ABSTRACT
Reportedly, dysfunction of human pulmonary arterial smooth muscle cells (PASMCs) is associated with the pathogenesis of pulmonary arterial hypertension (PAH). Herein, the role of miR-509-5p in hypoxia-induced PASMCs and the underlying mechanism were explored. PASMCs were cultured under both normoxia and hypoxia conditions. Quantitative real-time polymerase-chain reaction (qPCR) was employed for quantifying the expressions of miR-509-5p and DNMT1 mRNA in the serum of PAH patients and PASMCs. MiR-509-5p mimics and inhibitors were then, respectively, transfected into PAMSCs, and CCK-8 and Transwell assays were utilized to detect PASMCs’ proliferation and migration. Flow cytometry was executed for evaluating PASMCs’ apoptosis. Interrelation between miR-509-5p and DNMT1 was determined utilizing bioinformatics analysis and dual-luciferase reporter assay. Western blot assay was used to detect the expression of DNMT1 or SOD2. MiR-509-5p in serum samples of patients with PAH as well as hypoxia-induced PASMCs was significantly down-regulated, whereas DNMT1 was markedly up-regulated. MiR-509-5p mimics reduces the proliferation and migration of PASMCs, but promotes the apoptosis; conversely, miR-509-5p inhibitors exerted opposite effects. DNMT1 was identified as a target gene of miR-509-5p, and overexpression of DNMT1 reversed the biological functions of miR-509-5p in regulating the phenotypes of PAMSCs. MiR-509-5p up-regulated the expression of SOD2 by down-regulating DNMT1. MiR-509-5p regulates the proliferation, migration and apoptosis of PASMCs, and restoration of miR-509-5p may be a promising strategy to treat PAH.
1. Introduction
Pulmonary arterial hypertension (PAH), characterized with progressive deterioration of pulmonary artery vasoconstriction, threatens the life of the patients [Citation1], with morbidity in adults approximately 15/1, 000, 000 [Citation2]. At present, therapeutic strategies are focusing on reversing pulmonary vasoconstriction by targeting prostacyclin, endothelin or nitric oxide, however, the effects are not satisfactory [Citation3]. Thereupon, clarifying the mechanism of PAH pathogenesis and unveiling novel therapeutic targets are urgent. Abnormal proliferation and migration of pulmonary artery smooth muscle cells (PASMCs) have been reported to be significant features of pulmonary vascular remodeling, as well as one of the main pathological factors triggering PAH [Citation1,Citation4], yet the explicit regulatory mechanism of PASMC dysfunction remains unclear.
MicroRNAs (miRNAs) negatively regulate gene expression by targeting specific messenger RNAs (mRNAs), and inducing their degradation or inhibiting their translation [Citation5]. Many miRNAs are associated with the occurrence and development of PAH. For example, miR-143-3p facilitates PASMC migration and miR-145 promotes PASMC proliferation, both of which facilitate the pathogenesis of PAH [Citation2]. Overexpression of miR-140-5p blocks the proliferation and migration of PASMCs through inhibiting tumor necrosis factor-α [Citation6]. MiR-509-5p has been linked to many human diseases, especially cancers [Citation7–10]. For instance, miR-509-5p exerts a tumor-suppressive effect by down-regulating the expression of TRIB2 in osteosarcoma [Citation7]. MiR-509-5p is also remarkably down-regulated in pancreatic cancer tissues and cell lines, while augmented expression of miR-509-5p represses the proliferation, migration and invasion of pancreatic cancer cells by targeting MDM2 [Citation8], and importantly MDM2-mediated ubiquitination of angiotensin-converting enzyme 2 contributes to the development of PAH [Citation11]. Nonetheless, whether miR-509-5p regulates the proliferation and migration of PASMCs requires further delineation.
DNA methylation mediates the down-regulation of genes involved in cell proliferation and apoptosis [Citation12]. DNA methyltransferase 1 (DNMT1) is a crucial regulator for DNA methylation [Citation13]. DNMT1 is associated with multiple cardiopulmonary diseases. For instance, by comparison with normal fibroblasts, expression of DNMT1 in lung fibroblasts of idiopathic pulmonary fibrosis (IPF) is markedly augmented; miR-17 ~ 92 is capable of targetedly down-regulating DNMT1, thereupon regulating the pathogenesis of pulmonary fibrosis [Citation14]. In recent years, DNMT1 has been reported to be linked to the pathogenesis of PAH by hypermethylation of CpG Islands within the promoter of superoxide dismutase 2 (SOD2) and down-regulating SOD2 expression [Citation15], while its regulatory mechanism remains unclear.
In the present study, we detected an expression level of miR-509-5p in the serum of patients with PAH, and hypoxia-induced PASMCs. We demonstrated that miR-509-5p was notably down-regulated in the serum of PAH patients, and PASMCs cultured in hypoxic conditions. Additionally, we investigated the effects of miR-509-5p and DNMT1 on the proliferation and migration of PASMC and the regulatory mechanism among miR-509-5p, DNMT1 and SOD2, and demonstrated that miR-509-5p repressed the dysregulation of PASMCs via repressing DNMT1 and upregulating SOD2.
2. Materials and methods
2.1 Clinical samples
Forty patients with PAH (arterial blood gas analysis, arterial oxygen partial pressure ≤60 mmHg) were recruited to Zhongshan Hospital from May 2017 to May 2019. Forty healthy volunteers who underwent physical examinations were recruited as controls. 5 mL of vein blood derived from each enrolled subject was maintained in a blood collection tube containing EDTA, centrifuged at 4°C and 12,000 rpm for 15 min, and the collected plasma was cryopreserved in a low-temperature refrigerator at −80°C.
2.2 Cell culture and transfection
Human PASMCs were procured from the Shanghai Cell Center of Chinese Academy of Sciences (Shanghai, China). The cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA) and 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen, New York, CA, USA). The cells in normoxia group were cultivated in an incubator at 37°C, 5% CO2 and 21% O2, while the cells in hypoxia group were cultivated in an incubator at 37°C with 5% CO2 and 3% O2.
DNMT1 overexpression plasmid, miR-509-5p mimics, miR-509-5p inhibitors and their negative controls were provided by GenePharma (Shanghai, China), and they were, respectively, transfected into PASMCs utilizing Lipofectamine®3000 (Invitrogen, Carlsbad, CA, USA) in conformity with the manufacturer's protocols.
2.3 Quantitative real-time polymerase chain reaction (qPCR)
The TRIzol (Invitrogen, Carlsbad, CA, USA) method was employed to extract the total RNA of PASMCs. RNA reverse transcription and qPCR were performed with an RNA reverse transcription kit (TaKaRa, Dalian, China) and a SYBR® Premix Ex Taq™ II kit (TaKaRa, Dalian, China), respectively, and the target genes were amplified in ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). β-actin and U6 were employed as the internal references, and primer sequences are shown in .
Table 1. PCR primers utilized in this research
2.4 Cell counting kit-8 (CCK-8) assay
2 × 103 cells from each group were inoculated into each well of a 96-well plate. Followed by 24 h of culture, 10 μL of CCK-8 solution (Beyotime, Shanghai, China) was supplemented to each well. Next, the cells were incubated for consecutive 4 h, and the absorbance at 450 nm wavelength was measured utilizing a microplate reader. With the same method, the absorbance values of the cells were detected at 24 h, 48 h, 72 h and 96 h, respectively.
2.5 Transwell assay
The PASMCs in each group were trypsinized and resuspended in serum-free DMEM. The cell concentration was adjusted to 1 × 105 cells/mL. Then, 200 μL of cell suspension was added into the upper compartment of each Transwell chamber (pore size, 8 µM; Corning, NY, USA), while 500 μL of DMEM containing 10% FBS was supplemented to lower compartment, and then the cells were cultivated at 37°C and 5% CO2 for 48 h. Next, PASMCs remaining on the upper side of the membrane were removed, and the migrated cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Ultimately, the stained cells were counted under a microscope.
2.6 Flow cytometry
The apoptosis of PASMCs was detected with an Annexin V‐FITC/propidium iodide (PI) Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA). The cells were rinsed with phosphate buffer saline (PBS) twice, supplemented with 400 μL of loading buffer, then added with 10 μL of AnnexinV-FITC staining solution and 5 μL of PI staining solution, respectively. Followed by incubation at 4°C in the dark for 30 min, the cells were immediately detected employing a flow cytometer (Beckman Coulter, Fullerton, CA, USA).
2.7 Western blot
Total protein was extracted with RIPA buffer (Beyotime, Shanghai, China). Next, SDS-PAGE was performed, and then the protein was transferred to PVDF membrane (Millipore, Billerica, MA, USA). The PVDF membrane was then blocked with 5% skimmed milk for 2 h at room temperature, and incubated with primary antibody (anti-DNMT1 antibody, mouse monoclonal antibody, Abcam, ab13537, concentration 1:1000; anti-SOD2 antibody, mouse monoclonal antibody, Abcam, ab74231, concentration 1:1000) at 4°C overnight. The membrane was then rinsed with TBST 3 times, each time for 15 min. Subsequently, the membrane was incubated with a secondary antibody (goat anti-mouse IgG, Abcam, ab205719, concentration 1:2000) at room temperature for 2 h, rinsed with tris buffered saline tween (TBST) for 3 times, and ultimately, the Enhanced Chemiluminescence Western blotting Substrate (Dongguan Biotech, Shandong, China) was used to develop the protein bands.
2.8 Dual-luciferase reporter gene assay
The sequences of DNMT1 3ʹUTR containing the binding site for miR-509-5p was predicted utilizing bioinformatic analysis and amplified employing PCR. Subsequently, the amplified fragment was inserted into pmiR-GLO report vector (Promega, Madison, WI, USA), constructing DNMT1 wild-type (WT) reporter plasmid. A part of nucleotides were mutated for constructing DNMT1 mutant (MUT) reporter plasmid. PASMCs were transiently transfected with the reporter plasmids, together with miR-509-5p mimics or mimics NC, and 48 h later, the luciferase activity of the cells in each group was detected utilizing a dual-luciferase reporter assay system (Promega, Madison, WI, USA).
2.9 Enzyme-linked immunosorbent assay (ELISA)
The levels of DNMT1 protein in the serum of the patients and healthy controls were examined with a DNMT1 ELISA kit (Solarbio, Beijing, China) according to the manufacturer’s instruction.
2.10 Statistical analysis
SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The measurement data were expressed by mean ± standard deviation (x ± s). Independent sample t-test was employed for comparison between two groups of measurement data, and one-way ANOVA was used for comparison among multiple groups. The difference was deemed to be statistically significant when P < 0.05.
3. Results
3.1 MiR-509-5p was aberrantly lowly expressed in PAH
By analyzing the published datasets GSE44145 and GSE97842, expression of miR-509-5p was observed to be down-regulated in arterial blood samples of patients with PAH () by comparison with that of healthy people; meanwhile, the expression of miR-509-5p in lung tissue of mice treated with monocrotaline (a PAH model) was down-regulated by comparison with that of wild-type mice (). The results of the bioinformatics analysis suggested that miR-509-5p might play a crucial role in PAH progression. For further determining whether miR-509-5p was involved in the pathogenesis of PAH, expression of miR-509-5p in serum samples of patients with PAH as well as hypoxia-induced human PASMCs was quantified employing qPCR. As shown, expression of miR-509-5p in serum samples of patients with PAH was remarkably lower than that of healthy subjects (). Consistently, the expression of miR-509-5p in hypoxia-treated PASMCs was significantly down-regulated ().
Figure 1. Expression of miR-509-5p in GSE44145 and GSE97842. (a) Expression of miRNAs in GSE44145. miRNAs with Log2 fold change (FC)>1.5 were marked Orange, miRNAs with log2FC <-1.5 were marked blue, and miR-509-5p was marked red. (b) Expression of miRNAs in GSE97842. miRNAs with Log2FC>1.5 were marked Orange, miRNAs with log2FC <-1.5 were marked blue, and miR-509-5p was marked red.
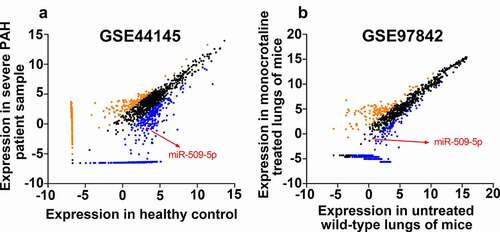
Figure 2. Expression characteristics of miR-509-5p in serum samples of PAH patients and PASMCs under hypoxia. (a) Expression of miR-509-5p in serum of PAH patients and healthy subjects was detected by qPCR. (b) Expression of miR-509-5p in PASMC under normoxia and hypoxia was detected by qPCR. *** denotes p < 0.001.
3.2 MiR-509-5p attenuated proliferation and migration of PASMCs and facilitated apoptosis
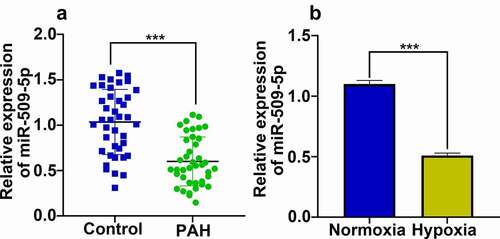
Subsequently, miR-509-5p overexpression and inhibition models were established by transfecting miR-509-5p mimics or miR-509-5p inhibitors into PASMCs, respectively (). Through CCK-8 and Transwell experiments, miR-509-5p mimics were observed to markedly suppress the proliferation and migration of PASMCs; flow cytometry revealed that overexpression of miR-509-5p was capable of promoting the apoptosis of PASMCs; meanwhile, miR-509-5p inhibitors exerted opposite effects, facilitating the proliferation and migration, and repressing the apoptosis of PASMCs (). These data suggested that miR-509-5p exhibited a crucial role in regulating the dysfunction of PASMCs.
Figure 3. Effect of miR-509-5p on the phenotypes of PASMCs. (a) Cell models with overexpressed or reduced miR-509-5p were established in PASMCs. (b, c) CCK-8 assay was employed for detecting the effect of miR-509-5p overexpression or inhibition on the proliferation of PASMCs. (d) Effect of miR-509-5p overexpression or inhibition on the migration of PASMCs was assessed with Transwell assay. (e) Effect of miR-509-5p overexpression or inhibition on PASMC apoptosis was detected by flow cytometry. *, **, *** represent p < 0.05, p < 0.01 and p < 0.001 respectively. In (b), *, ** indicate p < 0.05 and p < 0.01 by comparison with normoxia group. & indicates that p < 0.05 by comparison with hypoxia+mimics NC group. In (c), *, ** indicate p < 0.05 and p < 0.01 by comparison with normoxia group. &, && represent p < 0.05 and p < 0.01 respectively by comparison with hypoxia+inhibitors NC group.
3.3 DNMT1 was one of the downstream targets of miR-509-5p
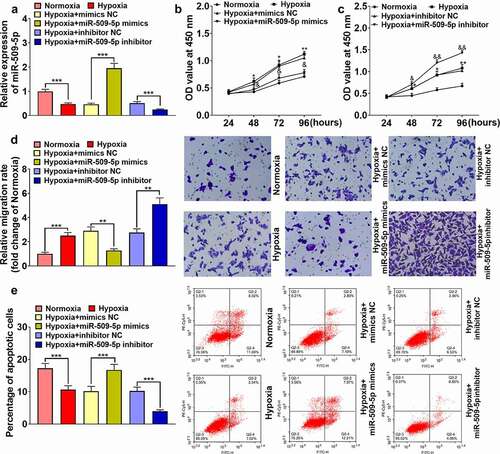
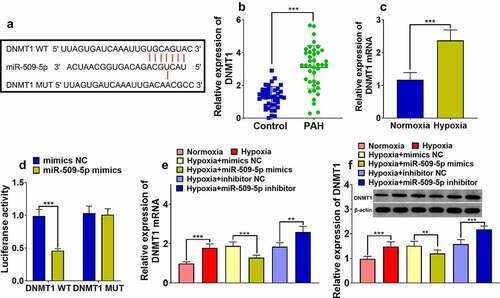
To further clarify the explicit mechanism regarding miR-509-5p affecting PASMCs, the downstream targets of miR-509-5p were predicted by the TargetScan database, and DNMT1 was predicted to be one of the candidate targets of miR-509-5p (). The expression level of DNMT1 was quantified employing ELISA, the results of which revealed that DNMT1 was remarkably augmented in the serum samples of patients with PAH; additionally, DNMT1 mRNA was up-regulated in PASMCs under hypoxia ()). In the dual-luciferase reporter gene assay, miR-509-5p mimics were revealed to markedly weaken the luciferase activity of wild-type DNMT1 reporter, whereas it had no obvious effect on the mutant reporter ()). Furthermore, through qPCR and Western blot, miR-509-5p was proved to suppress DNMT1 expression in PASMC at mRNA and protein levels ()). These data implied that miR-509-5p was capable of down-regulating DNMT1.
Figure 4. Expression of DNMT1 was targetedly inhibited by miR-509-5p. (a) Binding site between miR-509-5p and DNMT1 3ʹUTR was predicted by TargetScan database. (b) Expression of DNMT1 in the serum of patients with PAH and healthy subjects was detected by ELISA. (c) Expression of DNMT1 mRNA in PASMCs under normoxia and hypoxia was detected by qPCR. (d) The targeted binding relationship between miR-509-5p and DNMT1 was verified by dual-luciferase reporter gene assay. (e) Expression of DNMT1 mRNA in miR-509-5p overexpression and low expression models was quantified by qPCR. (f) Expression of DNMT1 in miR-509-5p overexpression and low expression models was detected by Western blot. ** and *** represent p < 0.01 and p < 0.001 respectively.
3.4 DNMT1 counteracted the effects of miR-509-5p on PASMCs
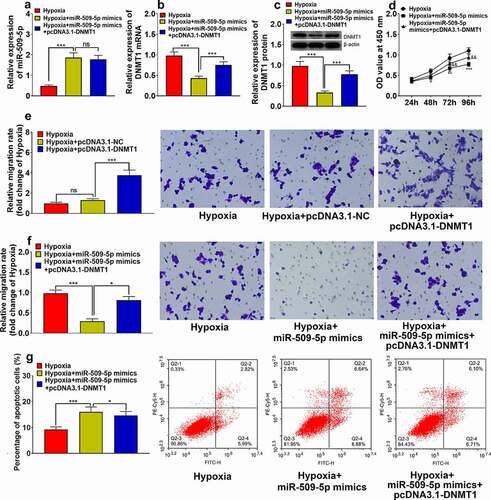
MiR-509-5p mimics and pcDNA3.1-DNMT1 were co-transfected into PASMCs to validate the functional interaction between miR-509-5p and DNMT1. Expression of miR-509-5p and DNMT1 was quantified through qPCR and Western blot assay. It was revealed that the expressions of DNMT1 mRNA and protein were significantly increased in PASMCs transfected with pcDNA3.1-DNMT1, whereas overexpression of DNMT1 exhibited no significant effect on the expression of miR-509-5p, indicating that miR-509-5p had a unidirectional regulatory function on DNMT1 ()). Then, pcDNA3.1-DNMT1 or pcDNA3.1-NC was transfected into PASMCs to investigate the impact of DNMT1 on cell proliferation and migration. The results of CCK-8 assay, Transwell assay and flow cytometry indicated that, the impact of miR-509-5p on suppression of cell proliferation, migration and facilitation of cell apoptosis was counteracted by co-transfected pcDNA3.1-DNMT1 ()), suggesting that miR-509-5p exhibited a protective role in PAH by down-regulating DNMT1.
Figure 5. Overexpression of DNMT1 counteracted effect of miR-509-5p on PASMC. (a) Expression of miR-509-5p in PASMCs was detected by qPCR after the co-transfection of miR-509-5p and pcDNA3.1-DNMT1. (b) Expression of DNMT1 mRNA in PASMCs was assessed by qPCR after the transfection. (c) Expression of DNMT1 in PASMCs was detected by Western blot after the transfection. (d) CCK-8 assay was utilized for detecting PASMC proliferation after the transfection. (f) Transwell assay was employed for detecting PASMC migration after DNMT1 overexpression. Transwell assay was employed for detecting PASMC migration after the transfection. (g) The apoptosis of PASMCs was detected by flow cytometry after the transfection. *, **, *** represent p < 0.05, p < 0.01 and p < 0.001 respectively. In (d), *, **, *** represent differences between hypoxia group and hypoxia+miR-509-5p mimics group (p < 0.05, p < 0.01, p < 0.001); && represents difference between hypoxia+miR-509-5p mimics group and hypoxia+miR-509-5p mimics+pcDNA3.1-DNMT1 group (p < 0.01).
3.5 miR-509-5p could upregulate SOD2 by repressing DNMT1
It is reported that DNMT1 participates in the pathogenesis of PAH by down-regulating SOD2 expression [Citation15], then we detected the expression of SOD2 in PASMCs. Western blot assay showed that the expression of SOD2 in hypoxia-treated PASMCs was significantly down-regulated compared with normoxia-treated PASMCs (Supplementary Figure 1A), and DNMT1 overexpression could significantly down-regulate the expression of SOD2 (Supplementary Figure 1B). miR-509-5p mimics upregulated the SOD2 expression and miR-509-5p inhibitor showed the opposite effect (Supplementary Figure 1C). DNMT1 overexpression could reverse the effect of miR-509-5p mimics on the expression of SOD2 (Supplementary Figure 1D).
4. Discussion
In recent years, increasing evidence supports that miRNAs is in close relationship with multiple cardiopulmonary diseases, including PAH. For example, miR-340-5p represses the pathogenesis of PAH induced by acute pulmonary embolism by suppressing the expression of IL-1β and IL-6 [Citation16]. Inhibition of miR-138-5p restores the expressions of KCNK3 and SLC45A3, attenuates monocrotaline-induced PAH [Citation17]. Another study reports that miR-182-3p is significantly up-regulated in PAH patients and experimental rodent models induced by monocrotaline; miR-182-3p mimics aggravate the pathological progression of PAH in rats [Citation18]. Additionally, miRNAs can be used as potential biomarkers for PAH diagnosis. For instance, the expression of circulating miR-17 of PAH patients is significantly up-regulated, and its expression level is associated with 6-minute walk distance, pulmonary artery pressure, and right atrial pressure of the patients [Citation19]. MiR-509-5p is recently demonstrated to exhibit crucial regulatory functions in several types of tumors, and its specific role in different tumors is heterogeneous. For instance, in osteosarcoma and pancreatic cancer, miR-509-5p is capable of suppressing proliferation and migration of cancer cells, exerting a tumor-suppressive role [Citation7,Citation8], whereas in papillary thyroid cancer, miR-509-5p is capable of targetedly down-regulating SFRP1 to potentiate proliferation and invasion of papillary thyroid cancer cells, exhibiting a cancer-promoting role [Citation20]. In the present study, we explored the expression characteristics and biological function of miR-509-5p in PAH. The expression of circulating miR-509-5p was demonstrated to be aberrantly lowly expressed in the serum of PAH patients, and it was also underexpressed in hypoxia-treated PASMCs. Furthermore, it was revealed that miR-509-5p repressed the proliferation and migration of PASMCs and facilitated their apoptosis, consistent with its inhibitory effects on cell proliferation and migration in osteosarcoma and pancreatic cancer [Citation7,Citation8]. In light of the aforesaid findings, it is indicated that miR-509-5p possesses the potential as a diagnostic biomarker or therapeutic target for PAH.
DNA methyltransferases are a class of pivotal regulators in epigenetics. In mammals, DNMT1, DNMT3A and DNMT3B are involved in DNA methylation to regulate a series of physiological and pathological processes, and DNA methylation of gene promoters is an epigenetic modification that facilitates transcriptional suppression, thus regulating cell proliferation, apoptosis and differentiation [Citation21,Citation22]. In recent years, the relationship between DNMT1 and PAH has gained growing attention. Importantly, DNMT1 expression is up-regulated in PASMCs of PAH patients, modulating the hypermethylation of CpG Islands in SOD2 promoter, hence down-regulating SOD2 expression and facilitating the proliferation of PASMCs, as well as inhibiting apoptosis [Citation15]. It is reported that platelet-derived growth factor BB induces the expression of DNMT1 via activating PI3K signaling, enhancing the methylation of CpG Island and repressing miR-1281 expression, in turn promoting the proliferation and migration of PASMCs [Citation23]. The present work substantiated that DNMT1 overexpression promoted the migration of PASMCs. Additionally, we demonstrated that miR-509-5p was capable of suppressing DNMT1 expression in PASMCs at mRNA and protein levels. Furthermore, we demonstrated that miR-509-5p’s effect of promoting proliferation and migration, and suppressing apoptosis of PASMCs could be counteracted by overexpressed DNMT1, indicating that miR-509-5p had a unidirectional regulatory function on DNMT1. Our research validated that DNMT1 was a promising therapeutic target for PAH, as well as further disclosed the upstream mechanism of DNMT1 dysregulation in PAH. SOD2 is down-regulated in PAH and the silencing of SOD2 leads to excessive proliferation of PASMCs [Citation15]. DNMT1 inhibitor 5-azacytidine can decrease methylation of SOD2 [Citation24]. Notably, we found that SOD2 was downregulated in hypoxia-treated PASMCs, and miR-509-5p could upregulated SOD2 by down-regulating DNMT1.
There are several limitations in the present work. First, the other downstream targets of miR-509-5p, which may be associated with the progression of PAH, remain to be screened and verified. Secondly, our conclusion is only based on in vitro experiments, and animal models and in vivo experiments, as well as clinical investigation are needed to further validate our demonstrations. Thirdly, more patients should be enrolled, and the relationship between miR-509-5p expression and the clinical characteristics, as well as the relationship between miR-509-5p expression and the treatment response of PAH patients should be analyzed, which is crucial to evaluate the potential of circulating miR-509-5p as a biomarker for PAH. Finally, the downstream mechanism by which DNMT1/SOD2 axis regulates the phenotypes of PASMCs and the development of PAH needs further investigation in the following work.
To put it succinctly, we demonstrate through in vitro experiments that miR-509-5p in PASMCs is capable of attenuating the proliferation and migration of PASMCs, expediting apoptosis via repressing DNMT1, suggesting that miR-509-5p suppresses the development of PAH. Our research furnishes the theoretical basis of PAH pathogenesis, and provides useful clues for the diagnosis and treatment of PAH.
Ethics statement
The protocol of human sample collection was approved by the Zhongshan Hospital. Written informed consents were acquired from each participating patient and the sample collection was conducted in accordance with the principles of the Declaration of Helsinki.
Supplemental Material
Download TIFF Image (736.5 KB)Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Zhao M, Chen N, Li X, et al. MiR-19a modulates hypoxia-mediated cell proliferation and migration via repressing PTEN in human pulmonary arterial smooth muscle life. Sci. 2019 Dec 15;239:116928.
- Deng L, Blanco FJ, Stevens H, et al. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015 Oct 23;117(10):870–883.
- Orriols M, Gomez-Puerto MC, Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol Life Sci. 2017 Aug;74(16):2979–2995.
- Liu G, Hao P, Xu J, et al. Upregulation of microRNA-17-5p contributes to hypoxia-induced proliferation in human pulmonary artery smooth muscle cells through modulation of p21 and PTEN. Respir Res. 2018 Oct 10;19(1):200.
- Caruso P, Dunmore BJ, Schlosser K, et al. Identification of MicroRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation. 2017 Dec 19;136(25):2451–2467.
- Zhu TT, Zhang WF, Yin YL, et al. MicroRNA-140-5p targeting tumor necrosis factor-α prevents pulmonary arterial hypertension. J Cell Physiol. 2019 Jun;234(6):9535–9550.
- Guo J, Wu Q, Peng X, et al. miR-509-5p inhibits the proliferation and invasion of osteosarcoma by targeting TRIB2. Biomed Res Int. 2019 Dec 16;2019:2523032.
- Li X, Li Y, Wan L, et al. miR-509-5p inhibits cellular proliferation and migration via targeting MDM2 in pancreatic cancer cells. Onco Targets Ther. 2017 Sep 11;10:4455–4464.
- Wang P, Deng Y, Fu X. MiR-509-5p suppresses the proliferation, migration, and invasion of non-small cell lung cancer by targeting YWHAG. Biochem Biophys Res Commun. 2017 Jan 22;482(4):935–941.
- Ma N, Zhang W, Qiao C, et al. The tumor suppressive role of MiRNA-509-5p by targeting FOXM1 in non-small cell lung cancer. Cell Physiol Biochem. 2016;38(4):1435–1446.
- Shen H, Zhang J, Wang C, et al. MDM2-mediated ubiquitination of angiotensin-converting enzyme 2 contributes to the development of pulmonary arterial hypertension. Circulation. 2020 Sep 22;142(12):1190–1204.
- Gopisetty G, Ramachandran K, Singal R. DNA methylation and apoptosis. Mol Immunol. 2006 Apr;43(11):1729–1740.
- Hong L, Sun G, Peng L, et al. The interaction between miR-148a and DNMT1 suppresses cell migration and invasion by reactivating tumor suppressor genes in pancreatic cancer. Oncol Rep. 2018 Nov;40(5):2916–2925.
- Dakhlallah D, Batte K, Wang Y, et al. Epigenetic regulation of miR-17~92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013 Feb 15;187(4):397–405.
- Zhang Y, Xu J. MiR-140-5p regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation, apoptosis and differentiation by targeting Dnmt1 and promoting SOD2 expression. Biochem Biophys Res Commun. 2016 Apr 22;473(1):342–348.
- Ou M, Zhang C, Chen J, et al. Overexpression of MicroRNA-340-5p inhibits pulmonary arterial hypertension induced by APE by downregulating IL-1β and IL-6. Mol Ther Nucleic Acids. 2020 May 22;21:542–554.
- Le Ribeuz H, Courboulin A, Ghigna MR, et al. In vivo miR-138-5p inhibition alleviates monocrotaline-induced pulmonary hypertension and normalizes pulmonary KCNK3 and SLC45A3 expression. Respir Res. 2020 Jul 16;21(1):186.
- Sun L, Lin P, Chen Y, et al. miR-182-3p/Myadm contribute to pulmonary artery hypertension vascular remodeling via a KLF4/p21-dependent mechanism. Theranostics. 2020 Apr 25;10(12):5581–5599.
- Li H, Yang Z, Gao F, et al. MicroRNA-17 as a potential diagnostic biomarker in pulmonary arterial hypertension. J Int Med Res. 2020 Jun;48(6):300060520920430.
- Yang C, Wang Y, Yang W, et al. MiR-509-5p improves the proliferative and invasive abilities of papillary thyroid carcinoma cells by inhibiting SFRP1. Arch Med Sci. 2019 Jul;15(4):968–978.
- Zhang J, Yang C, Wu C, et al. DNA methyltransferases in cancer: biology, paradox, aberrations, and targeted therapy. Cancers (Basel). 2020 Jul 31;12(8):E2123.
- Yang L, Luo P, Song Q, et al. DNMT1/miR-200a/GOLM1 signaling pathway regulates lung adenocarcinoma cells proliferation. Biomed Pharmacother. 2018 Mar;99:839–847.
- Li Y, Li L, Qian Z, et al. Phosphatidylinositol 3-Kinase-DNA methyltransferase 1-miR-1281-histone deacetylase 4 regulatory axis mediates platelet-derived growth factor-induced proliferation and migration of pulmonary artery smooth muscle cells. J Am Heart Assoc. 2018 Mar 7;7(6):e007572.
- Kim GH, Ryan JJ, Marsboom G, et al. Epigenetic mechanisms of pulmonary hypertension. Pulm Circ. 2011 Jul-Sep;1(3):347–356.
