ABSTRACT
lncRNA CASC9 expression was involved in a variety of diseases and exerted a protective role against inflammation and sepsis-induced injury. However, the role of CASC9 in severe pneumonia remains unclear. This study aimed to explore the potential diagnostic role of lncRNA CASC9 in severe pneumonia. The CASC9 expression levels were measured by RT-qPCR. The receiver operating characteristic curve (ROC) was conducted to evaluate the clinical diagnostic value of CASC9 in severe pneumonia. LPS-induced human lung fibroblast MRC-5 was used to establish the pneumonia model and then transfected with CASC9 overexpression vectors to evaluate the influence of CASC9 on cell viability and apoptosis. The inflammatory cytokines IL-1β, TNF-α, IL-6 levels were detected using a commercial enzyme-linked immunosorbent assay (ELISA). Pearson correlation analysis was used to explore the correlation between CASC9 expression and clinical data. The relative expression of CASC9 was downregulated in serum samples of severe pneumonia patients. The low expression of CASC9 in severe pneumonia was negatively correlated with several clinical data. The CASC9 had the relatively high area under ROC curve (AUC) values for distinguishing severe pneumonia from pneumonia children and healthy control. The elevated expression of CASC9 accelerated cell viability and diminished apoptosis in LPS-induced MRC-5 cells. The CASC9 expression was decreased in serum samples of severe pneumonia, and upregulation of CASC9 facilitated LPS-induced cell viability and inhibited apoptosis. In summary, CASC9 might be a diagnostic predictor and might act as a crucial regulatory roles in the progression of severe pneumonia.
Introduction
Severe pneumonia is mostly caused by pathogenic microorganisms in children, particularly infants [Citation1]. On account of their immaturity of physiological and immune functions of infants’ respiratory systems, severe pneumonia is always accompanied by other system dysfunctions, such as acute lung injury, respiratory failure, heart failure, toxic encephalopathy, disseminated intravascular coagulation, and systemic inflammatory response syndrome [Citation2]. The rapid onset, severe illness, rapid progress, and high case fatality rate have become the current difficulties in treating patients with severe pneumonia, which can endanger life and health [Citation3]. Therefore, exploring more novel diagnostic tools may improve a reasonable judgment on patients’ conditions.
Long non-coding RNAs (lncRNAs), a class of non-coding RNAs, comprise more than 200 nucleotides in length [Citation4]. LncRNAs have important clinical significance and regulatory roles in a variety of diseases, including pneumonia, and are one of the vital research hotspots currently [Citation5–7]. The abnormal expression of lncRNAs is involved in major cellular biological processes, including cell viabilities, invasion, and apoptosis [Citation8,Citation9]. For instance, knockdown of lncRNA KCNQ1OT1 could diminish LPS-induced lung injury through inhibiting cell apoptosis and inflammation by regulating the miR-370/FOXM1 axis in childhood pneumonia [Citation10]. Many lncRNAs are reported to be involved in the progression of pneumonia, such as lncRNA NKILA [Citation11] and lncRNA MEG3 [Citation12]. A recent study reviewed the current knowledge on lncRNAs in acute lung inflammatory response, including lncRNA MALAT1, CASC2, and CASC9 [Citation13]. Cancer susceptibility candidate 9 (CASC9), which is upregulated in several types of cancers [Citation14–16], is also reported to regulate miR-195-5p/pyruvate dehydrogenase kinase 4 axis and protect lung epithelial cells from sepsis-induced injury [Citation17], whereas the studies on the correlation between CASC9 expression and pneumonia were elusive.
Herein, the serum CASC9 expression was detected in severe pneumonia children with respiratory failure, pneumonia patients, and healthy control. The clinical significance of CASC9 as a predictor of severe pneumonia was evaluated. In addition, the effects of CASC9 on the cellular activities and intracellular inflammatory factors were investigated in MRC-5 pneumonia cell model induced by LPS.
Materials and methods
Study patients and sample collection
A total of 288 individuals were enrolled in the current retrospective cohort study between January 2018 and December 2020 in Zhuji Maternity and Child Care Hospital. The cohort study individuals include 96 severe pneumonia children with respiratory failure, 102 pneumonia patients, and 90 healthy children who received a routine physical examination and were randomly chosen from the same hospital. The diagnosis of severe pneumonia was confirmed using the Pediatric Infectious Diseases Society/Infectious Diseases Society of America guideline [Citation18]. The inclusion criteria were as follows: 1) patients were diagnosed with severe pneumonia children with respiratory failure, 2) with negative virus antibody, mycoplasma antibody, and chlamydia antibody results, 3) had no congenital disease, 4) with complete clinical characteristic records. The vein blood samples were obtained from participants before treatment. The serum specimens were obtained and stored after centrifuging blood samples. This study was approved by the Ethics Committee of Zhuji Maternity and Child Care Hospital. All the legal guardians of included patients and healthy individuals signed written informed consent.
Cell culture
The human lung fibroblast cell MRC-5 was obtained from the American Type Culture Collection (ATCC; Manassas, USA). The cell lines were cultured in Minimal Essential Medium (MEM; Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS; Gibco), 100 μg/ml streptomycin, and 100 U/mL penicillin (Gibco) and maintained at 37°C in a humidified incubator with 5% CO2.
Model construction and cell transfection
MRC-5 cells were treated using 10 μg/mL LPS (Sigma-Aldrich) for 6 h to construct a pneumonia model as described elsewhere [Citation19]. The pcDNA3.1-CASC9 (CASC9) and empty pcDNA3.1 vector were obtained from Shanghai GenePharma (China). The vectors were transfected with LPS-induced MRC-5 cells with the help of the Lipofectamine 2000 kit.
RNA extraction and quantitative real-time PCR
The total RNAs were extracted from serum specimens and MRC-5 cells using TRIzol reagent (Invitrogen) and reverse-transcribed to cDNA using Prime Script RT reagent kit (Tiangen, China). Then, qRT-PCR was carried out using a High-capacity cDNA Reverse Transcription Kit (Thermo Fisher, USA). The sequences for PCR were as follows: CASC9, 5’-GCCAGTCTTACTCCCACCAC-3’ (forward) and 5’-ACTCCCACCCGAATATTGCG-3’ (reverse); GAPDH, 5’-GAAGGTGAAGGTCGGAGTCA-3’ (forward) and 5’-TTGAGGTCAATGAAGGGGTC-3’ (reverse). The expression of CASC9 was detected using qRT-PCR by normalized to internal reference gene GAPDH and indicated using the 2−ΔΔCt method.
Enzyme-linked immunosorbent assay (ELISA)
The inflammatory cytokines IL-1β, TNF-α, IL-6 were detected using commercial enzyme-linked immunosorbent assay (ELISA) kits (Sigma-Aldrich, Merck KGaA) in serum samples and LPS-MRC-5 cells.
CCK-8 assay for cell viability
The Cell Counting Kit-8 (CCK-8) assay was applied to detect cell viability. Cell suspension (5000 cells/well) were seeded into a 96-well plate and fostered for 24 h. Then, 10 μl CCK-8 reagent (Dojindo, Japan) was added to the cell plates and cultured for a further 2 h. The optical density (OD) value was measured at 450 nm with a microplate reader.
Cell apoptosis assay
The effects of CASC9 on MRC-5 cell apoptosis were measured using the flow cytometry analysis and quantified using a PI/Annexin V apoptosis kit. Briefly, cells were stained with 5 μl Annexin V-FITC and 20 ng/ml PI for 15 min in the dark. The apoptosis abilities were analyzed using flow cytometry (Becton-Dickinson, San Jose, CA, USA).
Statistical analysis
All the experiments were repeated at least three times from three independent experiments. The data are presented as mean values ± SD and analyzed using SPSS 20.0 software (SPSS, Chicago, USA) and GraphPad 7.0 software (La Jolla, CA, USA). The statistical difference was determined using a Two-Tailed Student's t-test or one-way ANOVA. The clinical predictive value of CASC9 was evaluated using the receiver operating characteristic curve (ROC) and the calculation of the area under the ROC (AUC). The statistical significance was validated by p < 0.05.
Results
The baseline characteristics manifestation
The baseline clinical data are listed in . This study enrolled a total of 288 subjects, including 90 healthy control, 102 pneumonia patients, and 96 severe pneumonia patients with respiratory failure. The severe pneumonia group was constituted of 58 males and 38 females with an average age of 4.36 ± 1.38 years old. No significant differences were observed in the sex, age, and body mass index (BMI) between severe pneumonia patients, pneumonia patients, and the healthy control group (p > 0.05, ). The laboratory item lymphocyte showed no significant difference among the three groups (p > 0.05, ). The other laboratory items showed statistical differences among three groups, including white blood cell (WBC), absolute neutrophils, C-reactive protein (CRP), lactate dehydrogenase, procalcitonin (PCT), IL-1β, TNF-α, and IL-6 (p < 0.05, ). These data suggested that severe pneumonia patients may have obvious damage and severe inflammation.
Table 1. Clinical data of the study population.
The levels of CASC9 in severe pneumonia patients
The levels of CASC9 were measured in the three groups using RT-PCR. The results in ()) displayed that serum levels of CASC9 were lower in the severe pneumonia group than both the pneumonia group (p < 0.001) and the healthy group (p < 0.001).
Figure 1. Expression of CASC9 in the serum samples from severe pneumonia children, pneumonia children, and healthy control. (a). The expression of CASC9 was decreased in severe pneumonia children compared with control as well as pneumonia patients. ***p < 0.001, ###p < 0.001. (b-f). Pearson correlation test revealed that the serum CASC9 expression levels were negatively correlated with WBC (b), absolute neutrophils (c), CRP (d), lactate dehydrogenase (e), and PCT (f). (g-i). The Pearson correlation test revealed that serum CASC9 expression has negative correlations with inflammatory cytokines IL-1β (g), TNF-α (h), and IL-6 (i).
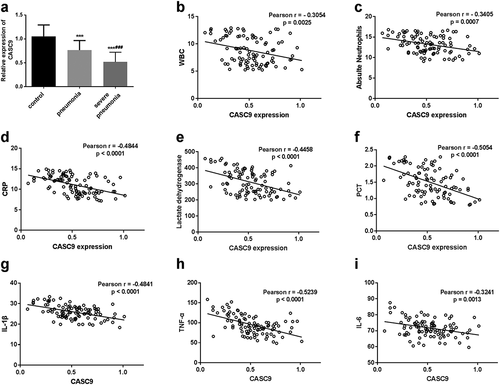
Moreover, taking into consideration the difference of laboratory items in severe pneumonia patients group, the correlation between CASC9 expression and these items was analyzed. The Pearson’s correlation analysis results in ) indicated that serum CASC9 levels were negatively correlated with WBC (r = −0.3054, p = 0.0025), absolute neutrophils (r = −0.3405, p = 0.007), CRP (r = −0.4844, p < 0.0001), lactate dehydrogenase (r = −0.4458, p < 0.0001), and PCT levels (r = −0.5054, p < 0.0001) in severe pneumonia patients. The correlation between CASC9 expression and inflammatory cytokines also showed negative correlations (p < 0.001 ())).
The clinical value of CASC9 in severe pneumonia
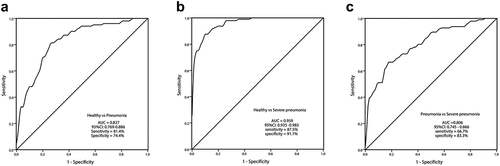
The diagnostic significance of CASC9 was evaluated by ROC curves. As shown in ), the AUC of CASC9 was 0.827 with a sensitivity of 81.4% and specificity of 74.4% for distinguishing pneumonia patients from healthy individuals. ) revealed that CASC9 exhibited the highest AUC (0.959) for distinguishing severe pneumonia patients from healthy groups (sensitivity of 87.5% and specificity of 91.1%). Between pneumonia patients and severe pneumonia patients, the AUC was 0.806 with a sensitivity of 66.7% and specificity of 83.3% ()). These results revealed that CASC9 expression may have diagnostic value in severe pneumonia.
Figure 2. The receiver operating characteristic (ROC) curves of CASC9 in the diagnosis of severe pneumonia. (a) The AUC of CASC9 prediction on pneumonia from healthy control was analyzed using ROC curve (AUC = 0.827). (b) The AUC of CASC9 prediction on severe pneumonia from healthy control was analyzed using ROC curve (AUC = 0.959). (c) The AUC of CASC9 prediction on severe pneumonia from pneumonia children was analyzed by ROC curve (AUC = 0.806).
Influence of CASC9 on cell models
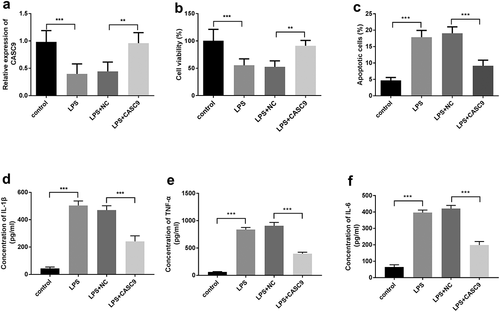
MRC-5 cells were incubated and induced using 10 μg/ml LPS to construct the pneumonia model, and then the cell model was transfected with pcDNA3.1-CASC9 to regulate the expression of CASC9 for evaluating the influence of CASC9 on cellular activities. After treatment with LPS, the CASC9 expression levels were decreased compared with control (p < 0.001), while the CASC9 levels were reversed after transfection with pcDNA3.1-CASC9 in the pneumonia cell model compared with LPS plus NC group (p < 0.01 ())). The cellular CCK-8 experiments illustrated that the treatment of LPS restained cell viability (p < 0.001 vs. control), while upregulation of CASC9 reversed cell viability compared with LPS+NC (p < 0.01 ())). The apoptosis assay indicated that LPS could increase cell apoptosis, while CASC9 overexpression can reduce LPS-induced cell apoptosis (p < 0.001 ())).
Figure 3. Influence of CASC9 in LPS-induced MRC-5 pneumonia cell model. (a) The expression of CASC9 was decreased in the LPS-induced MRC-5 cell model, while transfection of pcDNA3.1-CASC9 reversed the decreased expression of CASC9 by LPS. (**p < 0.01, ***p < 0.001). (b) Elevated expression of CASC9 could reverse the decreased cell viability induced by LPS. (c) Cell apoptosis of LPS-induced MRC-5 cells was increased while upregulation of CASC9 reversed the increased cell apoptosis by LPS. (d-f) Expression of inflammatory factors IL-1β (d), TNF-α (e), and IL-6 (f) were determined by ELISA assay. **p < 0.01, ***p < 0.001.
CASC9 upregulation reduces LPS-induced inflammatory cytokines in the cell model
The inflammatory cytokines IL-1β, TNF-α, and IL-6 in the MRC-5 cell model were detected using ELISA assay. As shown in ), the expression of IL-1β, TNF-α, and IL-6 was increased after treatment with LPS (p < 0.001), while increased expression of CASC9 diminished the LPS-induced rise (p < 0.001). The above results suggested that CASC9 is involved in the regulation of inflammatory response.
Discussion
Pneumonia is a lower respiratory tract infection in the lung and remains one of the major causes of childhood mortality [Citation20]. Severe pneumonia can finally lead to respiratory damage, multiple organ failure, and even paralysis due to the weakened immune system [Citation21].Early detection of pneumonia may achieve early treatment, thus improving clinical management. Herein, the statistical results showed that laboratory items have significant differences, including WBC, CRP, lactate dehydrogenase, PCT, IL-1β, TNF-α, and IL-6, suggesting that severe pneumonia children may have obvious damage and severe inflammation. The results were similar to a previous study, in which absolute neutrophils and CRP in children with M. pneumoniae pneumonia were increased compared with controls [Citation22].
Further, the relative expression of lncRNA CASC9 was down-regulated in severe pneumonia. A recent study indicated that CASC9 was downregulated in LPS induced human small airway epithelial cells (HSAECs) and upregulation of CASC9 could protect lung epithelial cells from sepsis-induced injury [Citation17]. Joining the difference of laboratory items in severe pneumonia patient groups, the Pearson’s correlation results revealed that CASC9 expression levels in severe pneumonia patients were negatively correlated with WBC, absolute neutrophils, CRP, lactate dehydrogenase, and PCT. A recent study revealed that lncRNA NNT-AS1 is upregulated in refractory Mycoplasma pneumoniae pneumonia (RMPP) compared with non-RMPP patients, and its expression has a positive correlation with CRP and PCT [Citation23]. The above information indicated that CASC9 expression may play a regulatory role in the progression of severe pneumonia.
Previous studies demonstrated that the dysregulation of lncRNAs could serve as diagnostic markers in diseases [Citation23–25]. For instance, lncRNA NORAD was highly expressed in the neonatal sepsis group compared with pneumonia controls and NORAD could act as a diagnostic indicator for neonatal sepsis patients [Citation26]. Herein, the clinical significance of CASC9 was also evaluated, and the results showed that CASC9 has high AUC values for distinguishing severe pneumonia patients from pneumonia patients and healthy controls. Previous investigation in cancers also revealed that CASC9 could act as a diagnostic or prognostic biomarker in several types of cancer [Citation27,Citation28]. These data suggested that CASC9 may also be a diagnostic predictor of severe pneumonia.
Furthermore, the upregulation of CASC9 increased cell proliferation and reduced cell apoptosis in MRC-5 cells model induced by LPS. Similarly, overexpression of CASC9 increased the viability of HSAECs, resulting in protecting lung epithelial cells from sepsis-induced injury [Citation17]. In another research, upregulated expression of CASC9 contributes to cell proliferation in non-small cell lung cancer via the miR-335-3p/S100A14 axis [Citation29]. Additionally, upregulation of CASC9 decreased the increased levels of inflammatory cytokines IL-1β, TNF-α, and IL-6 induced by LPS. The above available data revealed that increased CASC9 expression had beneficial effects on severe pneumonia. Moreover, downregulation of CASC9 could aggravate sepsis-induced acute lung injury via regulating miR-195-5p/PDK4 axis [Citation17]. Thus, we speculated that CASC9 may protect against severe pneumonia by regulating miR-195-5p/PDK4 axis.
There are some deficiencies and future topics in this study. Firstly, the clinical significance of CASC9 was preliminarily concluded, which needs to be confirmed in more patient samples. Secondly, control strategies are necessary for diseases [Citation30–32], which is difficult at present and will be taken into account in future researches. Finally, the detailed mechanism of CASC9 in severe pneumonia remains unclear, which will be explored in future studies.
In summary, serum lncRNA CASC9 expression was decreased in children with severe pneumonia and had a diagnostic indicator value. Overexpression of CASC9 could protect severe pneumonia through promoting cell viability and suppressing cell apoptosis of the pneumonia cell model, as well as decreasing the levels of the inflammatory cytokine. The present results provide preliminary insights into CASC9 as a novel diagnostic predictor and a potential target of therapeutic intervention in children with severe pneumonia. In future studies, the detailed mechanism of CASC9 in children with severe pneumonia will be explored.
Declarations and ethics statements
This study was approved by the Ethics Committee of Zhuji Maternity and Child Care Hospital. All the legal guardians of included patients and healthy controls signed written informed consent.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Waites KB. New concepts of mycoplasma pneumoniae infections in children. Pediatr Pulmonol. 2003 Oct;36(4):267–278.
- He J, Yuan R, Cui X, et al. Anemoside B4 protects against Klebsiella pneumoniae- and influenza virus FM1-induced pneumonia via the TLR4/Myd88 signaling pathway in mice. Chin Med. 2020;15(2):68.
- Wang L, Fan Y, Xu J, et al. The efficacy and safety of Tanreqing injection combined with western medicine for severe pneumonia: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020 Aug 28 99(35):e22010.
- Jarroux J, Morillon A, Pinskaya M. History discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008(1008):1–46.
- Liao C, Wang A, Ma Y, et al. Long non-coding RNA FOXP4-AS1 is a prognostic biomarker and associated with immune infiltrates in ovarian serous cystadenocarcinoma. Medicine (Baltimore). 2021 Oct 8 100(40):e27473.
- Song B, Dang H, Dong R. Differential expression of LOXL1-AS1 in coronary heart disease and its regulatory mechanism in ox-LDL-Induced human coronary artery endothelial cell pyroptosis. Cardiovascular drugs and therapy. 2021 Oct 11.
- Wang C, Liang G, Shen J, et al. Long non-coding RNAs as biomarkers and therapeutic targets in sepsis. Cardiovasc Drugs Ther. 2021;12:722004.
- Wang C, Zhao R, Zhang S. lncRNA XIST knockdown suppresses cell proliferation and promotes apoptosis in diabetic cataracts through the miR‑34a/SMAD2 axis. Mol Med Rep. 2022 Jan;25(1):7.
- Yin Z, Shen H, Gu CM, et al. MiRNA-142-3P and FUS can be sponged by long noncoding RNA DUBR to promote cell proliferation in acute myeloid leukemia. Front Mol Biosci. 2021;8:754936.
- Wang P, Zhang H, Zhao W, et al. Silencing of long non-coding RNA KCNQ1OT1 alleviates LPS-induced lung injury by regulating the miR-370-3p/FOXM1 axis in childhood pneumonia. BMC Pulm Med. 2021 Jul 23;21(1):247.
- Li Y, Wang W, Chen K, et al. Influence of LncRNA NKILA on bloodstream infection of hypervirulent Klebsiella pneumoniae and its ability to induce delayed neutrophil apoptosis. Evid Based Complement Alternat Med. 2021;6101078.
- Guo J, Zhang N, Liu G, et al. Upregulated expression of long non-coding RNA MEG3 serves as a prognostic biomarker in severe pneumonia children and its regulatory mechanism. Evid Based Complement Alternat Med. 2021 Dec;12(1):7120–7131.
- Chen C, He Y, Feng Y, et al. Long non-coding RNA review and implications in acute lung inflammation. Life Sci. 2021 Mar 15;269:119044.
- Chang J, Zhang Y, Ye X, et al. Long non-coding RNA (LncRNA) CASC9/microRNA(miR)-590-3p/sine oculis homeobox 1 (SIX1)/NF-κB axis promotes proliferation and migration in breast cancer. Bioengineered. 2021 Dec;12(1):8709–8723.
- Jiao Y, Liu Q, Zhao H, et al. Changes and prognostic value of lncRNA CASC9 in patients with advanced colon cancer after chemotherapy. Evid Based Complement Alternat Med. 2021; 1858974.
- Ning B, Guo S, Mei Y. Long non-coding RNA CASC9 promotes tumor progression in oral squamous cell carcinoma by regulating microRNA-545-3p/laminin subunit gamma 2. Bioengineered. 2021 Dec;12(1):7907–7919.
- Wang HR, Guo XY, Liu XY, et al. Down-regulation of lncRNA CASC9 aggravates sepsis-induced acute lung injury by regulating miR-195-5p/PDK4 axis. Inflammation Res. 2020 Jun;69(6):559–568.
- Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic Review. J Pediatric Infect Dis Soc. 2018 Dec 3;7(4):323–334.
- Cong S, Xiang L, Yuan X, et al. Notoginsenoside R1 up-regulates microRNA-132 to protect human lung fibroblast MRC-5 cells from lipopolysaccharide-caused injury. Int Immunopharmacol. 2019 Mar;68:137–144.
- GBD 2019 Under-5 Mortality Collaborators. Global, regional, and national progress towards sustainable development goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the global burden of disease study 2019. Lancet. (London England) 2021 Sep 4;398(10303):870–905.
- Mizgerd JP. Pathogenesis of severe pneumonia: advances and knowledge gaps. Curr Opin Pulm Med. 2017 May;23(3):193–197.
- Chu C, Lei X, Li Y, et al. High expression of miR-222-3p in children with mycoplasma pneumoniae pneumonia. Ital J Pediatr. 2019 Dec 16 45(1):163.
- Chen P, Huang Z, Chen L, et al. The relationships between LncRNA NNT-AS1, CRP, PCT and their interactions and the refractory mycoplasma pneumoniae pneumonia in children. Sci Rep. 2021 Jan 21;11(1):2059.
- Chen X, Liu Q, Chen J, et al. LncRNA RP11-248E9.5 and RP11-456D7.1 are valuable for the diagnosis of childhood pneumonia. Int J Gen Med. 2021;14:895–902.
- Jiang N, Meng X, Mi H, et al. Circulating lncRNA XLOC_009167 serves as a diagnostic biomarker to predict lung cancer. Clin Chim Acta. 2018 Nov;486:26–33.
- Zhang H, Li L, Xu L, et al. Clinical significance of the serum lncRNA NORAD expression in patients with neonatal sepsis and its association with miR-410-3p. J Inflamm Res. 2021;14:4181–4188.
- Sassenberg M, Droop J, Schulz WA. Upregulation of the long non-coding RNA CASC9 as a biomarker for squamous cell carcinoma. BMC Cancer. 2019 Aug 14;19(1):806.
- Zeng YL, Guo ZY, Su HZ, et al. Diagnostic and prognostic value of lncRNA cancer susceptibility candidate 9 in hepatocellular carcinoma. BMC Cancer. 2019 Dec 28;25(48):6902–6915.
- Zhao W, Chen T. Upregulated lncRNA CASC9 contributes to progression of non-small cell lung cancer through inhibition of miR-335-3p and activation S100A14 expression. Onco Targets Ther. 2020;13:6027–6036.
- Li W, Ji J, Huang L, et al. Bifurcations and dynamics of a plant disease system under non-smooth control strategy. Nonlinear Dyn. 2020;99(2209).
- Li W, Ji JC, Lihong H, et al. Global dynamics of a controlled discontinuous diffusive SIR epidemic system. Appl Math Lett. 2021 May 01;121:107420.
- Li W, Ji JC, Lihong H. Dynamics of a discontinuous computer worm system. Proceedings of the American Mathematical Society. 2020 June 13;148(10):1.
