ABSTRACT
Lysyl oxidase-like 2 (LOXL2) is a member of the lysine oxidase (LOX) family. Although its overexpression is known to play pivotal roles in carcinogenesis, its involvement in cervical cancer remains undefined. Here, we comprehensively explored the expression level and functional mechanism of LOXL2 in cervical cancer using bioinformatics and experimental methods. Bioinformatics analysis revealed that LOXL2 was significantly upregulated in cervical cancer compared to normal tissues. Enrichment analysis showed that most positively or negatively correlated genes of LOXL2 were correlated with extracellular matrix (ECM) formation and epithelial-mesenchymal transition (EMT). Further experiments confirmed that overexpression of LOXL2 greatly enhanced the malignant transformation abilities (e.g. proliferation, invasion, and migration) of cervical cancer cells via mediation of EMT. Furthermore, the small molecule inhibitor of LOXL2 ((2-Chloropyridin-4-yl) methanamine hydrochloride) significantly decreased the invasive ability of cervical cancer by reversing the process of LOXL2-induced EMT. In summary, LOXL2 may be a promising diagnostic and therapeutic biomarker for cervical cancer, and its small molecule inhibitor may be an effective anti-tumor drug.
Abbreviations: LOXL2 Lysyl oxidase-like 2; LOX lysine oxidase; CI confidence interval; HR hazard ratio; ECM extracellular matrix; EMT epithelial-mesenchymal transition; OS overall survival; IC50 median inhibitory concentration; PPI protein-protein interaction.
KEYWORDS:
Introduction
Cervical cancer is one of the most common fatal malignancies in women, which seriously threatens women’s health [Citation1,Citation2]. Persistent infection of high-risk HPVs is the main leading cause of cervical cancer, whose onco-proteins (E5, E6 and E7) can regulate host cell proliferation, apoptosis, differentiation, immune escape [Citation3–7]. It is estimated that there will be approximately 13800 new cases of cervical cancer in the United States in 2020, and 4290 new deaths [Citation8]. Furthermore, in many developing countries, a large proportion of cervical cancer patients are diagnosed at an advanced-stage with an unfavorable five-year overall survival (OS) due to inadequate and underdeveloped treatment and prevention (e.g. HPV vaccine) strategies [Citation9]. At present, treatments for advanced-stage patients, such as radiotherapy and chemotherapy, are nonspecific and less effective at controlling recurrence or distant metastasis. Although blocking PD1/PDL-1 is recommended as a targeted treatment for advanced cervical cancer, not all patients benefit from this strategy due to the low positive rate of PD1/PD-L1 in cervical cancer [Citation10–12]. Therefore, the identification of effective and specific therapeutic targets for cervical cancer is critical for improving prognosis in advanced-stage patients.
As a member of the lysine oxidase (LOX) family, lysyl oxidase-like 2 (LOXL2) can catalyze the covalent crosslinking of collagen and elastin by secretion into the extracellular environment, ultimately improving the synthesis and stability of the extracellular matrix (ECM) [Citation13–15]. Differentially expressed LOXL2 is not only related to nonmalignant tumors (e.g. cardiovascular diseases and fibrosis), but also plays key roles in the occurrence and development of cancer by affecting the biological functions of cell growth, adhesion, migration, and invasion [Citation16–20]. LOXL2 is over-expressed in oral squamous cell carcinoma, breast cancer, colon cancer, and head and neck squamous cell carcinoma, and is significantly associated with poor prognosis [Citation21–24]. Furthermore, several inhibitors targeting LOXL2 can impede the invasive ability of cancer cells in vitro and in vivo (e.g. PSX-C1, AB0023) [Citation25,Citation26]. These findings therefore indicate that LOXL2 may be a promising therapeutic target in cancers.
Currently, there is still a considerable knowledge gap regarding functional underlying mechanism of LOXL2 in cervical cancer. As such, we explored LOXL2 expression and its significance in cervical cancer using bioinformatics. We then validated the preliminary findings at the in vivo and in vitro level, and explored the effects of a specific LOXL2 inhibitor on the biological functions of cervical cancer cells.
Materials and methods
Bioinformatics analysis
In our study, we first used the ONCOMINE database (https://www.oncomine.org/resource/login.html#) to explore the expression level of LOXL2 in cervical cancer, and further measured its significances on the prognosis using the Kaplan–Meier plotter (http://kmplot.com/analysis/index.php?p=service&cancer=pancancer_rnaseq) [Citation27]. We then explored gene mutation characteristics of LOXL2 using the c-Bio Cancer Genomics Portal (c-BioPortal) (https://www.cbioportal.org/) and the COSMIC database (http://cancer.sanger.ac.uk) [Citation27–32]. We also identified positively or negatively correlated genes of LOXL2 in cervical cancer using the LinkedOmics (http://www.linkedomics.org/), and further performed enrichment analysis on these correlated genes to identify possible signaling pathways mediated by LOXL2 in cervical cancer using the FunRich3.1.3 [Citation33,Citation34].
Cells culture
The human cervical cancer cell lines HELA and SIHA were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% fetal bovine serum (SH30406.05, Hyclone) and antibodies (100 U/ml penicillin plus streptomycin). They were cultured at 37°C in an incubator containing 5%CO2.
RNA interference
SIHA and HELA cell lines were cultured in a six-well plate to 50% density, then transfected with Ubi-MCS-3FLAG-CBh-gcGFP-IRES-puromycin lentiviral particles purchased from Gene Chem (Shanghai, China), and then screened with 0.4 μg/ml puromycin (A1113803, Gibco) for 2 wk. Over-expression of LOXL2 (NM_002318) was further confirmed by qRT-PCR and western blot.
Western blot
After the cells were collected, RIPA lysis buffer (P0013B, Beyotime) containing 1% cocktail (04693132001, Roche) was added to extract the total protein. 30 μg protein was used to SDS-PAGE (8,012,011, BioSci) gel electrophoresis, then transferred protein onto PVDF membrane (10,600,023, GE) at 4°C for 1.5 hours, and seal with 5% skim milk at room temperature for 1 hour. Then, diluted LOXL2 (A4708, ABclonal, 1:1000), Snail1 (A11794, ABclonal,1:1000), E-Cadherin (A11492, ABclonal, 1:1000), N-Cadherin (A19083, ABclonal, 1:1000), GAPDH (A19056, ABclonal, 1:1000), Caspase 8 (66,093-1-Ig, Proteintech, 1:1000), Bim (ab32158, abcam, 1:1000), Bak (ab32371, abcam, 1:2000) were used to incubate with PVDF membrane at 4°C overnight. After the first antibody was washed by tris-buffered saline-Tween (TBST), the HRP Goat anti-rabbit IgG(H + L) (AS014, ABclonal,1:5000) was used to incubate with PVDF membrane at 37°C for 1 hour. Finally, the western bright ECL HRP substrate (K-12045- C20, Advansta) luminescence was used to detect signals of PVD membrane, and further analysis was performed by Image Lab (v4.1).
RNA extraction, PCR and qRT-PCR
Trizol reagent (15,596,026, Invitrogen) was used to extract the total RNA of the collected cell residue, then the HiScript II Q RT SuperMix (R223-01, Vazyme) was used to synthesize the cDNA. Finally, under the guidance of manufacturer’s constructions. Finally, the expression levels of targeted genes were detected by Bio-Rad CFX96 Real-Time System manager (C1000 Thermal Cycler). GAPDH was regarded as an internal reference. The specific primer sequences of targeted genes were shown in Supplementary Table S1.
Colony formation assay
One thousand cervical cancer cells were cultured in each six-well plate with a complete DMEM for 14 d. These six-well plates were washed by phosphate buffer saline (PBS) for three times after the medium was discarded, and then fixed by 4% paraformaldehyde for 10 minutes and stained by 0.5% crystal violet for 10 minutes.
Wound healing assay
5*10^5 cervical cancer cells were planted in each six-well plate. After the cells adhered the next day, a 200 μl pipette tip was used to draw a vertical cross in each plate, and washed them with PBS for three times. Then the cells were cultured with serum-free medium. The degree of cell migration to the scratched area was observed at 0 hour and 48 hours, respectively.
Transwell invasion and migration assay
Invasion
First, mixed Matrigel was prepared on ice according to the ratio of serum-free medium: Matrigel = 15:1, then 100 μl mixed Matrigel was added to the upper chamber of transwell chamber (3422, Corning Incorporated Costar, 8.0 µm pore size). 200 μl of serum-free medium containing 5*10^4 cells and 600 μl complete DMEM were added to the upper chamber and lower chamber, respectively. The upper chamber was taken out after incubation for 24 hours, take out the chamber, sucked up the excess liquid in the upper chamber, and then was fixed by 4% paraformaldehyde for 10 minutes and stained by 0.1% crystal violet for 30 minutes.
Migration
The other steps are the same as the invasion assay except that the mixed Matrigel was not needed in the upper chamber.
Five fields of every chamber were selected for analysis, and the magnification of the upright microscope was 100X.
Xenografts in nude mice
The animal experiment of our study was approved by the Animal Experiment Ethics Committee of Tongji Medical College. Thirty female BALB/c nude mice (4 wk) were obtained from Beijing HFK Biological Science Co., Ltd. (Beijing, China) and raised in the standard specific-pathogen free (SPF) animal laboratory established by Tongji Hospital. Firstly, 2*10^7 cells with or without over-expressing LOXL2 were injected into the subcutaneous (left armpit) of each mouse. The tumor volume of mice was measured and recorded every 3 d for 2 wk after tumorigenesis.
Secondly, 2*10^7 cells (SIHA of HELA) were injected into the subcutaneous (left armpit) of each mouse, the LOXL2 inhibitor ((2-Chloropyridin-4-yl) methanamine hydrochloride) (HY-101771A, MCE) was prepared according to the manufacturer’s requirements and was administered intraperitoneally at the dose of 10 mg/kg once every 2 d for 3 wk.
Twenty-one d after the nude mice were given the drug, a professionally trained and qualified experimenter used the cervical dislocation method to euthanize the nude mice for the weight of nude mice is less than 200 g according to the AVMA guidelines (2020 edition).
Cell proliferation assay and flow cytometry for cell apoptosis
One thousand cells were planted in each pore of 96-well plate. After the cells adhered to the wall, LOXL2 inhibitor with different concentrations were added into the corresponding pore according to the experimental design. The cell viability was measured by the Cell Counting Kit-8 (CCK-8, Dojindo Laboratories) (absorbance = 450) at 0 hours, 24 hours, 48 hours, 72 hours and 96 hours, respectively. SIHA and HELA cells were harvested after being treated with different concentrations of LOXL2 inhibitor, and further measured by an Annexin V APC (BD Pharmingen, 550,474) and 7-AAD (BD Pharmingen, 559,925). All experiments were performed three times.
3-dimensional (3D) organotypic coculture model assay
Firstly, 50 μl Matrigel was added into the upper chamber of each transwell pore, and incubated at 37°C overnight to make the Matrigel completely solidified. Secondly, a mixture of 50 μl Matrigel, 20 μl fetal bovine serum, 10 μl rat tail collagen, 10 μl 10X serum-free DMEM, and 10 μl 3T3 cell suspension were added into the upper chamber, and then incubated at 37°C for 48 hours. 100 μl serum-free DMEM containing 3*10^4 SIHA or HELA was added into the upper chamber of each pore, and 450 μl complete DEME containing LOXL2 inhibitor was added to the lower chamber. The medium in lower chamber was renewed once a day for 2 wk. Finally, the upper chamber was fixed with 10% paraformaldehyde for 10 minutes and then analyzed.
Statistical analysis
In our study, each group of experiments was repeated three times. Graphpad 6.0 was used for statistical analysis. The experimental results were expressed as the means ± standard deviation (s.d.). The differences between the two groups were compared by independent Student’s t-test. * P < 0.05; ** P < 0.01; *** P < 0.001; ****<0.0001 and ns – not significant.
Results
Overexpression of LOXL2 mRNA and its negative impact on prognosis in cervical cancer
Here, LOXL2 mRNA expression was first explored in cervical cancer using the ONCOMINE dataset. As shown in ), LOXL2 was highly expressed in various cancers, including cervical cancer. Two independent studies (i.e. Scotto Cervix, fold change = 2.375, P = 9.00E-06; Biewenga Cervix, fold change = 1.372, P = 0.005) consistently showed overexpression of LOXL2 in cervical cancer compared to normal tissues, as verified by meta-analysis of two cervical cancer studies (median rank = 2570.0, P = 0.017) (). Prognostic analysis using Kaplan-Meier Plotter indicated that higher expression of LOXL2 in cervical cancer was significantly correlated with poorer OS (Hazard ratio (HR) = 3.17, 95% confidence interval (95% CI) = 1.85–5.43, P = 9.9E-06) and progression-free survival (HR = 4.47, 95% CI = 1.86–10.75, P = 0.00027) ()). Further Kaplan–Meier analysis revealed that up-regulated LOXL2 also had negative effects on OS and progression-free survival in cervical cancer under different clinical subtypes (e.g. grade I/II, high/low mutation burden) and immunocytes abundance (e.g. CD8 + T cells, B cells, Eosinophils) using the Kaplan-Meier Plotter ().
Figure 1. The characteristics of LOXL2 in cervical cancer regarding mRNA expression, prognostic significance, and genomic mutation. (a) The numbers in each box represent the studies that differentially expressed LOXL2 in different cancers from ONCOMINE database. Red represents overexpression and blue represents down-expression, and LOXL2 was significantly overexpressed in cervical cancer. (b), (c) Two studies and the meta-analysis of three cervical cancer studies consistently revealed LOXL2 was significantly up-regulated in cervical cancer compared to normal tissues from ONCOMINE database. (d) Overexpression of LOXL2 was closely correlated with poor prognosis (OS and PFS) in cervical cancer using the Kaplan-Meier Plotter. (e) The mutation frequency of LOXL2 in TCGA (PanCancer Altas, n = 297) using the c-BioPortal. (f) The pie charts represent the mutation types of LOXL2 in cervical cancer using the COSMIC database. OS: overall survival; PFS: progression-free survival. *P < 0.05; ****P < 0.0001.
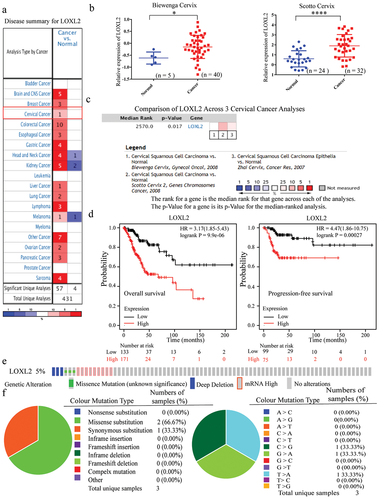
Table 1. The prognostic values of LOXL2 expression in different subtypes regarding clinical parameters in cervical cancer.
Table 2. The prognostic values of LOXL2 expression in different subtypes regarding immunocytes in cervical cancer.
Mutation characteristics of LOXL2 in cervical cancer
The mutation characteristics of LOXL2 in cervical cancer were next explored using c-BioPortal. As seen in ), the mutation frequency of LOXL2 in the Cancer Genome Atlas (TCGA) (PanCancer Atlas, n = 297) was 5%, and mRNA up-regulation was the main genetic alternation. As shown in the pie chart in ), the mutation types of LOXL2 in cervical cancer were further analyzed using the COSMIC database. Results showed that the frequency of mutation was low, and missense substitution (66.67%) was the most common mutation type. In addition, C > G (33.33%), G > A (33.33%) and T > A (33.33%) were the only three nucleotide changes.
Identification of genes correlated with LOXL2 and enrichment analysis
The LinkedOmics database was used to identify genes that were positively or negatively correlated with LOXL2 in cervical cancer. As shown in the volcano plot in ), 10,475 and 9429 genes were positively and negatively correlated with LOXL2, respectively. The top 50 genes positively and negatively correlated with LOXL2 are displayed in ), respectively. A protein–protein interaction (PPI) network was constructed using STRING ()). Further enrichment analysis of these 100 genes was performed using FunRich3.1.3. As shown in ), LOXL2 and its co-expressed genes were mainly enriched in the process of ECM, extracellular, and ECM structural constituents. Further analysis indicated that they were also primarily involved in the EMT biological pathway.
Figure 2. Identification of genes correlated with LOXL2 and their enrichment analysis in cervical cancer. (a) 10,475 and 9,429 identified genes were positively and negatively correlated with LOXL2 in cervical cancer using the LinkedOmics. (b) The top 50 positively correlated significant genes. (c) The top 50 negatively correlated significant genes. (d) The protein–protein interaction network of these top 100 correlated significant genes constructed by the STRING.
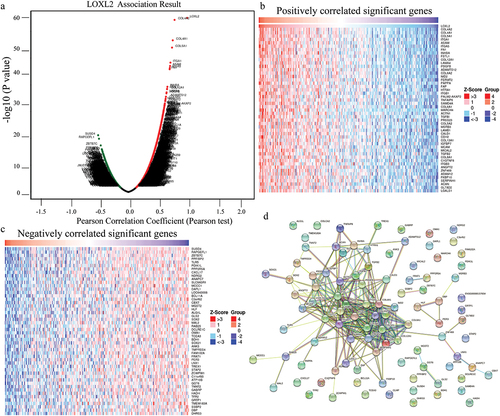
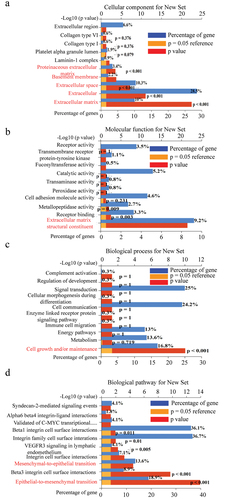
Figure 3. Functional enrichment analysis of genes correlated with LOXL2 using FunRich3.1.3. (a) The cellular component analysis for correlated genes. (b) Molecular function analysis for correlated genes. (c) Biological process analysis for correlated genes. (d) Biological pathway analysis for correlated genes.
LOXL2 promoted malignant transformation of cervical cancer lines by inducing EMT in vitro and in vivo
The above findings were validated in cervical cancer cells at the in vitro and in vivo level. First, LOXL2 expression levels in SIHA and HELA cell lines were increased via lentiviral transfection, as confirmed by quantitative real-time polymerase chain reaction (qRT-PCR) and western blotting ()). Transwell (migration and invasion) and wound healing assays consistently demonstrated that the SIHA and HELA cells exhibited higher migration ability after transfection with the LOXL2-lentvirus than after transfection with the empty lentivirus ()). Furthermore, based on the colony formation assay, up-regulated LOXL2 also significantly improved the viability of SIHA and HELA cells after transfection with LOXL2-lentivirus. ()). Further analysis based on qRT-PCR and western blotting indicated that the key molecules (e.g. E-cadherin, N-cadherin and Snail1) of the EMT signaling pathway changed significantly in the SIHA and HELA cells overexpressing LOXL2-lentivirus ()).
Figure 4. LOXL2 promoted malignant transformation of cervical cancer lines by inducing EMT. (a) Representative fluorescent images of SIHA and HELA after transfected with lentivirus. (b), (c), (d), (e) Up-regulated LOXL2 significantly promoted invasive, migrative and proliferative capability of SIHA and HELA. (f) and (g) mRNA and protein levels of key molecules (e.g. E-cadherin, N-cadherin, Snail1) in the EMT signaling pathway significantly changed after transfected with LOXL2-lenti-virus. (h) and (i) Over-expression of LOXL2 significantly promoted tumor growth in subcutaneous tumor model of SIHA cells in nude mice. EMT: epithelial-mesenchymal transition. ** P < 0.01; *** P < 0.001; ****P < 0.0001.
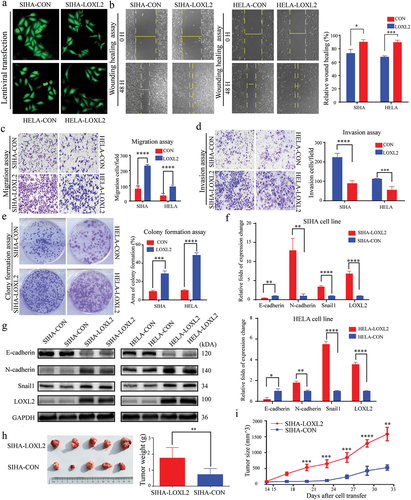
To explore whether overexpression of LOXL2 affects tumor growth in vivo, a xenotransplantation model was constructed in nude mice by subcutaneous injection of SIHA cells transfected with LOXL2-lentivirus or empty lentivirus. Both tumor weight and tumor size were much larger in nude mice over-expressing LOXL2, indicating that up-regulation of LOXL2 significantly enhanced tumor growth in cervical cells ()). In summary, LOXL2 promoted malignant transformation of cervical cancer cells (SIHA and HELA) by inducing EMT at the in vitro and in vivo levels.
LOXL2 small molecule inhibitor reversed the malignant transformation of cervical cancer cells by restraining LOXL2-induced EMT.
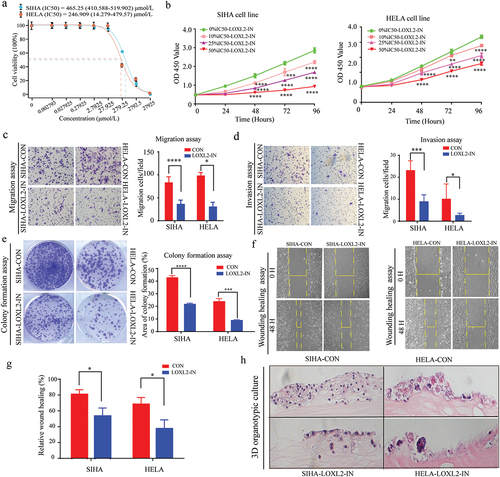
The efficiency of a specific LOXL2 inhibitor, i.e. (2-Chloropyridin-4-yl) methanamine hydrochloride, which has shown promise as an inhibitor of LOXL2 in vitro [Citation35], was explored in cervical cancer cells. First, we measured the median inhibitory concentration (IC50) of (2-Chloropyridin-4-yl) methanamine hydrochloride in SIHA and HELA cells. As shown in ), the IC50 values for the SIHA and HELA cells were 465.25 (410.588–519.902) μmol/L and 246.909 (14.279–479.57) μmol/L, respectively. We set up three different drug concentrations, and the corresponding drug concentrations of 10%IC50, 25%IC50% and 50%IC50 in SIHA and HELA were 46.5 μmol/L, 116.25 μmol/L, 232.5 μmol/L and 25 μmol/L,62.5 μmol/L, 125 μmol/L, respectively. The viability and proliferation of the SIHA and HELA cells decreased significantly with the increase in inhibitor concentration ()). Further transwell migration/invasion, colony formation, and wound healing assays all indicated that the LOXL2 inhibitor significantly restrained the migratory ability, invasiveness, and proliferation of SIHA and HELA cells ()). We next constructed a three-dimensional (3D) organic co-culture model to evaluate the effects of the LOXL2 inhibitor on the growth of SIHA and HELA cells. Results showed significant inhibition of the growth of the cervical cancer cells ()).
Figure 5. Small molecule inhibitor of LOXL2 inhibited the malignant transformation of cervical cancer in vitro. (a) The IC50 of (2-chloropyridin-4-yl) methanamine hydrochloride in SIHA and HELA cells. (b) Small molecular inhibitor of LOXL2 significantly inhibit viability of SIHA and HELA cells. (c), (d), (e), (f), (g) LOXL2 inhibitor significantly inhibited invasive, migrative and proliferative capability of SIHA and HELA. (h) 3D organic co-culture model consistently demonstrated that the small molecular inhibitor decreased the growth of cervical cancer cells. IC50: median inhibitory concentration; 3D: 3-dimensional. * P < 0.05; ** P < 0.01; *** P < 0.001; ****P < 0.0001.
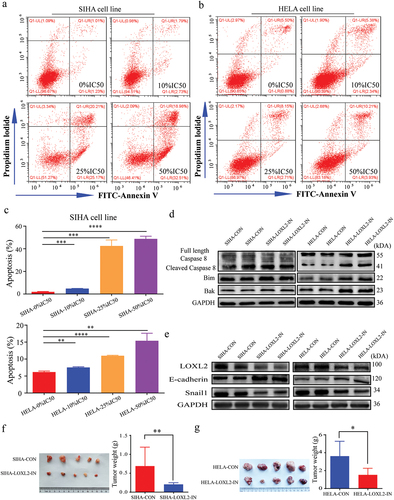
Furthermore, the proportion of apoptotic SIHA and HELA cells significantly elevated with the increase of inhibitor concentration ()). Western blotting indicated that the key molecules of apoptosis and the EMT signaling pathway also changed accordingly with the use of (2-Chloropyridin-4-yl) methanamine hydrochloride ()). Animal experiments consistently indicated that the LOXL2 inhibitor significantly decreased tumor growth ()). Overall, the above findings strongly suggested that this LOXL2 small molecule inhibitor could reverse malignant transformation and promote apoptosis of cervical cancer cells by restraining LOXL2-induced EMT.
Figure 6. Small molecule inhibitor of LOXL2 promoted apoptosis and tumor growth by repressing the EMT. (a), (b), (c) LOXL2 inhibitor significantly promoted apoptosis of SIHA and HELA cell lines. (d) and (e) The key molecules in apoptosis and EMT signaling pathway significantly changed in the SIHA and HELA cells after treated with LOXL2 inhibitor for 48 hours at the protein level. (f) and (g) LOXL2 inhibitor significantly decreased tumor growth in subcutaneous tumor model in nude mice. EMT: epithelial-mesenchymal transition. * P < 0.05; ** P < 0.01; ****P < 0.0001.
Discussion
Our previous research indicated that down-regulation of LOXL2 may play a role in cervical cancer by mediating the EMT signaling pathway [Citation36]. However, our prior study only included simple in vitro experiments, and did not explore or verify this theory in depth. In the current study, LOXL2 was found to be significantly overexpressed in cervical cancer compared to normal tissues using the online public databases from multiple dimensions. Further analysis revealed that LOXL2 may be involved in ECM formation and may play a pivotal role in mediating EMT using a completely different analysis strategy. These findings were verified by increasing the expression of LOXL2 in SIHA and HELA cells in vitro and in vivo. We also explored the effects of a LOXL2-selective inhibitor ((2-Chloropyridin-4-yl) methanamine hydrochloride) on the malignant transformation ability of the SIHA and HELA cells. Surprisingly, the LOXL2-selective inhibitor significantly decreased malignant transformation ability (e.g. viability, migration, invasion), and delayed cervical tumor growth at the in vitro and in vivo level. Thus, based on the above results, LOXL2 may be a promising marker for targeted therapy in cervical cancer, with its selective inhibitor also exhibiting great application potential.
EMT refers to the transformation of epithelial cells to mesenchymal cells, which involves changes in cell morphology and biological functions [Citation37,Citation38]. During this transformation, epithelial cells completely or partially lose their own characteristics and gradually acquire the molecular functions of mesenchymal cells, with cell-mesenchymal and cell–cell interactions changing accordingly [Citation37,Citation39]. EMT can be a complete or partial process. The former often occurs during the development of embryo and tissue, whereby epithelial cells lose their polarity and cell-to-cell connections, and gradually acquire a mesenchymal phenotype (e.g. mesoderm and neural crest) [Citation38–40]. The latter often occurs in a variety of diseases, including malignancies, and is accompanied by the dissolution of adhesion and tight junction proteins between epithelial cells, degradation of cell basement membranes, cell polarity loss, cell morphological changes, and ECM remodeling. EMT is reversible, incomplete, and dynamic in cancer [Citation41,Citation42]. Tumor cells gain the greater ability to infiltrate and invade the basement and vascular membranes, which is the first step of distant metastasis [Citation41,Citation43–45]. Our research strongly suggest that LOXL2 plays a pivotal role in cervical cancer by mediating EMT, and its small molecule inhibitor can strongly inhibit the growth of cervical cancer cells both in vitro and in vivo level. Thus, this study provides a new perspective for the control of distant metastasis in postoperative cervical cancer patients by combining existing radiotherapy or chemotherapy regimens with LOXL2 inhibitors.
For many malignant tumors, chemotherapy regimens (e.g. cytotoxic and molecular targeted drugs) remain the leading therapeutic treatments [Citation46]. However, tumor drug resistance is one of the most important factors restricting the effectiveness of chemotherapy and causing cancer-related mortalities. For example, studies have reported that tumor cells gradually acquire drug resistance during EMT in various kinds of cancer [Citation47–49]. Several small molecule inhibitors targeting EMT have been developed to test their effectiveness at reversing drug resistance at the in vitro and in vivo level (e.g. curcumin, salinomycin, and zidovudine) [Citation50,Citation51]. In this study, the small molecule LOXL2 inhibitor displayed considerable potential at reversing EMT, suggesting that it may be useful for improving drug resistance in advanced cervical cancer. This hypothesis will be tested in future studies on cervical cancer.
Our study indicated that overexpression of LOXL2 in cervical cancer was not driven by genome amplification ()). Previous studies have confirmed that HPV oncoproteins (E5, E6, and E7) could activate the HIF-1α, AP-1 transcription factor, and EGFR signaling pathway in different solid tumors [Citation52–55]. Furthermore, AP-1 and HIF-1α also were confirmed to up-regulated LOXL2 expression in solid tumors [Citation56,Citation57]. Hence, whether HPV oncoproteins were the key regulators of LOXL2 transcription in cervical cancer remains elusive, which needs to be further explored in future.
Conclusions
The present study confirmed that overexpression of LOXL2 promoted malignant transformation abilities by inducing EMT in cervical cancer. Of note, its small molecule inhibitor may be a new therapeutic option for cervical cancer.
Data availability of statement
The datasets analyzed in this study can be found in the ONCOMINE (https://www.oncomine.org/resource/login.html#), Kaplan-Meier Plotter (http://kmplot.com/analysis/index.php?p=service&cancer=pancancer_rnaseq), LinkedOmics (http://www.linkedomics.org/), the c-Bio Cancer Genomics Portal (https://www.cbioportal.org/), COSMIC (http://cancer.sanger.ac.uk).
Ethics approval
The animal experiment of our study was approved by the Animal Experiment Ethics Committee of Tongji Medical College.
Authors’ contribution
PW and CC were responsible for the study concept and design; SL and TP were involved in data collection, data screening, laboratory experiments, and statistical analysis; SL wrote the manuscript, and YM took charge of supervising the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (15.1 KB)Disclosure statement
No potential conflict of interest.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2073047
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Vu M, Yu J, Awolude OA, et al. Cervical cancer worldwide. Curr Probl Cancer. 2018;42:457–465.
- Scarth JA, Patterson MR, Morgan EL, et al. The human papillomavirus oncoproteins: a review of the host pathways targeted on the road to transformation. J Gen Virol. 2021;102:001540.
- Leechanachai P, Banks L, Moreau F, et al. The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene. 1992;7:19–25.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136.
- White EA, Münger K, Howley PM. High-risk human papillomavirus E7 proteins target PTPN14 for degradation. mBio. 2016;7. DOI:10.1128/mBio.01530-16
- James CD, Fontan CT, Otoa R, et al. Human papillomavirus 16 E6 and E7 synergistically repress innate immune gene transcription. mSphere. 2020;5. DOI:10.1128/mSphere.00828-19
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer. J Clinicians. 2020;70:7–30.
- Tsikouras P, Zervoudis S, Manav B, et al. Cervical cancer: screening, diagnosis and staging. J buon. 2016;21:320–325.
- Wimberly H, Brown JR, Schalper K, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3:326–332.
- Saglam O, Conejo-Garcia J. PD-1/PD-L1 immune checkpoint inhibitors in advanced cervical cancer. Integr Cancer Sci Ther. 2018;5. DOI:10.15761/ICST.1000272
- Wang Y, Li G. PD-1/PD-L1 blockade in cervical cancer: current studies and perspectives. Front Med. 2019;13:438–450.
- Vallet SD, Ricard-Blum S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays in Biochemistry. 2019;63:349–364.
- Shah MA, Scaman CH, Palcic MM, et al. Kinetics and stereospecificity of the lysyl oxidase reaction. J Biol Chem. 1993;268:11573–11579.
- Williamson PR, Kagan HM. Reaction pathway of bovine aortic lysyl oxidase. J Biol Chem. 1986;261:9477–9482.
- López B, González A, Hermida N, et al. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol. 2010;299:H1–9.
- Zibadi S, Vazquez R, Larson DF, et al. T lymphocyte regulation of lysyl oxidase in diet-induced cardiac fibrosis. Cardiovasc Toxicol. 2010;10:190–198.
- Soares MB, de Lima Rs, Rocha LL, et al. Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J Infect Dis. 2010;202:416–426.
- Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer–a prospect. J Cell Biochem. 2007;101:1338–1354.
- Finney J, Moon HJ, Ronnebaum T, et al. Human copper-dependent amine oxidases. Arch Biochem Biophys. 2014;546:19–32.
- Chung CH, Parker JS, Ely K, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006;66:8210–8218.
- Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017.
- Fong SF, Dietzsch E, Fong KS, et al. Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer. 2007;46:644–655.
- Akiri G, Sabo E, Dafni H, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666.
- Mahjour F, Dambal V, Shrestha N, et al. Mechanism for oral tumor cell lysyl oxidase like-2 in cancer development: synergy with PDGF-AB. 2019;8:34.
- Peng L, Ran YL, Hu H, et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669.
- Rhodes DRK-S-S, Mahavisno V, Varambally R, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180.
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404.
- Forbes SA, Beare D, Boutselakis H, et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017;45:D777–d83.
- Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2014;43:D805–D11.
- Lánczky A, Nagy Á, Bottai G, et al. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat. 2016;160:439–446.
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6.
- Pathan M, Keerthikumar S, Ang CS, et al. FunRich: an open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15:2597–2601.
- Vasaikar SV, Straub P, Wang J, et al. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2017;46:D956–D63.
- Hutchinson JH, Rowbottom MW, Lonergan D, et al. Small molecule lysyl oxidase-like 2 (LOXL2) inhibitors: the identification of an inhibitor selective for LOXL2 over LOX. ACS Med Chem Lett. 2017;8:423–427.
- Cao C, Lin S, Zhi W, et al. LOXL2 expression status is correlated with molecular characterizations of cervical carcinoma and associated with poor cancer survival via epithelial-mesenchymal transition (EMT) phenotype. Front Oncol. 2020;10:284.
- Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cell. 2016;166:21–45.
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428.
- Saitoh M. Involvement of partial EMT in cancer progression. J Biochem. 2018;164:257–264.
- Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486.
- Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629.
- Jolly MK, Ware KE, Gilja S, et al. EMT and MET: necessary or permissive for metastasis? Mol Oncol. 2017;11:755–769.
- Hugo H, Ackland ML, Blick T, et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383.
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829.
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110.
- Du B, Shim JS. Targeting Epithelial-Mesenchymal Transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21:965.
- Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530.
- McConkey DJ, Choi W, Marquis L, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344.
- Huang J, Li H, Ren G. Epithelial-mesenchymal transition and drug resistance in breast cancer (Review). Int J Oncol. 2015;47:840–848.
- Zhou Y, Liang C, Xue F, et al. Salinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the β-catenin/TCF complex association via FOXO3a activation. Oncotarget. 2015;6:10350–10365.
- Toden S, Okugawa Y, Jascur T, et al. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36:355–367.
- Luna AJ, Sterk RT, Griego-Fisher AM, et al. MEK/ERK signaling is a critical regulator of high-risk human papillomavirus oncogene expression revealing therapeutic targets for HPV-induced tumors. PLoS Pathog. 2021;17:e1009216.
- Morgan EL, Scarth JA, Patterson MR, et al. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ. 2021;28:1669–1687.
- Morgan EL, Macdonald A, Galloway DA. Autocrine STAT3 activation in HPV positive cervical cancer through a virus-driven Rac1-NFκB-IL-6 signalling axis. PLoS Pathog. 2019;15:e1007835.
- Zhang E, Feng X, Liu F, et al. Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells. PLoS One. 2014;9:e103440.
- Matsuoka K, Bakiri L, Wolff LI, et al. Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res. 2020;30:885–901.
- Wang M, Zhao X, Zhu D, et al. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36:60.
