ABSTRACT
Stroke is the most frequent cause of disability in developed countries. A common phenomenon of stroke, cerebral ischemia, is threatening many lives worldwide. In addition, ozone treatment was previously reported to exert functions in relieving brain injury. In the current study, the therapeutic effects of ozone on cerebral ischemia are investigated. A rat model of middle cerebral artery occlusion (MCAO) was established. The brain water content was calculated by weighing brain tissues, and the 2, 3, 5-triphenyltetrazolium chloride staining was performed to measure brain infarction volume in rats. A colorimetric assay was conducted to examine expression levels of malondialdehyde, superoxide dismutase, catalase, and glutathione in the rat hippocampus. Reverse transcription quantitative polymerase-chain reaction and Western blot analyses were employed to evaluate expression levels of Beclin1, LC3B, p62, and critical factors implicated in the NF-κB signaling pathway. We found that ozone significantly improved the survival rate of MCAO model rats, reduced the cerebral water content, and decreased the neurological scores of ischemic rats. Ozone markedly reduced cerebral ischemia-induced infarction in ischemic rats. Ozone decreased MDA levels and increased SOD, catalase, and GSH levels in the hippocampus of rats. Ozone significantly inhibited autophagy by decreasing Beclin1 and LC3B expression and increasing p62 expression. The ozone inactivated the NF-κB signaling pathway by decreasing the protein levels of TLR4, p-IKKβ, p-IKBα, and p-p65. We conclude that ozone treatment alleviates the brain injury in ischemic rats by suppressing autophagy and inactivating the NF-κB signaling pathway.
Introduction
Stroke is a medical condition that induces poor blood flow in the brain, which can be classified as ischemic and hemorrhagic stroke. As the most common form of stroke, ischemic stroke (also named cerebral infarction) is induced by occlusion of cerebral arteries [Citation1,Citation2]. More than 80% of stroke cases belong to ischemic stroke, which happens in the middle cerebral artery [Citation3]. Brain injury following ischemic stroke is caused by complex pathophysiological events such as oxidative stress, inflammation, apoptosis, nitrative stress, and excitotoxicity [Citation4]. Therapeutic strategies for brain injury have been investigated in recent years.
As a highly reactive compound, ozone (O3) is a triatomic molecule consisting of three oxygen atoms, serving as an oxidant. Since ozone was identified in 1840, it has been utilized in various fields such as room disinfection, teeth whitening, blood purification, and wound healing [Citation5]. Ozone generally mediates its effects by the generation of reactive oxygen species (ROS), triggering oxidative stress [Citation6]. Contradictively, the appropriate systemic administration of ozone induces an adaptive antioxidant response and further modulates oxidative stress and inflammation related to several conditions and drugs [Citation7,Citation8]. At present, ozone treatment has been demonstrated to be a promising method in the treatment of diverse diseases. Ozone treatment has been reported to decrease neuronal apoptosis and improve cognitive function in neonatal rats with hypoxic ischemic brain injury [Citation9]. Ozone therapy was found to attenuate oxidative stress in diabetes at a concentration of 0.42–0.84 mM and to relieve pain [Citation10]. The effectiveness of ozone therapy in chronic inflammatory diseases such as peripheral arterial occlusive disease and chronic heart failure has also been validated [Citation11]. Hence, we hypothesized that ozone may alleviate the ischemic stroke-induced brain injury by decreasing oxidative stress.
Malondialdehyde (MDA) is a lipid peroxidation marker and its high level denotes an elevated oxidative stress [Citation12]. Superoxide dismutase (SOD) and catalase (CAT) are enzymatic antioxidants, while glutathione (GSH) is a non-enzymatic antioxidant. SOD catalyzes the dismutation of O2·− to O2 and H2O2 and is the first line of enzymatic defense against ROS. CAT catalyzes the decomposition of H2O2 to produce water and molecular oxygen and plays a major role in protecting cells against further oxidative damage. In the present study, levels of MDA, SOD, CAT, and GSH in the hippocampal tissues of rats were detected.
Autophagy is a degradation process for unnecessary or damaged constituents in cells, which takes on significant functions in keeping the balance among cell viability, differentiation, and development [Citation13]. Beclin1 and LC3B are well-known critical molecules for autophagy, and their expression levels are positively correlated with autophagy. The autophagy adaptor protein p62 has been demonstrated to promote the selective degradation of protein cargo via autophagy, and its expression levels are negatively correlated with autophagy process [Citation14]. It has been demonstrated that stroke can be deteriorated by excessive autophagy [Citation15,Citation16]. ROS are key inducers for autophagy initiation [Citation17]. Intriguingly, autophagy is both a promoter and an “executioner” for ROS generation and cell apoptosis [Citation18–20]. Moreover, the therapeutic dosage of ozone inhibits autophagy [Citation21].
The nuclear factor-kappa B (NF-κB) signaling pathway takes on a critical role in brain development and has been indicated to be implicated in different neurological diseases [Citation22,Citation23]. NF-κB/Rel proteins are bound and suppressed by IkB proteins. After the IKK complex is activated, IκB is phosphorylated. The phosphorylation of IκB results in its ubiquitination and proteasomal degradation, releasing NF-κB/Rel complexes. Thus, NF-κB, a transcription factor, is released and promotes the expression of cytokines, cell adhesion molecules, and antiapoptotic proteins. In addition, toll-like receptor 4 (TLR4) and p65 exert significant functions in brain injury following ischemic stroke [Citation24]. It has been demonstrated that the IKK2/NF-κB signaling pathway is sufficient to protect neurons from traumatic brain injury [Citation25]. The inactivation of the NF-κB signaling pathway inhibits cognitive impairment and apoptosis of hippocampal neurons by inhibiting RCAN1 in hypoxic-ischemic rats [Citation26]. Quercetin alleviates brain injury in hypoxic-ischemic rats by suppressing the TLR4/NF-κB signaling pathway [Citation27].
In the current study, we investigated the effects and the underlying molecular mechanism of ozone on ischemia-induced brain injury utilizing a well-established middle cerebral artery occlusion (MCAO) rat model. This study may provide a novel insight into the function of ozone in ischemic stroke-induced brain injury.
Materials and methods
Animals
A total of 48 specific pathogen-free Sprague-Dawley rats weighing 250–300 g were purchased from the Institute of Zoology, Chinese Academy of Sciences. These rats were maintained in standardized environmental conditions (a 12-hour light/dark cycle at 25 ± 2°C) and were fed a commercial diet (CLEA, Shizuoka, Japan) and water ad libitum. This study was performed based on the guidance of Care and Use of Laboratory Animals of National Institute of Health, and was approved by the Ethics Committee of the Affiliated Changzhou NO. 2 People’s Hospital of Nanjing Medical University (Jiangsu, China). The rats were divided into four groups at random: MCAO group (n = 12), MCAO+ozone group (n = 12), sham group (n = 12), healthy control group (n = 12). Rats in the healthy control group received no treatment. Rats in the sham group received the similar procedures as those in the MCAO group excepting middle cerebral artery occlusion. Rats in the MCAO+ozone group received the MCAO surgery and were intraperitoneally injected with 2 mg/kg of ozone.
Middle cerebral artery occlusion (MCAO) rat model
Two days before the operation, the rats were dorsally depilated. After the intraperitoneal injection of 10% chloral hydrate (3 mL/kg), rats were anesthetized and received MCAO. The normal body temperature of the rats was maintained at 37°C with a heating mat under them. After the skin was sterilized, the rats were treated with a cervical median incision. The right side of the common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were isolated using forceps. The ECA was tied. A 0.25 mm nylon suture (Saintsun Pharma, Nanjing, China) was aseptically inserted from the right CCA to the ICA through the stump of ECA and gently advanced to occlude the middle cerebral artery. After the surgery, the rats recovered breathing and were kept in cages with food and water available. To evaluate neurological deficits in rats after stroke, neurologic tests were performed. The hallmark of a successful MCAO is that the rat showed the inability to extend the left limbs and walked in a circle to the contralateral side.
Measurement of brain water content
Whole brain tissues were instantly dissected from rats after anesthesia at the end of the animal experiments. The blood and water on the brain surface was absorbed by filter papers. Fresh brain tissues were weighed using an electronic analytical balance (Shanghai Yozoo Electronic Technology Co., Ltd.), which was recorded as wet weight. Next, the brain was baked in an electric thermostatic drier at 100°C for 48 h, and the brain weight was regarded as dry weight. The percentage of cerebral water content = (fresh brain weight – dry brain weight)/fresh brain weight × 100%.
Neurological impairment scoring
The neurological impairment score was determined by recording the behavior of the rats on day 5. The modified Neurological Severity Score test was utilized to evaluate the neurological function of rats in different groups. A scale of 0–4 grade was set and a higher score indicated a more severe brain injury. A score of 0 revealed no neurological impairment. Under a score of 1, the left limb of the rat flexed, suggesting a slight neurological impairment. When the rat walked in a circle to the left side but maintained normal at rest, a score of 2 was confirmed, indicating moderate neurological impairment. A score of 3 represented a severe symptom of neurological impairment, and correspondingly rats fell to the left side. Under a score of 4, rats showed no spontaneous activity and gradually lost consciousness, which are very severe symptoms of neurological impairment.
The 2, 3, 5-triphenyltetrazolium chloride (TTC) staining
After rats being sacrificed 5 days after MCAO, their brains were dissected. Brain tissues were sliced into five uniform 2-mm coronal sections. Next, the sections were placed into 24-well plates and incubated with 2% TTC stain in saline at 37°C for 15 minutes. After the sections were evenly stained, excess TTC was drained off. The slices were fixed with 10% neutral buffered formalin. The stained viable brain tissues were in deep red while the unstained infarcted tissues remained gray or white. Infarct volume and total brain volume were quantified with an ImageJ analysis system (NIH, Bethesda, MD, USA). Infarct volume percentage (%) = infarct volume/total brain volume × 100%.
The measurement of MDA, SOD, CAT, and GSH
To obtain rat hippocampal protein samples, the rat hippocampal tissues were minced into small pieces, and the tissues (10 mg) were homogenized with 100 μL of phosphate buffer solution. Afterward, the homogenate was centrifuged at 1000 g for 20 minutes. The supernatant was carefully preserved and stored at −80°C. The Lipid Peroxidation (MDA) Assay Kit (ab233471, Abcam), Superoxide Dismutase Activity Assay Kit (ab65354, Abcam), Glutathione Peroxidase Assay Kit (ab102530, Abcam) and Catalase Activity Kit (ab83464, Abcam) were utilized to evaluate MDA, SOD, GSH and catalase levels in the rat hippocampal tissues.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The rat hippocampal tissues were snap-frozen in liquid nitrogen. TRIzol reagent (1 mL; Invitrogen, Carlsbad, CA, USA) was applied to extract total RNA according to the manufacturer’s instructions. The extracted total RNA was reverse transcribed into cDNA utilizing the SuperScript II Reverse Transcriptase Kit (Invitrogen). RT-qPCR was performed using the SYBR Green PCR master mix (Thermo Fisher Scientific, Shanghai, China) and analyzed with the ABI 7500 instrument (Life Technology, Carlsbad, CA, USA). The 2−∆∆Ct method was utilized for analysis, and GAPDH was set up as an internal control. The primer sequences are as follows:
Beclin1
Forward: 5ʹ-CTCTCGTCAAGGCGTCACTTC-3ʹ
Reverse: 5ʹ-CCTTAGACCCCTCCATTCCTCA-3ʹ
p62
Forward: 5ʹ-TGTGTAGCGTCTGCGAGGGAAA-3ʹ
Reverse: 5ʹ-AGTGTCCGTGGTTCACCTTCCG-3ʹ
LC3B
Forward: 5ʹ-AGAGCGATACAAGGGTGAGAAG-3ʹ
Reverse: 5ʹ-AGGAGGAAGAAGGCTTGGTTAG-3ʹ
GAPDH:
Forward: 5ʹ-CTCCCATTCCRCCACCTTTG-3ʹ
Reverse: 5ʹ-CCACCACCCTGTTGCTGAG-3ʹ
Western blot analysis
The frozen rat hippocampal tissues were homogenized in the cell lysis buffer with protein inhibitors. The protein was extracted via electrophoresis using 8% sodium dodecyl sulfate-polyacrylamide gel. Next, the proteins were transferred to polyvinylidene difluoride membranes (0.45 immobilon-P; Millipore, Billerica, MA, USA). Afterward, 5% fat-free milk was applied to block the membrane. Subsequently, the membranes were incubated with primary antibodies (Abcam, Cambridge, MA, USA) in a cold-room at 4°C overnight, followed by incubation with second antibodies (Abcam). An iBind Automated Western System (Thermo Fisher) was utilized to visualize the target protein bands. The primary antibodies were anti-Beclin1 (ab210498; 1:1000), anti-p62 (ab155686; 1:1000), anti-LC3B (ab192890; 1:2000), Bcl-2 (ab194583; 1:1000), Bax (ab32503; 1:2000), cleaved caspase-3 (ab32042; 1:500), anti-TLR4 (ab13556; 1:1000), anti-p-IKKβ (ab194519; 1:500), anti-IKKβ (ab124957; 1:2000), anti-p-IKBα (ab92700; 1:1000), anti-IKBα (ab32518; 1:2000), anti-p-p65 (ab76302; 1:500), anti-p65 (ab16502; 1:2000), and anti-GAPDH (ab8245; 1:2000). GAPDH was deemed as an internal reference.
Statistical analysis
SPSS software 20.0 (IBM, Armonk, NY, USA) was utilized for statistical analysis. Data were recorded as the mean ± standard deviation. Each experiment was conducted for three technical repeats. Differences among groups were analyzed by Student’s t test and one-way analysis of variance followed by Tukey’s post hoc test. The values of p < 0.05 were considered statistically significant.
Results
Ozone alleviated the brain injury in cerebral ischemic rats
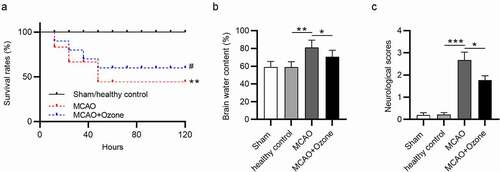
Survival rates of rats in different groups were detected within 5 days. In the sham control group and healthy control group, all rats were in good condition, and no significant changes were observed in survival rates. More than half of the rats died 40 hours later after MCAO surgery. The survival rates of rats were higher in the MCAO + ozone group compared with that in the MCAO group. Less than 50% of rats died in the MCAO + ozone group (). The brain water content of rats in four groups was measured, showing that rats treated with MCAO had the highest brain water content while ozone treatment decreased the percentage, and there were no significant changes between the sham control group and healthy control group (). Rats in the sham control group and healthy control group had no neurological deficits. The ischemic rats had a significantly increased neurological score at 2.8 and the ischemic rats administered with ozone has a decreased neurological score at 1.9 ().
Figure 1. Ozone decreased brain water content and neurological score as well as increased survival rate in MCAO model rats. (a) The survival rates of all rats in four groups: the sham group, the healthy control group, the MCAO group, the MCAO + ozone group within 5 days after MCAO surgery were measured. (b) The brain water content of all rats in the sham group, the healthy control group, the MCAO group, and the MCAO + ozone group was examined. (c) The averaged neurological score was determined according to the behaviors of rats in the sham group, the healthy control group, the MCAO group, and the MCAO + ozone group based on the modified Neurological Severity Score test. N = 12 in each group. *p < 0.05, **p < 0.01, ***p < 0.001.
Ozone decreased cerebral infarction volume in MCAO model rats
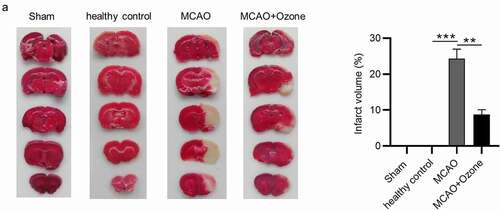
We assessed the cerebral infarction volume of all rats from four groups. Approximately 24% of pale infarcted area was identified in the total brains of ischemic rats, while ozone significantly decreased the percentage of infarcted area in total brains of ischemic rats ().
Figure 2. Ozone decreased cerebral infarction volume in MCAO model rats. (a).The cerebral infarcted area of rats in the Sham group, the healthy control group, the MCAO group and the MCAO + ozone group after TTC staining were shown. The percentage of cerebral infarcted area in total brain was also exhibited. N = 12 in each group. **p < 0.01, ***p < 0.001.
Ozone suppressed the oxidative stress in the hippocampus of ischemic rats
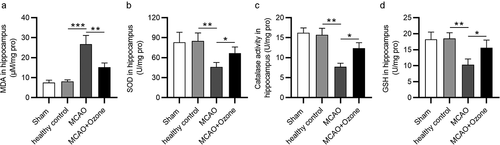
After a stroke, a high amount of ROS can cause apoptosis of hippocampal neurons. Since ROS levels are difficult to examine due to their short-life span, we examined the levels of oxidative stress markers in hippocampal tissues of the MCAO rats. Compared with the sham/healthy control group, MDA levels were markedly higher in ischemic rats. Rats in the MCAO + ozone group showed lower MDA levels than rats in the MCAO group (). Ozone reversed the significant decrease in hippocampal SOD, GSH, and catalase levels in MCAO model rats (). The results demonstrated that ozone suppressed oxidative stress in MCAO model rats.
Figure 3. Ozone reduced the ROS levels in the hippocampus of brain injury rats. (a)MDA levels in the hippocampus of rats in Sham group, healthy control group, MCAO group and MCAO + ozone group were detected by a Lipid Peroxidation (MDA) Assay Kit. (b-d) Levels of SOD, GSH and catalase in hippocampus of rats in Sham group, healthy control group, MCAO group and MCAO + ozone group were detected by corresponding Superoxide Dismutase Activity Assay Kit, Glutathione Peroxidase Assay Kit and Catalase Activity Kit. N = 12 in each group. *p < 0.05, **p < 0.01, ***p < 0.001.
Ozone inhibited the activation of autophagy induced by MCAO
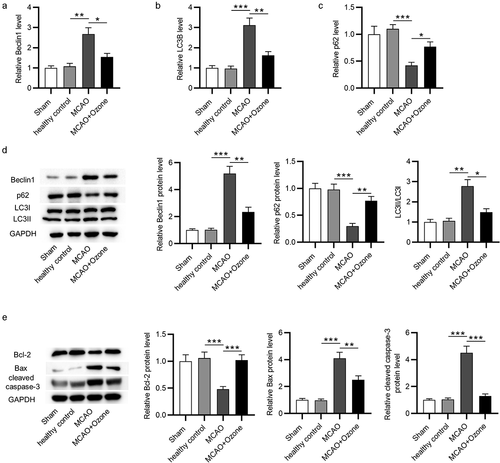
To evaluate the effects of ozone on autophagy in rats with brain injury, RT-qPCR and Western blot analyses were performed to examine the mRNA and protein expression of autophagy-associated critical factors in hippocampal tissues. According to the RT-qPCR analysis, MCAO induced the increase in Beclin1 mRNA expression and the elevation in ratio of LC3II/LC3I mRNA expression, and such effects were reversed by ozone (). In addition, ozone rescued the downregulated mRNA expression of p62 in MCAO rats (). Western blot analysis revealed that Beclin1 protein expression and the ratio of LC3II/LC3I protein expression were significantly increased, while p62 protein expression was markedly decreased in ischemic rats, and ozone reversed these changes (). Hippocampal Bcl-2 protein levels were lower, while Bax and cleaved caspase-3 protein levels were higher in ischemic rats than in sham or healthy control rats, and ozone reversed the changes in these proteins induced by MCAO (). The findings imply that the autophagy activated by MCAO is suppressed by ozone treatment.
Figure 4. Ozone inhibited the activation of autophagy induced by MCAO. (a-c) The mRNA expression levels of Beclin1, LC3B, and p62 in hippocampal tissues of rats in the sham group, the healthy control group, the MCAO group, the MCAO + ozone group were detected by RT-qPCR analysis. (d) Western blotting images of Beclin1, p62, LC3II, and LC3I proteins in hippocampal tissues of rats in the sham group, the healthy control group, the MCAO group, the MCAO + ozone group. Quantification of Beclin1 and p62 protein levels as well as the ratio of LC3II/LC3I protein levels was shown. N = 12 in each group. (e) Expression levels of Bax, Bcl-2, and cleaved caspase-3 proteins in hippocampal tissues of rats in the sham group, the healthy control group, the MCAO group, the MCAO + ozone group were evaluated by Western blotting. *p < 0.05, **p < 0.01, ***p < 0.001.
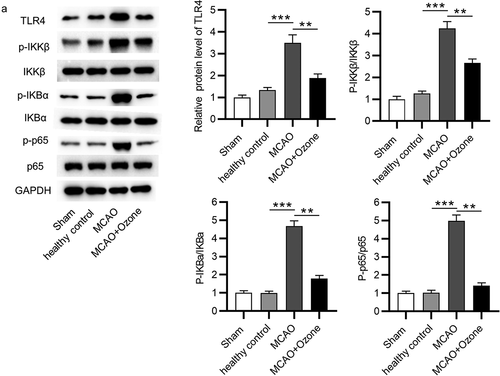
Ozone inactivated the NF-κB signaling pathway
To explore the effect of ozone on the NF-κB signaling pathway, a Western blot analysis was performed to determine both the mRNA and protein expression levels of key factors associated with the NF-κB signaling pathway. Western blot analysis revealed that ozone reversed the increase in protein expression levels of TLR4, the elevation in ratio of phosphorylated IKKβ/total IKKβ protein levels, ratio of phosphorylated IKBα/total IKBα protein levels and ratio of phosphorylated p65/p65 protein levels in MCAO model rats (). The results demonstrated that ozone inactivated the NF-κB signaling pathway.
Figure 5. Ozone inactivated the NF-κB signaling pathway. (A)Western blot analysis was utilized to detect expression levels of the NF-κB signaling pathway associated proteins including TLR4, p-IKKβ, IKKβ, p-IKBα, IKBα, p-p65, and p65 in hippocampal tissues of rats in the sham group, the healthy control group, the MCAO group, the MCAO + ozone group. N = 12 in each group. **p < 0.01, ***p < 0.001.
Discussion
Ozone is a critical oxidant gas that exerts functions in diverse aspects, such as pain alleviation [Citation28], anti-inflammation [Citation29] and immunomodulation [Citation30]. We investigated the effects of ozone in relieving brain injury in cerebral ischemic rats and the underlying mechanism. After the MCAO rat model was successfully established, the survival rates of all rats in four different groups were measured, which suggested that ozone treatment significantly improved the survival rate of MCAO model rats. According to the significantly reduced brain water content and cerebral infarction volume, as well as a significantly decreased neurological score based on behavioral observations of rats, ozone was verified to alleviate brain injury in MCAO model rats.
During the progression of ischemic stroke, oxygen, and glucose are prevented from entering brain cells, which can result in the generation of high amount of ROS from mitochondria [Citation31]. High production of ROS induces oxidative stress and the apoptosis of hippocampal neurons [Citation32]. Moreover, the ROS production is further enhanced by reperfusion, promoting the cell death induced by oxidative stress [Citation33]. Herein, we determined the influence of ozone on oxidative stress in rats treated with MCAO. Hippocampal MDA levels were higher, while SOD, GSH, and CAT levels were lower in MCAO model rats than in sham or healthy control rats. Ozone reversed the significant increase in MDA levels and the decrease in SOD, CAT, and GSH levels in hippocampal tissues of MCAO model rats, indicating that ozone alleviated the MCAO-induced oxidative stress in rats. Similar to the present study, previous studies revealed that medical ozone reduces oxidative stress in patients with multiple sclerosis [Citation34], in rats with testicular damage [Citation35] and in rabbits with organic damage [Citation36].
Autophagy has been reported to be activated or inactivated in ischemic stroke [Citation13,Citation37,Citation38]. In this study, compared with the sham or healthy control rats, MCAO model rats had higher hippocampal Beclin1 expression, higher LC3II/LC3I ratio, and lower hippocampal p62 expression, indicating that autophagy confers a harmful effect in ischemic stroke. Meanwhile, protein expression levels of apoptotic Bax and cleaved caspase-3 were significantly higher and that of anti-apoptotic Bcl-2 was significantly lower in MCAO model rats. Ozone reversed the changes in hippocampal expression profiles of these proteins caused by MCAO, showing that ozone suppressed autophagy and apoptosis in ischemic stroke. The NF-κB was activated by brain ischemia, and its activation may cause neuroinflammatory injury and neuronal apoptosis in rats treated with MCAO [Citation39,Citation40]. Our findings revealed that protein levels of TLR4 and ratios of p-IKKβ/IKKβ, p-IKBα/IKBα, p-p65/p65 were higher in hippocampal tissues of MCAO model rats, indicating the activation of the NF-κB pathway in brain ischemia, which is consistent with previous studies mentioned above. Furthermore, ozone inactivated the NF-κB signaling pathway by decreasing the expression levels of TLR4, p-IKKβ, p-IKBα, and p-p65. The suppressive effect of ozone on the NF-κB signaling pathway was supported by many other studies [Citation41–43]. Contradictively, some reports have revealed that ozone activated the NF-κB signaling pathway [Citation44,Citation45], and such contradictions might be tissue- or cell-specific and concentration-dependent. The NF-κB signaling pathway can induce oxidative stress and autophagy [Citation46,Citation47], which suggested that the inhibitory effects of ozone on oxidative stress and autophagy in hippocampal tissues of ischemic rats might be dependent on the NF-κB signaling pathway.
Overall, ozone improved the survival rates of MCAO rats and alleviated the brain injury of ischemic rats by suppressing oxidative stress and autophagy through inactivating the NF-κB signaling pathway. The present study may provide new insight of ozone application in treating ischemic stroke and improving the survival rates of patients with ischemic stroke.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn). 2017 Feb;23(1,Cerebrovascular Disease):15–39.
- Kuklina EV, Tong X, George MG, et al. Epidemiology and prevention of stroke: a worldwide perspective. Expert Rev Neurother. 2012 Feb;12(2):199–208.
- Chugh C. Acute ischemic stroke: management approach. Indian J Crit Care Med. 2019 Jun;23(Suppl 2):S140–s146.
- Khoshnam SE, Winlow W, Farzaneh M, et al. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017 Jul;38(7):1167–1186.
- Suh Y, Patel S, Kaitlyn R, et al. Clinical utility of ozone therapy in dental and oral medicine. Med Gas Res. 2019 Jul-Sep;9(3):163–167.
- Voter KZ, Whitin JC, Torres A, et al. Ozone exposure and the production of reactive oxygen species by bronchoalveolar cells in humans. Inhal Toxicol. 2001 Jun;13(6):465–483.
- Clavo B, Rodríguez-Esparragón F, Rodríguez-Abreu D, et al. Modulation of oxidative stress by ozone therapy in the prevention and treatment of chemotherapy-induced toxicity: review and prospects. Antioxidants (Basel). 2019 Nov 26;8(12). DOI:https://doi.org/10.3390/antiox8120588.
- Scassellati C, Galoforo AC, Bonvicini C, et al. Ozone: a natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res Rev. 2020 Nov;63:101138.
- Resitoglu B, Celik Y, Komur M, et al. The efficacy of ozone therapy in neonatal rats with hypoxic ischemic brain injury. Bratisl Lek Listy. 2018;119(2):81–85.
- Braidy N, Izadi M, Sureda A, et al. Therapeutic relevance of ozone therapy in degenerative diseases: focus on diabetes and spinal pain. J Cell Physiol. 2018 Apr;233(4):2705–2714.
- Bocci V, Zanardia I, Valacchi G, et al. Validity of oxygen-ozone therapy as integrated medication form in chronic inflammatory diseases. Cardiovasc Hematol Disord Drug Targets. 2015;15(2):127–138.
- Yalçınkaya E, Cakıroğlu Y, Doğer E, et al. Effect of follicular fluid NO, MDA and GSH levels on in vitro fertilization outcomes. J Turk Ger Gynecol Assoc. 2013;14(3):136–141.
- Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020 Aug;15(8):1388–1396.
- Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007 Dec 14;131(6):1149–1163.
- Wang P, Shao BZ, Deng Z, et al. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163-164:;98–117.
- Shi Q, Cheng Q, Chen C. The role of autophagy in the pathogenesis of ischemic stroke. Curr Neuropharmacol. 2020;19(5): 629–640. .
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011 Jan;36(1):30–38.
- Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011 Jul;21(7):387–392.
- Lu Y, Zhang R, Liu S, et al. ZT-25, a new vacuolar H(+)-ATPase inhibitor, induces apoptosis and protective autophagy through ROS generation in HepG2 cells. Eur J Pharmacol. 2016 Jan 15;771:130–138.
- Chen JJ, Chou CW, Chang YF, et al. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J Immunol. 2008 Jun 15;180(12):8030–8039.
- Wu MY, Xing CY, Wang JN, et al. Therapeutic dosage of ozone inhibits autophagy and apoptosis of nerve roots in a chemically induced radiculoneuritis rat model. Eur Rev Med Pharmacol Sci. 2018 Mar;22(6):1787–1797.
- Mémet S. NF-kappaB functions in the nervous system: from development to disease. Biochem Pharmacol. 2006 Oct 30;72(9):1180–1195.
- Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009 Sep;1(3):a001271.
- Qi J, Rong Y, Wang L, et al. Rab7b overexpression-ameliorated ischemic brain damage following tMCAO involves suppression of TLR4 and NF-κB p65. J Mol Neurosci. 2019 Jun;68(2):163–170.
- Mettang M, Reichel SN, Lattke M, et al. IKK2/NF-κB signaling protects neurons after traumatic brain injury. The FASEB Journal. 2018 Apr;32(4):1916–1932.
- Fang H, Li HF, Yang M, et al. NF-κB signaling pathway inhibition suppresses hippocampal neuronal apoptosis and cognitive impairment via RCAN1 in neonatal rats with hypoxic-ischemic brain damage. Cell Cycle. 2019 May;18(9):1001–1018.
- Wu M, Liu F, Guo Q. Quercetin attenuates hypoxia-ischemia induced brain injury in neonatal rats by inhibiting TLR4/NF-κB signaling pathway. Int Immunopharmacol. 2019 Sep;74:105704.
- Moreno-Fernández A, Macías-García L, Valverde-Moreno R, et al. Autohemotherapy with ozone as a possible effective treatment for Fibromyalgia. Acta Reumatol Port. 2019 Jul-Sep;44(3):244–249.
- Tartari APS, Moreira FF, Pereira M, et al. Anti-inflammatory effect of ozone therapy in an experimental model of rheumatoid arthritis. Inflammation. 2020 Jun;43(3):985–993.
- Franzini M, Valdenassi L, Ricevuti G, et al. Oxygen-ozone (O(2)-O(3)) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int Immunopharmacol. 2020 Nov;88:106879.
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005 Jun 1;38(11):1433–1444.
- Rodrigo R, Fernández-Gajardo R, Gutiérrez R, et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013 Aug;12(5):698–714.
- Orellana-Urzúa S, Rojas I, Líbano L, et al. Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des. 2020;26(34):4246–4260.
- Delgado-Roche L, Riera-Romo M, Mesta F, et al. Medical ozone promotes Nrf2 phosphorylation reducing oxidative stress and pro-inflammatory cytokines in multiple sclerosis patients. Eur J Pharmacol. 2017 Sep 15;811:148–154.
- Tusat M, Mentese A, Demir S, et al. Medical ozone therapy reduces oxidative stress and testicular damage in an experimental model of testicular torsion in rats. Int Braz J Urol. 2017 Nov-Dec;43(6):1160–1166.
- Guanche D, Zamora Z, Hernández F, et al. Effect of ozone/oxygen mixture on systemic oxidative stress and organic damage. Toxicol Mech Methods. 2010 Jan;20(1):25–30.
- Yu S, Yu M, He X, et al. KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell. 2019 Jun;18(3):e12940.
- Jiang J, Dai J, Cui H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed Pharmacother. 2018 Mar;99:583–590.
- Wu G, McBride DW, Zhang JH. Axl activation attenuates neuroinflammation by inhibiting the TLR/TRAF/NF-κB pathway after MCAO in rats. Neurobiol Dis. 2018 Feb;110:59–67.
- Yan L, Zhu T. Effects of rosuvastatin on neuronal apoptosis in cerebral ischemic stroke rats via Sirt1/NF-kappa B signaling pathway. Eur Rev Med Pharmacol Sci. 2019 Jun;23(12):5449–5455.
- Li J, Zeng T, Tang S, et al. Medical ozone induces proliferation and migration inhibition through ROS accumulation and PI3K/AKT/NF-κB suppression in human liver cancer cells in vitro. Clin Transl Oncol. 2021;23(9): 1847–1856 .
- Zeng J, Lei L, Zeng Q, et al. Ozone Therapy Attenuates NF-κB-mediated local inflammatory response and activation of Th17 cells in treatment for psoriasis. Int J Biol Sci. 2020;16(11):1833–1845.
- Simonetti V, Quagliariello V, Franzini M, et al. Ozone exerts cytoprotective and anti-inflammatory effects in cardiomyocytes and skin fibroblasts after incubation with doxorubicin. Evid Based Complement Alternat Med. 2019;2019:2169103.
- Tian L, Yan J, Li K, et al. Ozone exposure promotes pyroptosis in rat lungs via the TLR2/4-NF-κB-NLRP3 signaling pathway. Toxicology. 2021 Feb 28;450:152668.
- Fei X, Bao W, Zhang P, et al. Inhalation of progesterone inhibits chronic airway inflammation of mice exposed to ozone. Mol Immunol. 2017 May;85:174–184.
- Jastreboff PJ, Brennan JF, Coleman JK, et al. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci. 1988 Dec;102(6):811–822.
- Wang B, Mao JH, Wang BY, et al. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett. 2020 Oct 1;489:87–99.
