ABSTRACT
Platelets can protect from lipopolysaccharide-induced septic shock by inhibiting inflammation, but it is unknown whether platelets have an anti-atherosclerotic effect. The aim of this study was to investigate the effect of platelet transfusion on atherosclerosis (AS) in a mouse model of AS. Apolipoprotein E deficiency (ApoE−/−) mice were fed with a high-fat diet (HFD) for 8 weeks to establish a mouse model of AS. Mice weekly underwent bi-weekly injection with or without platelets during AS induction (HFD+platelet). Hematoxylin–eosin (H&E), Oil Red O, and Sudan IV stainings were used to assess pathological and morphological changes in the aortic tissue. Lipid levels, and liver and kidney function were examined using an automatic biochemical analyzer. Immune histochemical assays were used to detect the infiltration and distribution of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), IL-6, and monocyte chemotactic protein-1 (MCP-1) in the aortic arch. Western blot and enzyme-linked immunosorbent assay (ELISA) were used to examine the expression levels of TNF-α, IL-1β, IL-6, and MCP-1 in the aorta or the peripheral blood, respectively. Compared with the HFD group, AS pathological lesions from the aortic arch in the HFD+platelet group were significantly smaller and alterations in the lipid metabolism were also less pronounced. Furthermore, TNF-α, IL-1β, IL-6, and MCP-1 levels were all significantly reduced in mice that received platelet injection. Platelets transfusion can effectively ameliorate lipid metabolism, suppress the inflammatory response in the vascular wall, and inhibit the development of AS in mice.
Introduction
Atherosclerosis (AS) is a complex and systemic disease accompanied by chronic inflammation and characterized by an accumulation of high-cholesterol lipid plaques under the intimal layer of large and medium-sized arteries [Citation1]. This accumulation of low-density lipoprotein cholesterol (LDL-C) leads to endothelial cell activation, recruitment of smooth muscle cells and macrophages, and aggregation of other cells. Subsequently, macrophages collect and ingest LDL, transforming it into foam cells, leading to various stages of AS development, and gradually generating an atheromatous plaque [Citation2]. Recent studies have shown that inflammation is the core driving factor during the progression of AS, and macrophages are known to play an essential role in chronic inflammatory processes [Citation3,Citation4]. Macrophages and other inflammatory cells can infiltrate and release tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), IL-6, monocyte chemotactic protein-1 (MCP-1), and other cytokines in the lipid accumulation loci, promoting atheromatous plaque generation and AS development [Citation5]. The various platelet main components, and complex signaling pathways involved in platelet activation and its downstream cascade reaction have remained elusive, making it difficult to fully unravel the mechanisms underlying the role of platelets in physiology and pathology [Citation6,Citation7]. It was generally believed that platelets play a promoting role in the development of AS, but the links between AS and platelets have not been fully elucidated. However, recent work has suggested that platelets can inhibit the macrophage-dependent inflammatory responses, and negatively regulate the expression of IL-6, TNF-α, and other inflammatory factors closely associated with the pathogenesis of AS [Citation8]. These findings suggest that the links between platelets and AS may be different than previously appreciated. However, whether platelets at certain concentrations have an anti-atherosclerotic effect is still unknown. Therefore, we carried out an investigation of the role of platelets in the inflammatory response and AS in aortic tissue in a mouse model of AS.
Materials and methods
Mouse platelets preparation and transfusion
Blood was collected from the right ventricle of isoflurane-anesthetized C57BL/6 J mice (8–10 weeks) using 1/7 volume of ACD as an anticoagulant containing 85 mM trisodium citrate, 83 mM dextrose, and 21 mM citric acid [Citation9]. After standing for 30 min, platelets were washed once with CGS (0.12 M sodium chloride, 0.0129 M trisodium citrate, 0.03 M D-glucose, pH 6.5), centrifuged at 3500 rpm for 10 min at room temperature, resuspended in saline at a concentration of 5 × 10 9 /ml, and then incubated for 1 h at 22°C before transfusion. For platelet injection, 1 × 10 9 purified platelets resuspended in 0.2 ml saline were injected into the tail vein twice a week in the morning around 9–10 o’clock. Mice in the control group received the same volumes of normal saline at the same injection site.
AS induction in mice
Eight- to ten-week-old male apolipoprotein-E-deficient (ApoE−/−) and C57BL/6 J mice were purchased from Beijing Weitonglihua Experimental Animal Technology Co., Ltd. (Beijing, China). Mice were bred and maintained in the Zhejiang Academy of Medical Sciences (Hangzhou, China). All mice were housed in an environment with a temperature of 22 ± 1°C, relative humidity of 50 ± 1%, and a light/dark cycle of 12/12 h. All animal experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
ApoE−/− mice were randomly divided into a high-fat diet group (HFD, n = 10) and a platelet transfusion group (HFD+platelet, n = 10). Mice in both groups were fed with a normal chow diet for 1 week and then fed with HFD (Clinton/Cybulsky D12108; contains 21% fat and 1.25% cholesterol, w/w) for 8 weeks. Mice in the HFD+platelet group received platelet transfusion as described above. For platelet counts, whole blood (100 μl) was obtained from the retro-orbital plexus of isoflurane-anesthetized mice (IsoFlo, Abbott Laboratories, Abbott Park, IL). Platelet count was performed with a TEK8500 H4-0502 multispecies hematology analyzer.
Blood chemistry and cytokine measurements analysis
After food was withheld for 12 h, mice were anesthetized by an intraperitoneal injection of 2,2,2-tribromoethanol (Sigma Chemical Co., St. Louis, MO). Blood samples (700 ~ 800 μl for each mouse) were obtained from the retro-orbital plexus. Blood samples were centrifuged at 3500 rpm for 10 min, and the upper serum was collected and frozen at −20°C for later analysis. Fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (Scr), and blood urea nitrogen (BUN) levels were measured using an automatic biochemical analyzer (AU5421, Olympus, Tokyo, Japan). TNF-α, IL-1β, and IL-6 were measured by enzyme-linked immunosorbent assay (ELISA) (NeoBioscience, Beijing, China) according to the manufacturer’s instructions.
Blood pressure measurement
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were measured using a noninvasive tail-cuff system (BP-98A, Softron, Tokyo, Japan) as described previously [Citation10].
Histology and morphometric analysis
At the end of the experiments, aortas were excised quickly and rinsed with normal saline. Whole aortas were fixed with 4% paraformaldehyde for 24 h, which were paraffin embedded and sliced into 4 μm sections. Hematoxylin–eosin (H&E) staining (G1121, Solable) of the aortic arch samples was performed to inspect the aortic media’s shape, arrangement, and atherosclerotic plaque under a light microscope (BX52, Olympus, Tokyo, Japan). Then, the aortic media and atherosclerotic plaque distribution in each section were assessed using the Image-Pro Plus 5.0 analysis software (Media Cybernetics, Houston, TX).
For the assessment of the atherosclerotic plaque, two staining methods were used in this study. Oil Red O staining was used to examine the aortic arch, and the Sudan IV staining was used to examine the entire aortic tree. Vessel frozen sections (4 μm) were stained with Oil Red O (Sigma, St. Louis, Missouri, USA) for 10 min, washed with 60% isopropanol for 1 min, and then counterstained with hematoxylin for 30 s. Frozen aortic trees were stained with Sudan IV (Solarbio, Beijing, China) for 2 min, washed with distilled water for 1 min, and the atherosclerotic plaque area was analyzed using the Image-Pro Plus 5.0 analysis software (Media Cybernetics, Houston, TX).
Immunohistochemistry
Immunohistochemistry was carried out as previously described [Citation11]. Briefly, the aortic arch sections were deparaffinized in xylene and rehydrated in a gradient of ethanol to distilled water. Endogenous peroxidase activity was blocked using 0.6% hydrogen peroxide in methanol for 15 min. Nonspecific antibody binding was blocked using 5% bovine serum albumin, and sections were incubated with rabbit anti-TNF-α antibody, anti-IL-1β antibody, anti-IL-6 antibody, and MCP-1 antibody (Cell Signaling Technology, Inc., USA). Sections were then washed, and biotin-conjugated anti-rabbit IgG was used followed by incubation with the streptavidin-biotin-HRP system (DakoCytomation). Antibody binding was visualized by 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (Sigma, St Louis, Missouri, USA) staining and counterstaining with hematoxylin. No staining was detected in slides incubated without the primary antibody. The images were visualized under a light microscope (BX52, Olympus, Tokyo, Japan) and quantified with Optimas (Media Cybernetics, version 6.2).
Western blot analysis
Individual aortas were homogenized using Pierce’s RIPA‐buffer (500 µl/50 mg of tissue) with PMSF protease inhibitor, a protease inhibitor cocktail (Cell Signaling Technology, Inc., USA), and phosphatase inhibitor cocktail (Becton, Dickinson and Company, USA), and incubated on ice for 30 min. Homogenized samples were centrifuged at 10,000 g for 10 min at 4°C, and clear supernatants were collected. Protein concentrations were determined by the BCA assay. Fifty micrograms of protein extract was loaded and electrophoresed on 8–15% SDS polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). The membranes were subsequently probed with anti-TNF-α, anti-IL-1β, anti- IL-6, anti-MCP-1, and anti-β-actin antibodies (Cell Signaling Technology, Inc., USA) and then with a secondary antibody linked with horseradish peroxidase. The enhanced chemiluminescence (ECL) method was used to detect the conjugated horseradish peroxidase. The optical density for each ladder was obtained using the ImageJ software, and relative changes were calculated.
Statistical analysis
The SPSS 20.0 statistical software was used for statistical analysis. The data were presented as means ± standard deviation (SD) and were analyzed using one-way ANOVA or Student’s t-tests (unpaired or paired). A P value <0.05 was considered statistically significant.
Results
Platelet transfusion has no effect on general characteristics of ApoE−/− mice
Platelets from randomly selected mice from the two groups (HFD and HFD+platelet group) were counted twice a week before and during modeling time. Platelet counts were not statistically significant between groups before modeling, although counts increased by ~25% after transfusion with 1 × 109 platelets ().
Figure 1. Platelet transfusion from wild-type C57BL/6 mice significantly increased platelet count but had no other substantial effects. (a) Platelet count before and after platelet transfusion (48 h). (b) Weekly body weight. (c) Weekly water intake. (d) Weekly food intake.
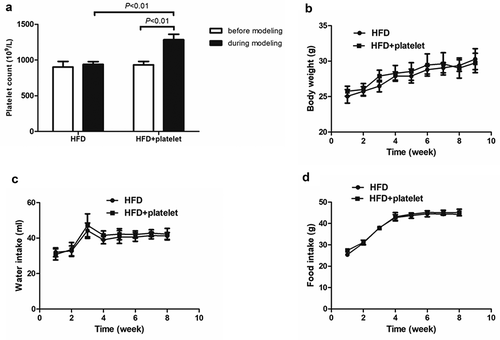
To the best of our knowledge, this study is the first to explore whether platelet transfusion can prevent atherosclerosis in ApoE−/− mice. It was investigated whether platelet transfusion from wild-type C57BL/6 mice had an effect on the general characteristics of ApoE−/− mice. As shown in , body weight, water intake, and food intake were similar throughout the experiment between the HFD and HFD+platelet group ().
Platelet transfusion affected platelet counts, but no other blood parameters
Although ApoE−/− mice were injected with purified platelets derived from the same genetic background (wild-type C57BL/6 mice), and the effect on the blood system, and liver and kidney function were investigated. White blood cells (WBCs), red blood cells (RBCs), and platelets were measured. There were no significant differences in WBC, lymphocyte (LYM), neutrophil (NEU), monocyte (MON), eosinophil (EOS), and basophil counts between the two groups (). In addition, RBC count, hemoglobin concentration (HGB), hematocrit (HCT), mean red blood cell volume (MCV), mean corpuscular hemoglobin amount (MCH), mean erythrocyte hemoglobin concentration (MCHC), red blood cell volume distribution width (RDW-CV), and red blood cell volume distribution width (RDW-SD) were similar between the HFD and HFD+platelet groups (). Finally, there was also no significant difference in mean platelet volume (MPV), platelet packed product (PCT), platelet distribution width (PDW), and large platelet count (P-LCC) between groups ().
Figure 2. Platelet transfusion from wild-type C57BL/6 mice had affected except platelet count, liver and kidney function, but had no other effect on blood system. (a) White blood cell counts. (b) Red-blood-cell-related indicators. (c) Platelet-cell-related indicators. (d) Liver enzyme levels. (e) Kidney function indicators. Abbreviations: HFD, high-fat diet; WBC, white blood cell; LYM, lymphocyte; NEU, neutrophils; MON, monocyte; EOS, eosinophils; BAS, basophils; RBC, red blood cell; HGB, hemoglobin concentration; HCT, hematocrit; MCV, mean red blood cell volume; MCH, mean corpuscular hemoglobin amount; MCHC, mean erythrocyte hemoglobin concentration; RDW-CV, red blood cell volume distribution width; RDW-SD, red blood cell volume distribution width; MPV, mean platelet volume, PCT, platelet packed product; PDW, platelet distribution width; P-LCC, large platelet count.
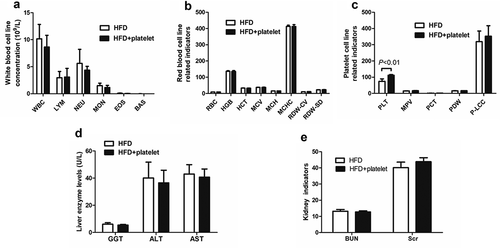
The effects of platelet transfusion on liver and kidney function in mice on HFD were also examined. Serum concentrations of liver enzymes including glutamyltransferase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were all within the normal range, and no differences between the two groups were observed (). Additionally, there was no significant difference in Scr and BUN between the HFD and HFD+platelet groups ().
Effect of platelet transfusion on risk factors for atherosclerosis
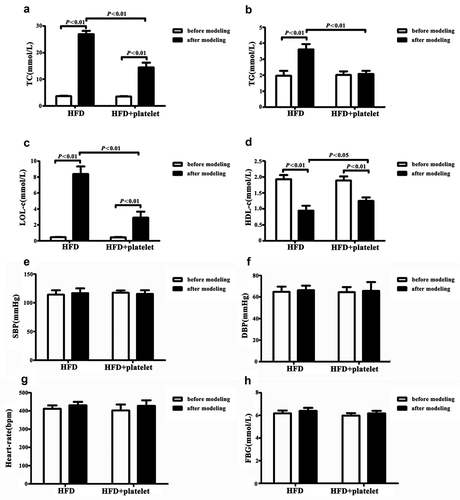
At the end of the experiment, atherosclerotic risk factors including lipid profile, blood pressure (BP), and fasting blood glucose (FBG) were examined in both groups. Lipid testing indicated that TC, TG, and LDL-C levels in the HFD group were significantly higher after modeling than pre-modeling, while HDL-C was significantly decreased (). Mice in the HFD+platelet group had higher levels of TC and LDL-C than those in the pre-modeling group, although there was no significant change in TG (). When compared with the HFD group, TC and LDL-C in the HFD+platelet group were found to be significantly decreased but not HDL-C (). These results suggest that platelets could ameliorate “bad cholesterol” and improve “good cholesterol” in ApoE−/− mice fed with HFD. However, platelet transfusion had no effect on BP, heart rate, and FBG ().
Figure 3. Alteration in lipid metabolism in atherosclerotic mice were significantly ameliorated by platelet intervention, but blood pressure and glucose levels remained unchanged. (a) Total cholesterol (TC) levels. (b) Triglycerides (TG) levels. (c) Low-density lipoprotein cholesterol (LDL-C) levels. (d) High-density lipoprotein cholesterol (HDL-C) levels. (e) Systolic blood pressure (SBP) levels. (f) Diastolic blood pressure (DBP) levels. (g) Heart rate levels. (h) Fasting blood glucose (FBG) levels. Abbreviations: HFD, high-fat diet.
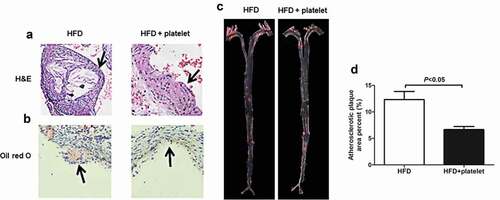
Platelet transfusion alleviates atherosclerotic plaque
H&E and Oil Red O stainings showed that the mice in the HFD group had a larger accumulation of lipids under the intima of the aorta, and more foam cells aggregations (). These aggregations developed into AS second-phase fibrous plaque stage, with the severe cases progressing into the third AS phase atheromatous plaque stage. However, this situation could be reversed by platelet transfusion, and the atherosclerotic plaques in the HFD+platelet group were all mild. Sudan IV staining also showed that the plaque area of the aortic tree in the HFD+platelet group was significantly smaller than that in the HFD group ().
Figure 4. Platelet transfusion from wild-type C57BL/6 mice suppressed plaque formation in the aorta from atherosclerotic mice. (a) Representative photographs of Hematoxylin–Eosin (H&E) staining. (b) Atherosclerotic plaque of aortic sections stained with Oil Red O staining. (c) Atherosclerotic plaque of the aortic tree stained with Sudan IV. (d) Quantitative analysis of the atherosclerotic plaque area in the aortic tree.
Platelet transfusion inhibits inflammatory factors production
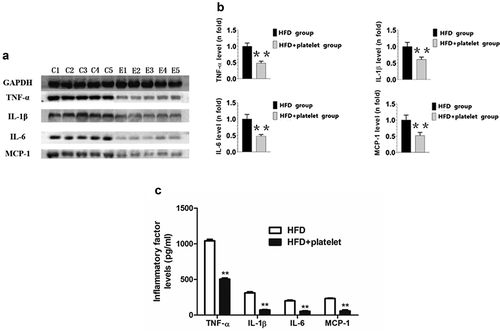
To investigate whether platelets could inhibit the production of inflammatory factors in ApoE−/− mice, immunohistochemical assays and immunoblot were used to analyze the distribution and infiltration of inflammatory factors in the aortic arch. ELISA assay was used to detect inflammatory factors in the peripheral blood. Immunohistochemical results showed that TNF-α, IL-1β, IL-6, and MCP-1 were distributed and unfiltered at the aortic arch plaque in mice in the HFD group, but were all significantly decreased in the HFD+platelet group (). In addition, Western blot results indicated that the expression of these inflammatory factors in the HFD+platelet group was significantly reduced after platelet transfusion (). A similar trend was observed by ELISA ().
Figure 5. Platelet transfusion from wild-type C57BL/6 mice inhibited infiltration of inflammatory factors in the aorta from atherosclerotic mice after fed with a high-fat diet (HFD) for 8 weeks. (a) and (c) Immunostaining for tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and monocyte chemoattractant protein-1 (MCP-1); the black arrow represents the lesion. (b) and (d) An enlarged view of the part pointed by the black arrow in (a) and (c) respectively; and the red arrow showing positive cells for each cytokine. (e)–(h) Quantitative analysis of TNF-α, IL-1β, IL-6, and MCP-1; cytokines + index means mean density, defined as positive integrated optical density (IOD)/pixel area of the tissue (AREA) for each immunohistochemical image. Abbreviations: HFD, high-fat diet.
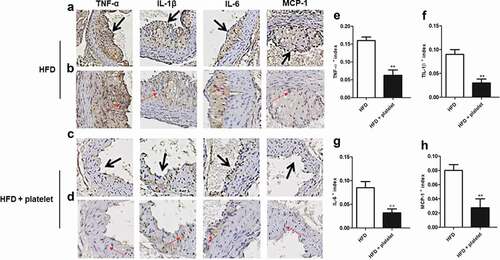
Figure 6. Platelet transfusion from wild-type C57BL/6 mice inhibited expression of inflammatory proteins in the aorta and in the peripheral blood from atherosclerotic mice; inflammatory cytokines were measured after fed with a high-fat diet (HFD) for 8 weeks with or without platelet transfusion. (a) Protein band diagram, C1–C5 represent the high-fat diet (HFD) group, E1–E5 represent the HFD+platelet group. (b) Western blot analysis of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and monocyte chemoattractant protein-1 (MCP-1). The ratio of TNF-α, IL-1β, IL-6, or MCP-1 proteins is set to “1” for the HFD group. (c) TNF-α, IL-1β, IL-6, and MCP-1 were measured by enzyme-linked immunosorbent assay (ELISA) from peripheral blood.
Discussion
The main findings of this study were as follows: (1) lipid metabolism in atherosclerotic mice was altered, and platelet intervention reduced TC, TG, and LDL-C levels, but elevated HDL levels. (2) The atheromatous plaque and inflammatory factors including TNF-α, IL-1β, IL-6, and MCP-1 in aortic tissue or peripheral blood from atherosclerotic mice improved upon exogenous platelet administration.
Lipid metabolism disturbance is a very typical feature of AS; they are often accompanied by increased levels of TC, TG, and LDL-C and decreased HDL-C in the body [Citation12,Citation13]. In addition, atherosclerotic plaques in the subintimal aorta are the most direct evidence of AS [Citation14]. Similar to the changes in blood lipids reported in previous atherosclerosis studies [Citation15,Citation16], the present study showed that TC, TG, and LDL-C levels were significantly increased following 8 weeks of HFD, and the HDL-C level was significantly reduced. In addition, H&E staining revealed a very obvious atheromatous plaque in the aortic tissue following 8 weeks of HFD, a morphological change that was consistent with the studies of Zhu and Geng et al. [Citation17,Citation18]. Taken together, these findings indicate that we successfully established a model of AS.
Based on epidemiological and clinical studies, TC, TG, LDL-C, and HDL-C levels are the landmark for evaluating AS. Among them, TC levels are correlated with the occurrence and development of cardiovascular and cerebrovascular diseases, especially in AS [Citation19]. Moderately increasing TG levels could result in cellular lipoprotein remnants overload and macrophage recruitment to ultimately recognize and clear these remnants. In turn, elevated TG levels also lead to the foam cells formation, plaque progression, and activation of inflammatory reactions in vivo, promoting the occurrence and development of AS [Citation20,Citation21]. In the blood, accumulated LDL-C undergoes oxidation and can enhance monocyte infiltration and foam cell formation, and is an early inducing factor of AS [Citation22,Citation23]. Nevertheless, HDL-C can effectively protect LDL-C from oxidative damage, impede the expression of adhesion molecules, and inhibit AS, suggesting an inhibiting effect on the development of AS [Citation24]. Interestingly, alterations in lipid metabolism in mice could be ameliorated by injecting platelets. Upon platelet injection, the TC, TG, and LDL-C levels in the HFD+platelet group decreased significantly, and HDL-C increased significantly when compared with the HFD group, indirectly suggesting that platelet intervention had an inhibitory effect on the occurrence and development of AS. These findings were confirmed in histopathology analysis. Histopathology revealed that the aortic arch AS lesion in mice from the HFD group had a large accumulation of lipids under the intima of the aorta, and developed into AS second-phase fibrous plaque stage, and in the severe cases, it progressed into the third AS phase atheromatous plaque stage. Interestingly, these results were reversed by platelet intervention, as the degree of lipid accumulation under the aorta intima and foam cell accumulation in the HFD+platelet group decreased, and the atheromatous plaque was mild in mice from the HFD+platelet group. These findings suggest that platelet transfusion significantly impeded the occurrence and development of AS.
Recent studies have shown that the occurrence and development of AS go through three stages: foam cell and lipid streak phase, fibrous plaque phase, and atheromatous plaque phase [Citation2]. In these stages, the release of various inflammatory factors and the occurrence of inflammatory response are the core factors that promote the formation and development of AS [Citation3–5]. MCP-1, TNF-α, IL-1β, and IL-6 are inflammatory markers that can trigger and promote the occurrence and development of AS. Among them, MCP-1 is recognized as an important cytokine with the ability of chemotaxis and activation of monocytes/macrophages. MCP-1 can cause a variety of inflammatory cells, especially monocytes, to aggregate in lesion loci and become associated with other inflammatory factors that can promote differentiation of monocytes into macrophages. MCP-1 can also promote macrophage migration, aggregation, and phagocytosis of ox-LDL, which in turn promotes macrophage transformation into foam cells. Furthermore, in the final phase of AS, MCP-1 can also induce plaque rupture [Citation25–27]. TNF-α can significantly increase the ability of LDL transport in endothelial cells and enhance the retention of LDL particles in the blood vessel wall, induce the proliferation of smooth muscle cells by releasing adhesion molecules, and stimulate the apoptosis of macrophages, smooth muscle cell, and other cells, eventually leading to the formation of a necrotic core in the final phase of AS [Citation28,Citation29]. IL-1β can strengthen inflammatory cells activation and endothelial cells adhesion, and promote the proliferation of vascular smooth muscle cells. Delphine et al. suggested that injection of IL-1β antibody can effectively reduce the formation of atheromatous plaque in a mouse model of AS [Citation30,Citation31]. IL-6 can damage the vascular endothelium, stimulate smooth muscle cells to secrete inflammatory mediators, and affect plaque stability [Citation32]. To examine the inflammatory response in AS lesions, we used immunohistochemical assays to analyze the distribution and infiltration of inflammatory cytokines in the aortic arch. In addition, we used immunoblot and ELISA to detect the levels of aortic inflammatory cytokines. Immunohistochemical analysis showed that the levels of TNF-α, IL-1β, IL-6, and MCP-1 in HFD group were distributed and unfiltered at the plaque of aortic arch but were significantly decreased after platelet transfusion in mice from the HFD+platelet group; immunoblot and ELISA analysis showed similar findings. Overall, these results indicate that arterial vascular inflammation in atherosclerotic mice can be effectively inhibited via platelet intervention.
To the best of our knowledge, this is the first report showing that platelet transfusion can lower “bad cholesterol” levels and increase “good cholesterol” levels. Additionally, this study is also the first to indicate that platelet transfusion can prevent AS in atherosclerotic mice. These findings differ from the traditional view that platelets promote the development of AS. However, the potential mechanisms underlying this phenomenon are poorly understood. Platelets play an important role in physiological hemostasis and pathological arterial thrombosis [Citation33]. In recent years, more and more evidence point out to platelets as an important component of the inflammatory immune system, and systemic inflammation may be accompanied by platelet activation in patients [Citation34,Citation35]. Recent studies have suggested a role for endocytic trafficking in platelet function. Platelets can endocytose cargo via two ways: clathrin-dependent endocytosis, which requires GTP hydrolysis and requires Dynamin and specific surface receptors, and clathrin-independent endocytosis, which may require Dynamin and occurs via caveolin- or RhoA-dependent pathways or may not need Dynamin and occurs via ADP-ribosylation factor 6 (Arf6)- or Cdc42-dependent pathways [Citation36]. Moreover, in 2019, Carestia et al. reported that platelets could sequester cytokines (TNF-α, IL-6, and IL-10) released by lipopolysaccharide (LPS)-stimulated monocytes [Citation37]. In that study, platelets were co-cultured with monocytes and stimulated with LPS at the same time. High levels of cytokines (TNF-α, IL-6, and IL-10) were measured in the absence of platelets, whereas no cytokines were detected in LPS-stimulated platelets. Then, platelets were collected after 24-h co-culture, and intracellular cytokines (TNF-α, IL-6, and IL-10) in platelets were observed by flow cytometry or confocal microscopy [Citation37]. These results suggest that sequestration may have led to reduced cytokines (TNF-α, IL-6, and IL-1) and “bad cholesterol” in the HFD+platelet group. However, future studies should be performed to validate this hypothesis.
In summary, this study revealed that platelets can effectively reduce TC, TG, and LDL-C levels, suppress the inflammatory response in the vascular wall, and inhibit the development of AS in a mouse model of AS. All together, these findings uncover a previously unappreciated role of platelets in AS.
Acknowledgments
We are grateful to all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shapiro MD, Fazio S. From lipids to inflammation: new approaches to reducing atherosclerotic risk[J]. Circ Res. 2016;118(4):732–749.
- Mclaren JE, Michael DR, Ashlin TG, et al. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy[J]. Prog Lipid Res. 2011;50(4):331–347.
- Williams JW, Giannarelli C, Rahman A, et al. Macrophage biology, classification, and phenotype in cardiovascular disease: JACC macrophage in CVD series (Part 1)[J]. J Am Coll Cardiol. 2018;72(18):2166–2180.
- Bories G, Leitinger N. Macrophage metabolism in atherosclerosis[J]. FEBS Lett. 2017;591(19):3042–3060.
- Ma J, Liu C, Yang Y, et al. C/EBPbeta acts upstream of NF-kappaB P65 subunit in Ox-LDL-induced IL-1beta production by macrophages[J]. Cell Physiol Biochem. 2018;48(4):1605–1615.
- Yeung J, Li W, Holinstat M. Platelet signaling and disease: targeted therapy for thrombosis and other related diseases[J]. Pharmacol Rev. 2018;70(3):526–548.
- van der Meijden P, Heemskerk J. Platelet biology and functions: new concepts and clinical perspectives[J]. Nat Rev Cardiol. 2019;16(3):166–179.
- Xiang B, Zhang G, Guo L, et al. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway[J]. Nat Commun. 2013;4(1):2657.
- Zhang G, Xiang B, Ye S, et al. Distinct roles for Rap1b protein in platelet secretion and integrin αIIbβ3 outside-in signaling[J]. J Biol Chem. 2011;286(45):39466–39477.
- Kanda T, Hayashi K, Wakino S, et al. Role of Rho-kinase and p27 in angiotensin II-induced vascular injury[J]. Hypertension. 2005;45(4):724–729.
- Lewis P, Stefanovic N, Pete J, et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice[J]. Circulation. 2007;115(16):2178–2187.
- Remmerie A, Scott CL. Macrophages and lipid metabolism[J]. Cell Immunol. 2018;330:27–42.
- Ai XM, Ho LC, Han LL, et al. The role of splenectomy in lipid metabolism and atherosclerosis (AS)[J]. Lipids Health Dis. 2018;17(1):186.
- Emini VB, Perrotta P, De Meyer G, et al. Animal models of atherosclerosis[J]. Eur J Pharmacol. 2017;816:3–13.
- Ding L, Chang M, Guo Y, et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism[J]. Lipids Health Dis. 2018;17(1):286.
- Li J, Lin S, Vanhoutte PM, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice[J]. Circulation. 2016;133(24):2434–2446.
- Zhu B, Zhai Y, Ji M, et al. Alisma orientalis beverage treats atherosclerosis by regulating gut microbiota in ApoE−/− mice. Front Pharmacol. 2020;11:570555.
- Geng J, Yang C, Wang B, et al. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway[J]. Biomed Pharmacother. 2018;97:941–947.
- Gotto AJ, Brinton EA. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update[J]. J Am Coll Cardiol. 2004;43(5):717–724.
- Rosenson RS, Davidson MH, Hirsh BJ, et al. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease[J]. J Am Coll Cardiol. 2014;64(23):2525–2540.
- Dron JS, Hegele RA. Genetics of triglycerides and the risk of atherosclerosis[J]. Curr Atheroscler Rep. 2017;19(7):31.
- Chen T, Huang W, Qian J, et al. Macrophage-derived myeloid differentiation protein 2 plays an essential role in ox-LDL-induced inflammation and atherosclerosis[J]. EBioMedicine. 2020;53:102706.
- Du Y, Li X, Su C, et al. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice[J]. Br J Pharmacol. 2020;177(8):1754–1772.
- Kosmas CE, Martinez I, Sourlas A, et al. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease[J]. Drugs Context. 2018;7:212525.
- Kizilay MO, Lora M, Shum-Tim D, et al. A proinflammatory secretome mediates the impaired immunopotency of human mesenchymal stromal cells in elderly patients with atherosclerosis[J]. Stem Cells Transl Med. 2017;6(4):1132–1140.
- Basurto L, Gregory MA, Hernandez SB, et al. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women[J]. Exp Gerontol. 2019;124:110624.
- Hamlat-Khennaf N, Neggazi S, Ayari H, et al. [Inflammation in the perivascular adipose tissue and atherosclerosis][J]. C R Biol. 2017;340(3):156–163.
- Zhang Y, Yang X, Bian F, et al. TNF-alpha promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-kappaB and PPAR-gamma[J]. J Mol Cell Cardiol. 2014;72:85–94.
- Tay C, Liu YH, Hosseini H, et al. B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation[J]. Cardiovasc Res. 2016;111(4):385–397.
- Gomez D, Baylis RA, Durgin BG, et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice[J]. Nat Med. 2018;24(9):1418–1429.
- Khan R, Rheaume E, Tardif JC. Examining the role of and treatment directed at IL-1β in atherosclerosis. Curr Atheroscler Rep. 2018;20(11):53.
- Yeh CC, Wu JY, Lee GL, et al. Vanadium derivative exposure promotes functional alterations of VSMCs and consequent atherosclerosis via ROS/p38/NF-kappaB-mediated IL-6 production[J]. Int J Mol Sci. 2019;20(24):6115.
- Qiao J, Wu X, Luo Q, et al. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis[J]. Haematologica. 2018;103(9):1568–1576.
- Morrell CN, Aggrey AA, Chapman LM, et al. Emerging roles for platelets as immune and inflammatory cells[J]. Blood. 2014;123(18):2759–2767.
- Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation[J]. Front Immunol. 2018;9:2712.
- Banerjee M, Whiteheart SW. The ins and outs of endocytic trafficking in platelet functions[J]. Curr Opin Hematol. 2017;24(5):467–474.
- Carestia A, Mena HA, Olexen CM, et al. Platelets promote macrophage polarization toward pro-inflammatory phenotype and increase survival of septic mice[J]. Cell Rep. 2019;28(4):896–908.
