T, Wong W, Zhou E, et al. Biological impact of xeno-free chemically defined cryopreservation medium on amniotic epithelial cells. Stem Cell Res Ther. 2016;7(1):8.
- Qiu C, Ge Z, Cui W, et al. Human amniotic epithelial stem cells: a promising seed cell for clinical applications. Int J Mol Sci. 2020;21(20):1–26.
- Konishi H, Urabe S, Teraoka Y, et al. Porphyromonas gingivalis, a cause of preterm birth in mice, induces an inflammatory response in human amnion mesenchymal cells but not epithelial cells. Placenta. 2020;99:21–26.
- Miki T. A Rational Strategy for the Use of Amniotic Epithelial Stem Cell Therapy for Liver Diseases. Stem Cells Transl Med. 2016;5(4):405–409.
- Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180(2):599–607.
- Naeem A, Dakshanamurthy S, Walthieu H, et al. Predicting new drug indications for prostate cancer: the integration of an in silico proteochemometric network pharmacology platform with patient-derived primary prostate cells. Prostate. 2020;80(14):1233–1243.
- Parasido E, Avetian GS, Naeem A, et al. The sustained induction of c-MYC drives nab-paclitaxel resistance in primary pancreatic ductal carcinoma cells. Mol Cancer Res. 2019;17(9):1815–1827.
- Yuan H, Myers S, Wang J, et al. Use of Reprogrammed Cells to Identify Therapy for Respiratory Papillomatosis. N Engl J Med. 2012;367(13):1220–1227.
- Uchida Y, Ferdousi F, Zheng YW, et al. Global Gene Expression Profiling Reveals Isorhamnetin Induces Hepatic-Lineage Specific Differentiation in Human Amniotic Epithelial Cells. Front Cell Dev Biol. 2020;8:1–17.
- Yang P-J, Yuan W-X, Liu J, et al. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation [Internet]. Acta Pharmacologica Sinica. 2018,39(8), 1305-1316. Chicago. Available from: www.chinaphar.com.
- Koike C, Zhou K, Takeda Y, et al. Characterization of amniotic stem cells. Cell Reprogram. 2014;16(4):298–305.
- Magatti M, Pianta S, Silini A, et al. Isolation, culture, and phenotypic characterization of mesenchymal stromal cells from the amniotic membrane of the human term placenta. Methods in Molecular Biology. Humana Press Inc 2016; 233–244
- Ochsenbein-Kölble N, Bilic G, Hall H, et al. Inducing proliferation of human amnion epithelial and mesenchymal cells for prospective engineering of membrane repair. J Perinat Med. 2003;31(4):287–294.
- de O PJD, de Melo GB, Jáp G, et al. Ultrastructural and growth factor analysis of amniotic membrane preserved by different methods for ocular surgery. Arq Bras Oftalmol. 2007;70(5):756–762.
- Kruse FE, Joussen AM, Rohrschneider K, et al. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefe’s Arch Clin Exp Ophthalmol. 2000;238(1):68–75.
- Füst Á, Pllinger É, Stündl A, et al. Both freshly prepared and frozen-stored amniotic membrane cells express the complement inhibitor CD59. Sci World J. 2012;2012:815615.
- Niknejad H, Deihim T, Peirovi H, et al. Serum-free cryopreservation of human amniotic epithelial cells before and after isolation from their natural scaffold. Cryobiology. 2013;67(1):56–63.
- Srinivasan RC, Strom SC, Gramignoli R. Effects of Cryogenic Storage on Human Amnion Epithelial Cells. Cells. 2020;9(7):1–13.
- Tricoli L, Naeem A, Parasido E, et al. Characterization of the effects of defined, multidimensional culture conditions on conditionally reprogrammed primary human prostate cells. Oncotarget. 2018;9(2):2193–2207.
- Bembi B, Comelli M, Scaggiante B, et al. Treatment of sphingomyelinase deficiency by repeated implantations of amniotic epithelial cells. Am J Med Genet A. 1992;44(4):527–533.
- Yeager AM, Singer HS, Buck JR, et al. A therapeutic trial of amniotic epithelial cell implantation in patients with lysosomal storage diseases. Am J Med Genet A. 1985;22(2):347–355.
- Scaggiante B, Pineschi A, Sustersich M, et al. Successful therapy of Niemann-Pick disease by implantation of human amniotic membrane. Transplantation. 1987;44(1):59–61.
