ABSTRACT
Rrp14 is a conserved protein that plays an important role in rRNA processing and ribosomal biogenesis. In Schizosaccharomyces pombe, the rrp14 gene is split into SPAC8C9.10 c (rrp14) and SPBC947.07 (rrp1402). Although the SPAC8C9.10 c gene is not essential for S. pombe survival, deletion of the gene causes the yeast cells to grow sick and to exhibit decreased rRNA transcription. We identified a novel Pol5 protein that physically interacts with the Rrp14 protein. Taking advantage of the Pil1 co-tethering assay, we found that Rrp14 facilitates the nucleolus translocation of Pol5, and the 7-RINAWN-12 motif of the Rrp14 protein is responsible for the interaction between Pol5 and Rrp14. Since deletion of the 7-RINAWN-12 motif affects rRNA transcription, we thus propose that Rrp14 affects rRNA transcription by facilitating the nucleolus translocation of Pol5.
Introduction
The SURF6 gene is the sixth member of the surfeit locus, which was first identified as a cluster of six genes in mice [Citation1–3]. SURF6 gene-encoding proteins are detected exclusively in the nucleolus. Although these proteins have demonstrated a high capacity for nucleic-acid binding in vitro, the Surf6 protein does not appear to contain any known nucleic-acid binding motifs. Therefore, a specific function was proposed for Surf6 family proteins, which are unrelated to any known nucleolus proteins containing nucleic-acid binding motifs [Citation4]. Fusion of the highly conserved C-terminal domain of Surf6, even from distantly related species, with green fluorescent protein (GFP) resulted in the targeting of GFP in the nucleolus in mouse cells, as detected by microscopy. However, the truncated form of the Surf6 protein lacking this domain fused with GFP could still be detected in the nucleolus, suggesting that additional nucleolus-targeting signals exist in the Surf6 protein besides those in the C-terminal conserved domain [Citation4].
In budding yeast, RRP14 is a homologue of the human SURF6 gene. The RRP14 gene-encoding protein was originally found to be associated with proteins involved in the development of cell polarity and the processing of pre-ribosomal RNA [Citation5–7]. Saccharomyces cerevisiae with RRP14 gene deletion consistently showed abnormal polarity and defects in ribosomal biogenesis [Citation8,Citation9]. Moreover, the role of the Surf6 family of proteins in ribosomal biogenesis was found to be conserved in mammalian cells. Although the detailed mechanism remains elusive, Surf6 family proteins appear to be involved in rRNA transcription, maturation, and 60S ribosomal subunit assembly [Citation7–11].
In S. pombe, however, the rrp14 gene is split into two different genes, namely SPAC8C9.10 c (rrp14) and SPBC947.07 (rrp1402), which are conserved in the N-terminal and C-terminal, respectively. SPAC8C9.10 c (rrp14) is regarded as a non-essential gene in S. pombe, making S. pombe an ideal model organism to study the function of the N-terminal rrp14. In the present study, we hypothesized that the growth defect in S. pombe caused by rrp14 gene deletion is associated with a reduction in the rRNA transcription level. We also identified Pol5, which is important for rRNA transcription and ribosome biogenesis, as a novel protein that interacts with Rrp14. Taking advantage of the Pil1 co-tethering assay developed recently (Unpublished data from Du’s Lab), we were able to map the Rrp14 motif responsible for the association with Pol5. We believe that Rrp14 affects rRNA transcription at least partially through facilitating the nucleolus translocation of Pol5.
Materials and methods
Yeast strains and mediums
The strains used in this study are listed in Supplementary Table 1. The strategy for gene deletion and tagging was based on homology recombination and polymerase chain reaction (PCR) as previously described [Citation12], and the primers and plasmids used are listed in Supplementary Table 2 and Supplementary Table 3, respectively. Colony PCR was used to verify proper integration. The standard Pombe culture medium used in this study consisted of Yeast Extract with Supplements (YES) and Edinburgh minimal media (EMM), as previously described [Citation13]. The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9) system was used to induce rrp14 mutagenesis and the strategy applied was cloning-free and gap repair-based, as described recently [Citation14]. Briefly, gapped plasmid containing the Cas9-coding sequence and the 5′ region of the ura4 marker was acquired through the linearization of pDB4280 by NotI digestion. The single guide RNA (sgRNA) insert was amplified from atemplate containing the 3′ region of the ura4 marker, the hammerhead ribozyme sequence, and the sgRNA scaffold sequence acquired with NotI-digested pDB4282. The Rrp14 sgRNA target sequence was amplified with the primers listed in Supplementary Table 2. Following transformation using the lithium acetate method [Citation15], the expression of the gap-repaired plasmid induced the cleavage sites, as indicated in Supplementary Fig 1. The exact mutations were confirmed by sequencing. The plasmids used for the Pil1 co-tethering assay were constructed and are listed in Supplementary Table 3. pDual-Pnmt41-Pil1-mCherry-target gene plasmids were integrated into the chromosomal Ars1 locus following linearization with NotI digestion, and pDual-Pnmt41-target gene-GFP plasmids were integrated into the chromosomal Ars1 locus following linearization with MluI digestion. All the strains used for the Pil1 co-tethering assay were incubated for at least 14 hrs before imaging.
Tetrad dissection
Diploid S. pombe cells were plated on sporulation agar (SPA) plates. Following sporulation, tetrad dissection was performed with the TDM50 tetrad dissection microscope (Micro Video Instruments, Avon, USA). The spores were then plated onto YES plates and incubated for 3 days at 32°C.
Spot assay
The indicated strains growing at the mid-log phase (optical density [OD] at 595 nm: 0.5–0.6) were diluted to OD595 0.1, then subjected to serial five-fold dilutions with YES medium and plated onto YES plates. The plates were incubated at 32°C and images were obtained 48 hrs later.Each spot assay was performedat least three times.
Immunoprecipitation and Western blot analysis
Approximately 100 OD595 units were collected and immediately frozen in liquid nitrogen. The S. pombe cell pellets were suspended in 1 ml of lysis buffer (50 mM of Tris pH 7.5, 150 mM of NaCl, 0.1% NP-40, 1 mM of DTT, 10% glycerol, and a protease inhibitor tablet). The cells were lysed by bead-beating for 60 s on ice, performed five times with 60 s intervals. The supernatant was separated by centrifugation at 14,000 rpm and 4°C for 20 min, subjected to a co-immunoprecipitation assay with GFP-Trap (Chromotek), and incubated at 4°C for 3 hrs. The resulting beads were washed three times with washing buffer (50 mM of Tris pH 7.5, 150 mM of NaCl, and 1% Triton X-100). The beads were then eluted in 2X sodium dodecyl sulfate (SDS) buffer (10 mM of Tris pH 7.5, 1 mM of EDTA, and 1% SDS). The samples were loaded onto a gel for SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were transferred onto a nitrocellulose membrane (Whatman, Piscataway, NJ, USA). The membrane was blocked with 5% milk for 1 hr at room temperature and blotted with GFP antibody (Roche) and mCherry antibody (Huaxingbio).
Pil1 co-tethering assay
In the fission yeast S.pombe, endogenous Pil1 forms linear eisosome filaments at the cell cortex; therefore, the recombinant Pil1-mcherry-Pol5 can be attached to the plasma membrane through filamentous anchoring. Rrp14 and different truncated forms of Rrp14, as indicated, were fused with a GFP tag at the C-terminus and co-expressed with Pil1-mcherry-Pol5, respectively. Microscopy was then used to detect the co-localization of GFP and mCherry either in the nucleolus or at the cell cortex. The co-expression of Pil1-mCherry and Rrp14-GFP was used as the control to exclude the possibility of physical interaction between these two recombinant proteins.
GFP purification coupled with affinity purification mass spectrometry (AP-MS) analysis
Protein GFP purification and MS analysis were performed as previously described. Briefly, cell lysates prepared using the bead-beating lysis method were incubated with GFP-Trap for 3 hrs. After incubation, the beads were washed with lysis buffer and then eluted by incubation with an elution buffer (1% SDS, 100 mM Tris, pH 8.0) at 65°C. Eluted proteins were precipitated with 20% trichloroacetic acid for at least 1 hr. Protein precipitates were washed three times using ice-cold acetone and then dissolved in 8 M urea and 100 mM Tris at pH 8.5, reduced with 5 mM tris(2-carboxyethyl)phosphine for 20 min, and alkylated with 10 mM iodoacetamide for 15 min in the dark. The samples were digested with trypsin overnight at 37°C. Formic acid was added to a final concentration of 5% to stop the digestion reaction. Liquid chromatography with tandem MS (LC-MS/MS) analysis was performed on an Easy-nLC II high performance liquid chromatograph (Thermo Fisher Scientific) coupled to an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific). Peptides were loaded on a pre-column (100 µM ID, 4 cm long, packed with C12, 10 µm, 120 Å resin from YMC) and separated on an analytical column (75 µM ID, 10 cm long, packed with Luna C18, 3 µm, 100 Å resin from Phenomenex) using an acetonitrile gradient from 0–28% in 100 min at a flow rate of 200 nl/min. The top eight most intense precursor ions from each full scan (resolution 60,000) were isolated for CID MS2 (normalized collision energy 35) with a dynamic exclusion time of 60 s. MS/MS fragment ions were detected with a linear ion trap in the normal scan mode. Precursors with less than 2+ or unassigned charge states were excluded. The MS/MS spectra were searched with Prolucid against an S. pombe protein database. The search results were filtered with DATASelect.
Microscopy
Cells were grown at the mid-log phase (OD 595:0.6) after incubating in EMM medium for at least 14 hrs. Afterward, 1 μl cell cultures in liquid medium were imaged under a cover-slip with a wide-field microscope. Images were captured with the Z-series and processed by iterative deconvolution in SoftWoRx (Applied Precision).To determine the effects of rrp14 mutations on Pol5 translocation, five high-power fields were randomly selected and the fluorescence intensity of Pol5-GFP at the nucleolus and the total GFP fluorescence intensity from whole cells were obtained. The averageratio of the fluorescence intensityof nucleolar pol5-GFPto the fluorescence intensity of total intracellular GFP was analyzed foreach strain. We used one-way ANOVA tests to determine the statistical significance.
Quantitative reverse transcription PCR
Three OD log-phase cells were collected and frozen in liquid nitrogen. Total RNA was extracted as previously described and RT-PCR was performed as recommended by the manufacturer. Two microliters of cDNA was used for PCR (TAKARA RR902) or qPCR(TIANGEN FP205) following DNAaseI treatment as recommended by the manufacturer, and the primers used are listed in Supplementary Table 2. The data were analyzed with GraphPad Prism 5 software. Fold induction values were calculated for each amplicon as described previously [Citation11] and the results were expressed as the mean ± standard error of the mean (SEM). The Student t-test was used to compare the values for rrp14Δ and rrp14(7–12Δ) with those for WT. Differences were considered significant at a p-value less than 0.05. The results of three qRT-PCR independent experiments were averaged.
Results
Rrp14 physically interacts with Pol5 in the nucleolus
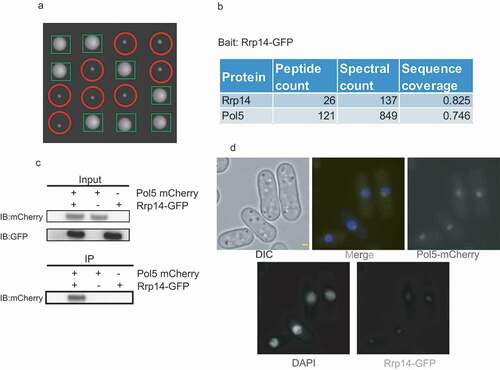
Rrp14 and Rrp1402 from S. pombe are respectively conserved in the N-terminal and C-terminal regions in S. cerevisiae, similar to Surf6 protein in humans. From this perspective, S. pombe can be an ideal model organism to study the function of the N-terminal domain of Rrp14/Surf6 under natural conditions. To explore the function of Rrp14 in the growth of S. pombe cells, we deleted one rrp14 allele in diploid S. pombe cells. The spores were then plated onto YES plates following tetrad dissection and allowed to grow for 3 days. The colony sizes of the rrp14Δ strains detected were much smaller than those of WT rrp14 (), indicating rrp14 plays an important role in the growth of S. pombe. To better characterize the function of rrp14, we decided to purify the Rrp14 protein and identify its interacting proteins. We tagged Rrp14 endogenously at the C-terminus with a GFP tag. We then used GFP-Trap agarose beads to purify Rrp14 and its associated protein from the whole cell extract (WCE) of the Rrp14-GFP Pombe strain as previously described [Citation16]. Consistent with the previously published report that Rrp14 interacted with 60S ribosomal subunits [Citation6,Citation7,Citation10], mass spectrometry (MS) analysis of purified Rrp14-GFP showed that many 60S ribosomal subunits can be co-purified with Rrp14 (Supplementary Table4). Interestingly, Pol5, a nucleolar protein reported to be important for rRNA transcription in budding yeast [Citation17], was detected at a high spectral number following Rrp14-GFP purification (). To further confirm the physical interaction between Pol5 and Rrp14, we performed a co-immunoprecipitation assay from the WCE of strains endogenously expressing Pol5-mCherry and Rrp14-GFP. Consistent with our MS analysis data, Pol5-mCherry could be co-purified with Rrp14-GFP (). We then examined the subcellular location of Pol5 and Rrp14 and found that Rrp14-GFP co-localized with Pol5-mCherry in the nucleolus, exclusively revealed by immunofluorescence microscopy (). We thus propose that Pol5 physically interacts with Rrp14 in the nucleolus.
Figure 1. a. Deletion of rrp14 caused S.pombe cells to grow significantly slower. Tetrad dissection analysis indicated that rrp14-deletion spores formed much smaller sized colonies than those of WT. Following tetrad dissection, the spores were allowed to grow on YES plates for another 3 days. The rrp14-deletion colonies (indicated by red circles) were identified based on their capacity to grow on YES plates containing hygromycin, as their colony sizes were much smaller than those of WT(indicated by green tangles). b. Pol5 was found to be associated with Rrp14 through MS analysis. c. Pol5-mCherry can be co-purified with Rrp14-GFP. Whole cell extracts of the indicated strain were incubated with GBP beads to purify Rrp14 and its associated protein. The resulting beads were then washed three times with lysis buffer. The elution from the beads after boiling was run on a gel. The mCherry signal could only be detected in the whole cell extracts of the strain containing both Rrp14-GFP and Pol5-mCherry. d. Rrp14-GFP co-localizes with Pol5-mCherry in the nucleolus, as detected under amicroscope.
Rrp14 facilitates the translocation of Pol5 into the nucleolus
To further explore the role of Rrp14 and its association with Pol5, the Pil1 co-tethering assay was performed. In this assay, we expressed recombinant Pil1-mCherry-Pol5 in S. pombe. The Pil1-mCherry region is responsible for the formation of a linear filament at the cell cortex in the recombinant protein and the fluorescence can be detected using fluorescence microscopy (). Given the physical interaction between Pol5 and Rrp14, we thought that the co-expression of Rrp14-GFP and Pil1-mCherry-Pol5 should result in the recruitment of Rrp14-GFP to the cell cortex. Strikingly, we found that Rrp14-GFP was still detected exclusively in the nucleolus when it was co-expressed with the Pil1-mCherry-Pol5 protein. However, Pil1-mCherry-Pol5 disappeared from the cell cortex and translocated into the nucleolus. Strains co-expressing either Pil1-Pol5-mCherry and GFP or Pil1-mCherry and Rrp14-GFP were used as controls to exclude the possibility of nonspecific interactions between Pol5 and GFP or Rrp14 and Pil1-mCherry (). In the control strain expressing both the recombinant Pil1-mCherry-Pol5 and GFP, the mCherry signal could be detected both at the cortex forming the linear filaments and in the nucleolus as predicted, due to the localization characteristics of Pil1 (eisosome) and Pol5 (nucleolus). Free GFP was widely detected throughout the cell with an enriched signal in the nucleolus. In the other control strain co-expressing Pil1-mCherry and Rrp14-GFP, Pil1-mCherry was detected exclusively at the cell cortex while Rrp14-GFP was detected in the nucleolus. These results indicated that no interactions exist between Pil1-Pol5-mCherry and GFP or Pil1-mCherry and Rrp14-GFP, suggesting that Rrp14 over-expression may target Pol5 in the nucleolus. The potential role of Rrp14 in facilitating the translocation of Pol5 into the nucleolus was thus proposed. We endogenously tagged the pol5 gene with mCherry at its C-terminus either in the WT or rrp14-deleted S. pombe. Consistent with our hypothesis, Pol5-mCherry diffused into the cytoplasm in the rrp14-deleted cells compared to the fluorescence in the WT S. pombe cells observed under the microscope (). Following the deletion of the rrp14 gene, the average fluorescence intensity of nucleolar Pol5-GFP decreased to about 1/4 of that of WT strains().These results further confirmed the important role of Rrp14 in facilitating the nucleolar localization of Pol5.
Figure 2. Pil1 co-tethering assay indicates that Rrp14 facilitates the translocation of Pol5 into the nucleolus. In the strain co-expressing Pil1-mCherry-Pol5 and GFP, recombinant Pil1-mCherry-Pol5 protein can be detected both at the cell cortex with the formation of a linear filament (Cell cortex) and in the nucleolus(Cell cross-section).The expression of Rrp14-GFP causesPil1-mCherry-Pol5 protein to translocate from the eisosomes into the nucleolus, but has no effect on the localization of Pil1-mCherry. Scale bars, 1 µm.
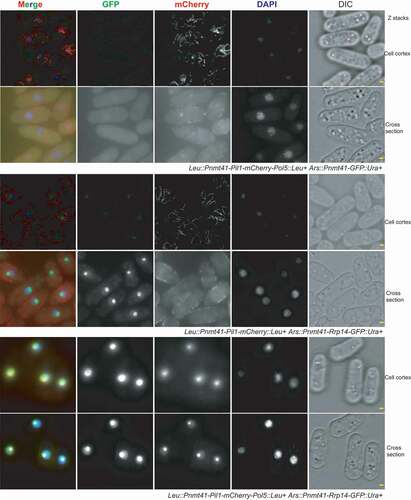
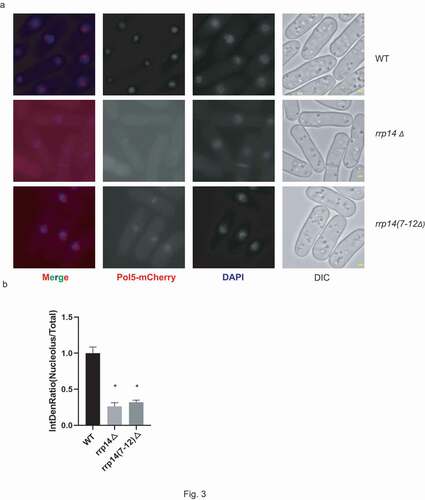
Figure 3. a. Mutation of Rrp14 causes Pol5 to diffuse into the cytoplasm. Pol5-mCherry can be detected exclusively in the nucleolus in WT strains, and deletion of the rrp14 gene or rrp14(7–12Δ) causes Pol5-mCherry to diffuse into the cytoplasm. Scale bars, 1 µm. B. The average fluorescence intensity of nucleolar Pol5-mCherry was analyzed following Rrp14 mutation. For each indicated strain, a minimum of five high-power fields were randomly selected. The average ratio of the fluorescence intensity of nucleolar Pol5-GFP to the fluorescence intensity of total intracellular GFP from the indicated strains was calculated and normalized to the relative intensity of the WT strain. The data are presented as the mean ± SEM. (*) indicates the values that differ significantly (p < 0.05).
Identification of the motif responsible for Rrp14’s association with Pol5
e wished to map the domain responsible for Rrp14’s interactions with Pol5 using the Pil1 co-tethering assay. We first divided the full-length Rrp14 into two truncated forms, N-Rrp14 (amino acids 1–78) and C-Rrp14 (amino acids 79–115), as indicated in . C-Rrp14-GFP was detected exclusively in the nucleolus when it was co-expressed with Pil1-mCherry, similar to the findings when co-expressed with Pil1-Pol5-mCherry. It seems that C-Rrp14 does not interact with Pol5 physically, though nuclear location sequences still exist in C-Rrp14, which allow its translocation into the nucleolus. On the other hand, N-Rrp14 was co-localized with Pol5, with linear filaments forming at the cortex, but not when it was co-expressed with Pil1-mCherry (Supplementary Fig 2). Interestingly, N-Rrp14 was unable to target the Pil1-Pol5-mCherry in the nucleolus since Pil1-Pol5-mCherry could still form cortical filaments even when co-expressed with N-Rrp14. We thus speculated that N-Rrp14 is responsible for the association of Rrp14 with Pol5, while C-Rrp14 plays a role in facilitating the nuclear translocation of the Pol5 binding with N-terminal Rrp14.
Figure 4. a. Schematic illustration of the different truncated forms of Rrp14. b. Pil1 co-tethering assay indicates that rrp14(1–6Δ) expression can still facilitate the translocation of Pol5 into the nucleolus from eisosomes. In contrast, Pil1-mCherry-Pol5 can still form linear filaments at the plasma membrane when co-expressed with rrp14(1–12Δ). c. Complementation assay with rrp14Δstrains. Introduction of the full-length Rrp14 and the truncated Rrp14 lacking the N-terminal 1–6 amino acids (rrp14 (1–6Δ)) can rescue the growth defect in the rrp14Δ strains, but rrp14 (1–12Δ), rrp14 (1–18Δ), rrp14 (1–24Δ), and rrp14 (1–30Δ) cannot.
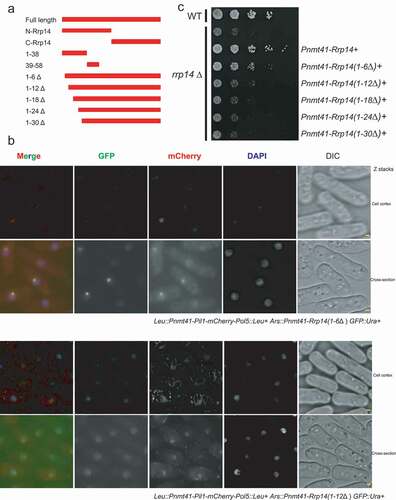
We next created different truncated versions of Rrp14 fused with GFP, as indicated in , including the N-terminal region of Rrp14 encompassing amino acids 1–38 and 39–58, as well as truncated Rrp14 lacking the N-terminal amino acids 1–6, 1–12, 1–18, 1–24, and 1–30. We found that Rrp14(1-38)-GFP could still physically interact with Pil1-mCherry-Pol5 but not Rrp14(39–58) (Supplementary Fig 3), indicating that the N-terminal amino acids 1–38 in Rrp14 are indispensable for its association with Pol5. We further found that over-expressed Rrp14(1–6Δ) could still facilitate the translocation of Pil1-mCherry-Pol5 into the nucleolus, since Pil1-mCherry-Pol5 could be detected mainly in the nucleolus when it was co-expressed with Rrp14 (1–6Δ) (). Comparatively, truncated Rrp14s (1–12Δ, 1–18Δ, 1–24Δ, 1–30Δ) completely lost the capacity to target Pol5 in the nucleolus ( and Supplementary Fig 4). This finding suggested that the N-terminal region of Rrp14 encompassing amino acids 1–12, especially amino acids 7-RINAWN-12, is essential for Rrp14’s interactions with Pol5.
To explore whether and which truncated forms of Rrp14 can complement growth defects in S. pombe cells due to rrp14 gene deletion, we introduced the full length Rrp14 and the truncated forms of Rrp14 in a strain with the rrp14 gene deleted. Only the full-length Rrp14 and Rrp14(1–6Δ) could fully rescue the growth defect in the rrp14-deficient mutant. The truncated Rrp14 without the first 12 N-terminal residues lacked the capacity to fully complement the growth defect of the rrp14 gene deletion mutant (). Given the essential role of the N-terminal amino acids 1–12 of Rrp14 in its association with Pol5, this result suggests that the association of Pol5 with Rrp14 plays an important role in the growth phenotype of S. pombe.
Mutation disrupting the association of Rrp14 with Pol5 affects the growth of S. pombe
To better explore the role of the domain responsible for Rrp14’s association with Pol5 in the growth phenotype of S. pombe, we mutagenized 7–12 amino acid residues of Rrp14 using the CRISPR/Cas9 system. We used a cloning-free and gap repair-based strategy as previously described [Citation14]. Schematics for the cleavage sites induced by sgRNA are presented in Supplementary Fig 1. We acquired the rrp14(7–12Δ) mutant using donor DNA amplified by PCR. A series of additional spontaneous mutations adjacent to the cleavage sites was also acquired following Cas9 digestion without the addition of donor DNA, including rrp14(8Δ), rrp14(9Δ), and rrp14(10–12Δ), confirmed by sequencing. Unfortunately, for some unknown reason, we were unable to achieve spontaneous mutation containing the 7th amino acid even after adding the donor DNA containing the intended mutation. We next tagged Pol5 endogenously with the mCherry tag at the C-terminus in strains with different Rrp14 mutation backgrounds. We found that the mutation of amino acids 7–12 in Rrp14 caused the Pol5-mCherry signal to diffuse into the cytoplasm partially(), but the rrp14(8Δ), rrp14(9Δ), and rrp14(10–12Δ) mutations did not(Data not shown). It seems likely that the 7th amino acid arginine (R7) is essential for Rrp14’s association with Pol5. Moreover, we found that the R7 amino acid of Rrp14 from S. pombe is conserved as R8 in S. cerevisiae following alignment using the Clustal Omega program (Supplementary Fig 5). A spot assay was performed to compare the growth of the WT, rrp14Δ, and different mutation strains. Consistent with our complement growth assay results, rrp14 (7–12Δ) mutation, which was expected to disrupt Rrp14’s association with Pol5, caused the S. pombe cells to grow slowly ().
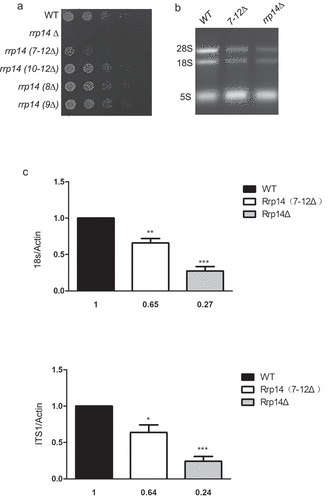
Figure 5. a. Deletion of the motif(7-RINAWN-12) responsible for the interaction of Rrp14 with Pol5 causes the cells to grow slowly. Strains harboring the indicated mutations in Rrp14 and WT strains were diluted to 0.1(OD595). Following serial five-fold dilution, the cells were plated onto YES plates and a photo was taken after 48-h incubation at 32°. b.The 28s and 18s rRNA levels in the rrp14Δ and rrp14(7–12Δ) strains were significantly decreased compared to those of the WT strain. The total RNAs from WT, rrp14Δ, and rrp14(7–12Δ) strains were extracted and stained with ethidium bromide after running on 2.0% agarose gel. c. The results of qRT-PCR analysis for the PCR products corresponding to the 18sRNA and ITS1. The bar graph illustrates the amount of 18sRNA/Actin and ITS1/Actin in rrp14Δ (gray columns), and rrp14(7–12Δ)(white columns) normalized to the values in WT cells (black columns). The values obtained are presented as the mean N ± SEM based on the results of three independent experiments.
The domain responsible for Rrp14’s association with Pol5 has a role in rRNA transcription
Accumulating evidence suggests that Pol5 has an important role as a transcription factor in rRNA transcription, although it was originally identified as a DNA polymerase through bioinformatics analysis. Given the important role of Rrp14 in rRNA transcription as reported previously, we wished to examine whether the disruption of Pol5 with Rrp14 affects rRNA transcription. We extracted the total RNA from WT, rrp14Δ, and rrp14 (7–12Δ) strains, and ran them on 2.0% agarose gel. Following ethidium bromide staining, we found that the 28s and 18s rRNA levels in the rrp14Δ and rrp14 (7–12Δ) strains were significantly decreased compared to those of the WT strain (). qRT-PCR was also performed to compare the transcription of 18sRNA and the intron between 18sRNA and 28sRNA (internal transcribed spacer 1 [ITS1]) using the indicated primers (Supplementary Table 2). Actin was used as the internal control. The data from qRT-PCR analysis corresponding to the 18sRNA and ITS1 are shown in . Consistent with the reported important role of Rrp14 in rRNA transcription, deletion of the rrp14 gene reduced the level of 18s and ITS1 transcription by more than half of that in the WT strain. Deletion of the 7-RINAWN-12 motif of Rrp14 also decreased the 18sRNA and ITS1 transcription level; thus, disruption of the interaction between Pol5 and Rrp14 can reduce rRNA transcription.
Discussion
Pol5 in yeast was originally identified as a B-type DNA polymerase. However, accumulating evidence indicates that Pol5 has an important role in ribosomal biogenesis [Citation17–21]. Although the detailed molecular mechanism is still uncertain, it has been speculated that Pol5 might regulate rRNA transcription, either as a transcription factor by binding to the enhancer region of rDNA or as an accessory factor important for ribosome biogenesis. In this study, we found that Rrp14 protein, a homologue of the N-terminal part of Rrp14 in S. cerevisiae, can interact physically with Pol5 in S. pombe. Deletion of rrp14 caused the cells to grow significantly slower with a reduced level of rRNA transcription, indicating an important conserved role for Rrp14 (N-terminal region of Rrp14/Surf6) in rRNA transcription.
The Pil1 co-tethering assay, a strategy developed recently to detect physical interactions between two proteins in vivo, was used in this study to detect physical interactions between the Rrp14 and Pol5 proteins. Compared with co-immunoprecipitation and yeast two-hybrid assays, which are the traditional strategies for detecting protein-protein interactions, the Pil1 co-tethering assay can visualize subcellular physical interactions in eisosomes or specific cellular localizations due to the distribution of the Pil1 protein and target proteins, respectively. Taking advantage of the Pil1 co-tethering assay, we found that overexpression of the Rrp14 protein caused the Pil1-mCherry-Pol5 protein to translocate from the cell cortex into the nucleolus. Thus, an assisting role for Rrp14 in the nucleolar localization of Pol5 appears likely. We further confirmed that the deletion of rrp14 resulted in the diffusion of the Pol5 protein into the cytoplasm from the nucleolus. Our results also suggested that the Pil1 co-tethering assay would be a useful method to explore the role of protein association in specific subcellular localization.
We found that deletion of the 7-RINAWN-12 motif in Rrp14 disrupted the association between Rrp14 and Pol5. rrp14(7–12Δ) strains also showed decreased rRNA transcription. Given the important role of Pol5 as a transcriptional factor in rRNA transcription, we believe that Rrp14 affects the rRNA transcription level, at least partially through its association with Pol5. Mybbp1a, the human orthologue of Pol5, is detected exclusively in the nucleolus, similar to Pol5, and is also important for RNA synthesis [Citation22,Citation23]. The export of Mybbp1a from the nucleolus upon nucleolar stress contributes to P53 acetylation and even apoptosis, indicating the physiological importance of the nucleolar location of Mybbp1a [Citation23,Citation24]. Interestingly, we found that Rrp14 can facilitate the nucleolar translocation of Pol5 in S. pombe. Given the homology of the Rrp14 and Pol5 proteins in S. pombe to the N-terminus of the Surf6 and Mybbp1a proteins in humans, it seems reasonable to believe that the Surf6 protein may have a similar function in facilitating the nucleolar translocation of Mybbp1a in humans.
Supplemental Material
Download Zip (8.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Magoulas C, Fried M. The Surf-6 gene of the mouse surfeit locus encodes a novel nucleolar protein. DNA Cell Biol. 1996;15(4):305–316.
- Armes N, Gilley J, Fried M. The comparative genomic structure and sequence of the surfeit gene homologs in the puffer fish fugu rubripes and their association with CpG-rich Islands. Genome Res. 1997;7(12):1138–1152.
- Magoulas C, Zatsepina OV, Jordan PW, et al. The SURF-6 protein is a component of the nucleolar matrix and has a high binding capacity for nucleic acids in vitro. Eur J Cell Biol. 1998;75(2):174–183.
- Polzikov M, Zatsepina O, Magoulas C. Identification of an evolutionary conserved SURF-6 domain in a family of nucleolar proteins extending from human to yeast. Biochem Biophys Res Commun. 2005;327(1):143–149.
- Drees BL, Sundin B, Brazeau E, et al. A protein interaction map for cell polarity development. J Cell Biol. 2001;154(3):549–571.
- Horsey EW, Jakovljevic J, Miles TD, et al. Role of the yeast Rrp1 protein in the dynamics of pre-ribosome maturation. Rna. 2004;10(5):813–827.
- Fatica A, Cronshaw AD, Dlakic M, et al. Ssf1p prevents premature processing of an early pre-60S ribosomal particle. Mol Cell. 2002;9(2):341–351.
- Oeffinger M, Fatica A, Rout MP, et al. Yeast Rrp14p is required for ribosomal subunit synthesis and for correct positioning of the mitotic spindle during mitosis. Nucleic Acids Res. 2007;35(4):1354–1366.
- Yamada H, Horigome C, Okada T, et al. Yeast Rrp14p is a nucleolar protein involved in both ribosome biogenesis and cell polarity. Rna. 2007;13:1977–1987.
- Shirai C, Takai T, Nariai M, et al. Ebp2p, the yeast homolog of Epstein-Barr virus nuclear antigen 1-binding protein 2, interacts with factors of both the 60 S and the 40 s ribosomal subunit assembly. J Biol Chem. 2004;279(24):25353–25358.
- Moraleva A, Magoulas C, Polzikov M, et al. Involvement of the specific nucleolar protein SURF6 in regulation of proliferation and ribosome biogenesis in mouse NIH/3T3 fibroblasts. Cell Cycle. 2017;16(20):1979–1991.
- Gregan J, Rabitsch PK, Rumpf C, et al. High-throughput knockout screen in fission yeast. Nat Protoc. 2006;1(5):2457–2464.
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823.
- Zhang XR, He JB, Wang YZ, et al. A cloning-free method for CRISPR/Cas9-mediated genome editing in fission yeast. G3. 2018;8(6):2067–2077.
- Forsburg SL, Rhind N. Basic methods for fission yeast. Yeast. 2006;23(3):173–183.
- Liu XM, Sun LL, Hu W, et al. ESCRTs cooperate with a selective autophagy receptor to mediate vacuolar targeting of soluble cargos. Mol Cell. 2015;59(6):1035–1042.
- Shimizu K, Kawasaki Y, Hiraga S, et al. The fifth essential DNA polymerase phi in Saccharomyces cerevisiae is localized to the nucleolus and plays an important role in synthesis of rRNA. Proc Natl Acad Sci U S A. 2002;99(14):9133–9138.
- Braun CM, Hackert P, Schmid CE, et al. Pol5 is required for recycling of small subunit biogenesis factors and for formation of the peptide exit tunnel of the large ribosomal subunit. Nucleic Acids Res. 2020;48(1):405–420.
- Gallagher JEG. Proteins and RNA sequences required for the transition of the t-Utp complex into the SSU processome. FEMS Yeast Res. 2019;19(1). DOI:https://doi.org/10.1093/femsyr/foy120
- Ramos-Saenz A, Gonzalez-Alvarez D, Rodriguez-Galan O, et al. Pol5 is an essential ribosome biogenesis factor required for 60S ribosomal subunit maturation in Saccharomyces cerevisiae. RNA. 2019;25(11):1561–1575.
- Yang W, Rogozin IB, Koonin EV. Yeast POL5 is an evolutionarily conserved regulator of rDNA transcription unrelated to any known DNA polymerases. Cell Cycle. 2003;2(2):120–122.
- Keough RA, Macmillan EM, Lutwyche JK, et al. Myb-binding protein 1a is a nucleocytoplasmic shuttling protein that utilizes CRM1-dependent and independent nuclear export pathways. Exp Cell Res. 2003;289(1):108–123.
- Yamauchi T, Keough RA, Gonda TJ, et al. Ribosomal stress induces processing of Mybbp1a and its translocation from the nucleolus to the nucleoplasm. Genes Cells. 2008;13(1):27–39.
- Kuroda T, Murayama A, Katagiri N, et al. RNA content in the nucleolus alters p53 acetylation via MYBBP1A. EMBO J. 2011;30(6):1054–1066.
