ABSTRACT
Osteoarthritis (OA) is a common degenerative disease characterized by reducing articular chondrocytes and destruction of joint matrix, it’s detailed pathogenesis remains unclear. Emerging evidences have demonstrated that long non-coding RNAs (lncRNAs) are closely related to the progression of OA. This study aims to explore the expression of long non-coding RNA LEMD1 antisense RNA 1 (LEMD1-AS1) in OA tissues and chondrocytes and investigate the possible mechanisms of LEMD1-AS1 in OA, which will provide a new target for the treatment of OA. In our study, LEMD1-AS1 and post-GPI attachment to protein (PGAP1) were lowly expressed, but miR-944 was highly expressed both in OA tissues and in Lipopolysaccharide (LPS) -treated chondrocytes detected by qRT-PCR. Over-expression of LEMD1-AS1 or down-regulation of miR-944 significantly promoted viability, proliferation and inhibited cell apoptosis, cell cycle arrest and inflammatory responses of chondrocytes treated with LPS by CCK-8, EdU, flow cytometry and an ELISA assay. Over-expression of LEMD1-AS1 or down-regulation of miR-944 remarkably increased the protein levels of PCNA, Ki-67, Cyclin A1, Cyclin B1, Cyclin D2 and Bcl-2, while decreasing the protein levels of p27, Bax, Cleaved-caspase-3 and Cleaved-caspase-9 in chondrocytes treated with LPS. LEMD1-AS1 bound to miR-944 and regulated its expression, and PGAP1 presented as a direct target gene of miR-944, which was confirmed by a dual-luciferase reporter assay. Inhibition of PGAP1 partially restored the effects of LEMD1-AS1/miR-944 on the proliferation, cell apoptosis, cell cycle distribution and inflammatory responses of LPS-treated chondrocytes. To conclude, the LEMD1-AS1/miR-944/PGAP1 axis may be a novel therapeutic candidate to target in OA treatment.
1. Introduction
Osteoarthritis (OA) is a common degenerative disease characterized by the reduction of articular chondrocytes and destruction of joint matrix [Citation1]. Severe OA leads to joint deformity and function [Citation2]. OA, the most common joint disease in elderly patients, mainly occurs in middle-aged and elderly people [Citation3]. Epidemiological statistics show that the prevalence rate of OA in people over 40 years old is about 30%, and reaches 60%-70% in people over 65 years old [Citation4]. With the acceleration of the aging population in China, the prevalence of OA has become a more and more serious social and public health problem [Citation5]. At present, there is no radical treatment for OA. The majority of clinical treatments are to control pain, correct deformity, restore joint function and improve the quality of life [Citation6,Citation7]. In view of the complexity of the pathological process and diverse mechanisms of OA, some new therapeutic schemes have been put into clinical application, such as chondrocyte transplantation, bone marrow mesenchymal stem cell transplantation, matrix induced autologous chondrocyte transplantation [Citation8–10]. Therefore, further study on the pathological mechanisms of OA and more effective targets for prevention and treatment for OA need to be identified.
Considering the typical heredity of OA, much attention has been focused on gene therapy with a good development prospect [Citation11]. It is an important topic in the field of OA treatment to further clarify the differential genes of OA and screen candidate genes with research value [Citation12]. The human genome project has gained more interest in long non-coding RNAs (lncRNAs) which are a subclass of non-coding RNAs (ncRNAs) with more than 200 nt in length, and are closely related to the occurrence and development of many diseases, including OA [Citation13]. Some scholars point out that lncRNAs play an important role in the pathogenesis of OA. Through gene chip and high-throughput DNA sequencing technologies, 2166 lncRNAs are up-regulated and 1472 lncRNAs are down-regulated in the process of OA, providing new hope for the clinical treatment of OA [Citation14]. In addition, SNHG9 specially binds with miR-34a to silence miR-34a, thus blocking the inhibitory effect of miR-34a on chondrocyte proliferation, which may play a positive role in alleviating OA [Citation15]. Moreover, BLACAT1 promotes the metalloproteinase induction of IL-1β, which may be a bridge between inflammatory factors and cartilage damage [Citation16]. Currently, many studies focus on the role of lncRNAs in OA, but there are still many unknowns in pathology. LEMD1-AS1, as a recently identified lncRNA, is one of the top 10 down-regulated lncRNA in OA from the GSE82107 dataset [Citation17]. LEMD1-AS1 presented low expression in OA based on the GEO dataset. Over-expression of LEMD1-AS1 inhibits the proliferation, migration and invasion of OC cells and the growth of transplanted tumor in nude mice depending on miR-183-5p-mediated TP53 expression [Citation18]. However, the detailed role and underlying mechanisms of LEMD1-AS1 in OA remains unclear.
In the present study, the expressions of LEMD1-AS1, miR-944 and PGAP1 were detected in cartilage tissues of OA patients and chondrocytes. Then the relationships between LEMD1-AS1 and miR-944, or miR-944 and PGAP1 were predicted by bioinformatics analysis, and further verified. This study indicating that LEMD1-AS1/miR-944/PGAP1 might be a novel therapeutic candidate target in OA treatment.
2. Materials and methods
2.1 Tissue samples
Thirty patients with knee osteoarthritis were selected in our community, including 14 males and 16 females. The patients were aged 40–60 years, with an average of (48.6 ± 6.1) years. The course of disease ranged from 2 to 8 years, with an average of (4.5 ± 1.3) years. Inclusion criteria: patients were aged 40–75 years; the research met the diagnostic criteria of knee osteoarthritis in the Guideline of Diagnosis and Treatment of Osteoarthritis (2010 Edition) [Citation19]. X-ray film showed that osteophyte was present in one knee. Informed consent was obtained. This research met the requirements of the Medical Ethics Committee of our hospital. Exclusion criteria: patients with secondary knee osteoarthritis, gout, or rheumatoid arthritis; patients with a history of knee surgery and neurological diseases of lower limbs; patients accompanied by coagulation dysfunction, systemic infectious diseases or knee joint infection; patients who had a history of vascular injury, tuberculosis, tumor, bone rigidity in knee joint or congenital malformation of lower limbs; patients accompanied by serious diseases such as endocrine, cardiovascular or cerebrovascular diseases; patients with cognitive dysfunction, mental disorder or communication disorder; patients who could not receive follow-up, were lost to follow-up, withdrew or died.
All patients voluntarily had signed the informed notice, and the Ethics Committee of Beicheng New District Hospital of Linyi people’s hospital had approved experimental procedures.
2.2 Isolation and culture of chondrocytes
The cartilage tissues were cut into small pieces and centrifuged at low speed (1, 000 r/min, 5 min). After 4-h digestion at 37°C with 0.2% type II collagenase, the supernatant was removed by low-speed centrifugation (1, 000 r/min, 5 min). The cell culture medium was used for cell precipitation, and the cells were cultured in a saturated humidity cell culture chamber with 5% CO2 at 37°C [Citation20].
2.3 Cell transfection
Pc-LEMD1-AS1, sh-LEMD1-AS1, miR-944 mimic/inhibitor, sh-PGAP1 and the corresponding negative control (NC) vectors were obtained from GenScript (Nanjing, China). Chondrocytes at a density of 3 × 105 cells per well were maintained in 6-well plates and transfected with pc-LEMD1-AS1 (50 nM), sh-LEMD1-AS1 (50 nM), miR-944 mimic/inhibitor (50 nM) and/or sh-PGAP1 (50 ng) using Lipofectamine 2000 (Invitrogen, GrandIsland, NY, USA). Transfection efficiency was examined by qRT-PCR after 48 h.
2.4 CCK-8 assay
Transfected chondrocytes (1 × 104 cells per well) were seeded in 96-well plates for 0 h, 24 h, 48 h and 72 h, respectively, and then treated with LPS (10 μg/mL) for 2 h. Then, cell viability was examined by CCK-8 kit (Beyotime Biotechnology, Shanghai, China) based on the specification. The optical density was detected at 490 nm by microplate reader (Tecan Infinite M200 Micro Plate Reader; LabX, Switzerland).
2.5 EdU assay
Transfected chondrocytes (4 × 104 cells per well) were cultured in 24-well plates for 48 h, and treated with LPS (10 μg/mL) for 2 h. Then cells were fixed with 4% paraformaldehyde, and permeabilized with Triton X-100. Afterward, cells were blocked with goat serum for 1 h, and stained according to the manufacturer’s instructions. The number of EdU-positive cells was counted in three random images per well by microscopy using a 100 × objective (Olympus, Tokyo, Japan).
2.6 Flow cytometry analysis
For cell apoptosis, transfected chondrocytes (2 × 105 cells per well) were cultured in 24-well plates for 48 h, and treated with LPS (10 μg/mL) for 2 h. Then the cells and culture medium were collected, and mixed with 5 µL Annexin V-FITC and 10 µL PI. After 10 min, the rate of apoptosis was detected by flow cytometry. For cell cycle, transfected chondrocytes from different groups were collected. Then the cells were fixed by 70% EtOH overnight, and stained by PI for 30 min. Finally, the distribution of cell cycle was detected by flow cytometry. Cells were analyzed with Flow Jo Software (FLOWJO, LLC, Ashland, OR, USA).
2.7 Enzyme-linked immunosorbent assay (ELISA)
The concentrations of TNF-α, IL-1β and IL-6, collected from chondrocytes were examined by TNF-α ELISA Kit (#ab181421, Abcam), IL-1β ELISA Kit (#ab46052, Abcam), IL-6 ELISA Kit (#ab46027, Abcam), MMP3 ELISA Kit (#ab16663, Abcam), MMP9 ELISA Kit (#ab100606, Abcam) and ADAMTS4 ELISA Kit (Chemicon, CA) respectively according to the manufacturer’s instructions.
2.8 RNA extraction and qRT-PCR analysis
Total RNA was extracted from chondrocytes according to the manufacturer’s protocol. Then total RNA was reverse transcribed into cDNA using RNeasy plus micro kit, which was used as starting materials for qRT-PCR on the Step One System (Life Technologies Corp). Primer sequences used were designed as follows: LEMD1-AS1 forward, 5’-AATGACCGCAATCCCAAGGT-3’, and reverse, 5’-GGTGAC TAGCAGTGCGTGAT-3’. MiR-944 forward, 5’-CGCGAGCAGGAAATTATTGTA-3’, and reverse, 5’-TATGCTTGTTCTCGTCTCTGTGTC-3’. U6 forward, 5’-CTCGCTTCGGCAGCACA-3’, and reverse 5’-AACGCTTCACGAATTTGCGT-3’. PGAP1 forward, 5’-CAACTTCGGCCTCAATGT C-3’, and reverse, 5’-TTCGTGGATGGTGAAGTCC-3’. β-actin forward, 5’-CCCGCGAGTACAACC TCTTG-3’, and reverse, 5’-GTCATCCATGGCGAACTGGTG-3’. All primer sequences were designed using Primer Premier software 4.0 (Premier, Canada). Conditions for qRT-PCR were used: 95°C for 10 min, 40 cycles of 95°C for 15s, and 60°C for 1 min. All target gene transcripts were normalized to U6 and β-actin using the 2−ΔΔCT method.
2.9 Western blot
Total protein from chondrocytes was isolated by RIPA buffer (Invitrogen, Carlsbad, CA, USA), and protein concentration was examined by BCA kit (GenScript, Nanjing, China). After being separated by 10% SDS-PAGE, the protein was transferred to PVDF membranes and blocked with 5% skim milk at room temperature for 1 h. Afterward, the membranes were cultured with the primary antibodies overnight at 4°C, followed by incubation with HRP-conjugated secondary antibody (1: 2, 000 dilution, ab6728) for another 2 h at room temperature. At last, protein blots were examined by ECL kit (Millipore, Bedford, MA, USA) and quantified using ImageJ software. The primary antibodies were listed as below: anti-PCNA (1: 1, 000, ab92552), anti-Ki-67 (1: 1, 000, ab16667), anti-Cyclin A1 (1: 1, 000, ab53699),anti-Cyclin B1 (1: 1, 000, ab32053),anti-Cyclin D2 (1: 1, 000, ab230883),anti-p27 (1: 1, 000, ab62364), anti-Bax (1: 1, 000, ab32503), anti-Bcl-2 (1: 1, 000, ab32124), anti-Cleaved-caspase-3 (1: 1, 000, ab32042), anti-Cleaved-caspase-9 (1: 1, 000, ab2324) and anti-β-actin (1: 2, 000, ab8227). All antibodies were obtained from Abcam (MA, USA). β-actin was used as an internal reference.
2.10 Dual-luciferase reporter assay
The sequence of wild type LEMD1-AS1 (LEMD1-AS1-WT), mutant type LEMD1-AS1 (LEMD1-AS1-Mut), 3’-UTR of PGAP1-WT and PGAP1-Mut were inserted into pmirGLO reporter vector (150 ng) (GenScript, Nanjing, China). Then, pmirGLO-LLEMD1-AS1-WT/Mut and pmirGLO-PGAP1-WT/Mut were co-transfected with miR-944 mimic or mimic NC into chondrocytes using Lipofectamine 2000 (Invitrogen, USA). The activity of luciferase was examined by a dual-luciferase reporter assay system (Promega, USA).
2.11 RNA pull-down analysis
Chondrocytes were incubated with biotin-labeled miR-944-WT or miR-944-Mut for 48 h. Then the lysate was incubated with magnetic beads (Invitrogen, Carlsbad, CA, USA) at 4°C for 3 h, and washed with precooled lysis buffer and high salt buffer solution (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0 and 50 mM NaCl) three times. Finally, the bound RNA was purified with Trizol. LEMD1-AS1 enrichment was analyzed by qRT-PCR [Citation21].
2.12 Statistical analysis
Data was analyzed by GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA) and presented as mean ± SD. N = 3 represents data shown are three technical replicates were used for each biological replicates. One-way analysis of variance (ANOVA) followed Tukey’s poc host was employed to compare differences among multiple groups. P< 0.05 was indicated as statistically significant.
3. Results
3.1 LEMD1-AS1 is down-regulated in OA tissues and LPS-treated chondrocytes
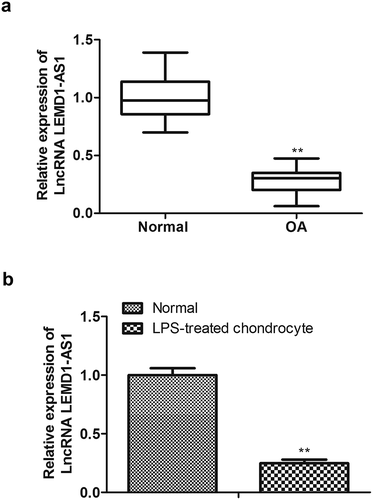
To explore the role of LEMD1-AS1 in OA, cartilage tissues of OA patients were collected, and qRT-PCR was performed. As shown in ), LEMD1-AS1 was notably down-regulated in OA tissues. Since the chondrocytes exhibited an essential role in the progression of OA, qRT-PCR was next used to assess the expression of LEMD1-AS1 in chondrocytes, which were isolated from cartilage tissues and were treated with LPS to induce inflammation. As displayed in ), LEMD1-AS1 was significantly decreased in chondrocytes treated with LPS. These data suggested that LEMD1-AS1 was notably low-expressed in OA tissues and LPS-treated chondrocytes.
Figure 1. LEMD1-AS1 is down-regulated in OA tissues and LPS-treated chondrocytes. (a) The expression of LEMD1-AS1 in cartilage tissues of OA patients was detected by qRT-PCR. (b) The expression of LEMD1-AS1in chondrocytes treated with LPS was detected by qRT-PCR . **P< 0.01 vs. normal group. All data were presented as mean ± SD. n = 3.
3.2 Up-regulation of LEMD1-AS1 promotes proliferation, inhibits apoptosis, and reduces cell cycle arrest of chondrocytes treated with LPS
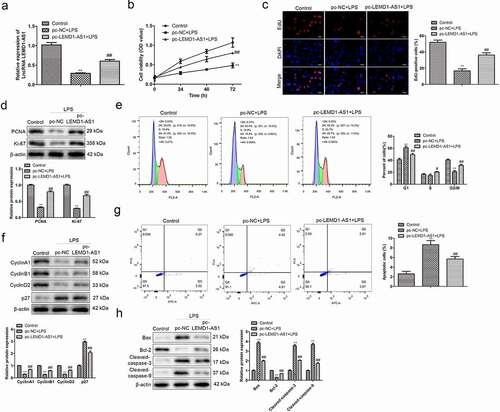
To assess the functional role of LEMD1-AS1, pc-LEMD1-AS1 was transfected in chondrocytes treated with LPS, and qRT-PCR was performed. As shown in ), LPS notably decreased the expression of LEMD1-AS1 in chondrocytes compared with the control group, while pc-LEMD1-AS1 remarkably increased the expression of LEMD1-AS1 in these chondrocytes. Functionally, CCK-8 was performed to evaluate the effects of LEMD1-AS1 on the viability of LPS-treated chondrocytes. The data of ) illustrated that LPS inhibited, while up-regulation of LEMD1-AS1 promoted the viability of chondrocytes treated with LPS. Next, EdU assay was carried out to determine the effects of LEMD1-AS1 on the proliferation of LPS-treated chondrocytes. The data of ) displayed that LPS inhibited proliferation of chondrocytes, while up-regulation of LEMD1-AS1 recovered the proliferation of chondrocytes treated with LPS. Western blot was used to evaluate the effects of LEMD1-AS1 on the expression levels of proliferation-related proteins. The data of ) showed that compared with the control group, LPS significantly inhibited the protein expressions of PCNA and Ki-67, while over-expression of LEMD1-AS1 promoted the levels of PCNA and Ki-67 in LPS-treated chondrocytes. Moreover, flow cytometry analysis was adopted to explore the effects of LEMD1-AS1 on cell apoptosis and cell cycle distribution of chondrocytes treated with LPS. The data of ) displayed that LPS promoted apoptosis and induced cell cycle arrest at G1 phase of chondrocytes, while up-regulation of LEMD1-AS1 notably reversed these phenomena. Furthermore, a Western Blot assay was used to evaluate the effects of LEMD1-AS1 the levels of cell apoptosis-related and cell cycle-related proteins. As shown in ), LPS decreased the protein levels of Cyclin A1, Cyclin B1, Cyclin D2 and Bcl-2, and increased the protein levels of p27, Bax, Cleaved-caspase-3 and Cleaved-caspase-9 in chondrocytes, while up-regulation of LEMD1-AS1 significantly reversed the effects of LPS. These data suggested that up-regulation of LEMD1-AS1 promoted proliferation, inhibited apoptosis, and reduced cell cycle arrest of chondrocytes treated with LPS.
Figure 2. Up-regulation of LEMD1-AS1 promotes proliferation, inhibits apoptosis, and reduces cell cycle arrest of chondrocytes treated with LPS. (a) qRT-PCR was performed to evaluate the LEMD1-AS1 expression in LPS-treated chondrocytes transfected with pc-LEMD1-AS1. (b) The viability of LPS-treated chondrocytes transfected with pc-LEMD1-AS1 was assessed by CCK-8 assay at indicated times. (c) The proliferation of LPS-treated chondrocytes transfected with pc-LEMD1-AS1 was evaluated by EdU assay. (d) The expression levels of proliferation-related proteins in LPS-treated chondrocytes transfected with pc-LEMD1-AS1, including PCNA and Ki-67, were evaluated by a Western Blot assay. (e) The cell cycle distribution of LPS-treated chondrocytes transfected with pc-LEMD1-AS1 was evaluated by flow cytometry analysis. (f) The expression levels of cell cycle-related proteins in LPS-treated chondrocytes transfected with pc-LEMD1-AS1, including Cyclin A1, Cyclin B1, Cyclin D2 and p27, were evaluated by a Western Blot assay. (g) The cell apoptosis of LPS-treated chondrocytes transfected with pc-LEMD1-AS1 was evaluated by flow cytometry analysis. (h) The expression levels of cell apoptosis-related proteins in LPS-treated chondrocytes transfected with pc-LEMD1-AS1, including Bax, Bcl-2, Cleaved-caspase-3 and Cleaved-caspase-9, were evaluated by Western Blot **P< 0.01 vs. control group, ##P< 0.01 vs. pc-NC + LPS group. All data were presented as mean ± SD. n = 3.
3.3 Up-regulation of LEMD1-AS1 ameliorates inflammatory responses of chondrocytes treated with LPS
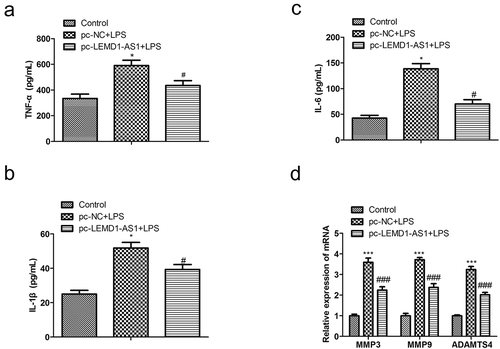
Moreover, to assess the effects of LEMD1-AS1 on the inflammatory responses in chondrocytes treated with LPS, an ELISA assay was carried out to evaluate the expression levels of TNF-α, IL-1β,IL-6 MMP3, MMP9 and ADAMTS4. As shown in ), the expressions of TNF-α, IL-1β,IL-6, MMP3, MMP9 and ADAMTS4 were obviously increased in chondrocytes treated with LPS, while over-expression of LEMD1-AS1 notably improved the effects of LPS. These data suggested that up-regulation of LEMD1-AS1 ameliorated inflammatory responses of chondrocytes treated with LPS.
Figure 3. Up-regulation of LEMD1-AS1 ameliorates inflammatory responses of chondrocytes treated with LPS. The secreted levels of (a) TNF-α, (b) IL-1β and (c) IL-6 in LPS-treated chondrocytes transfected with pc-LEMD1-AS1 were determined by ELISA. (d) The cells expression of proteolytic enzymes like MMPs, and ADAMTS and even terminal differentiation of chondrocytes when the LEDM1-AS1 treated. *P< 0.05 vs. control group, #P< 0.05 vs. pc-NC + LPS group. All data were presented as mean ± SD. n = 3.
3.4 Regulatory relationship between LEMD1-AS1 and miR-944
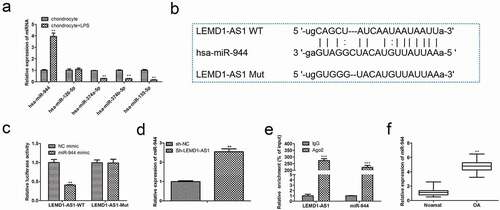
To verify whether LEMD1-AS1 could act as a competing endogenous RNA (ceRNA), bioinformatics tools were utilized to acquire miRNAs potentially binding LEMD1-AS1. As shown in , miR-944 was speculated to binding with LEMD1-AS1, since miR-944 was obviously up-regulated in LPS-treated chondrocytes. Then dual-luciferase reporter analysis was employed to validate the interactions between LEMD1-AS1 and miR-944. As expected, the miR-944 mimic distinctly weakened the luciferase intensity of chondrocytes transfected with LEMD1-AS1-WT ()). Moreover, to further explore the relationship between LEMD1-AS1 and miR-944, qRT-PCR was used to evaluate the expression of miR-944 in chondrocytes transfected with sh-NC or sh-LEMD1-AS1. The data of ) showed that the expression of miR-944 was obviously increased in chondrocytes transfected with sh-LEMD1-AS1. In parallel, RNA pull-down ascertained that LEMD1-AS1 was highly enriched in chondrocytes treated with the wild type of miR-944 ()). Furthermore, qRT-PCR was performed to evaluate the expression of miR-944 in cartilage tissues, and the data in ) indicated that miR-944 was highly expressed. These results suggested that LEMD1-AS1 served as a ceRNA for miR-944 in OA.
Figure 4. Regulatory relationship between LEMD1-AS1 and miR-944. (a) The candidate miRNAs regulated by LEMD1-AS1 were predicted. (b) The binding sites between LEMD1-AS1 and miR-944. (c) Dual-luciferase reporter analysis was employed to validate the coactions between LEMD1-AS1 and miR-944. **P< 0.01 vs. NC mimic group. (d) The expression of miR-944 in LPS-treated chondrocytes transfected with sh-LEMD1-AS1 was determined by qRT-PCR . **P< 0.01 vs. sh-NC group. (e) Relative enrichment of LEMD1-AS1 in chondrocytes was detected by RNA pull down. **P< 0.01 vs. IgG. (f) The expression of miR-944 in cartilage tissues of OA patients was detected by qRT-PCR . **P< 0.01 vs. normal group.All data were presented as mean ± SD. n = 3.
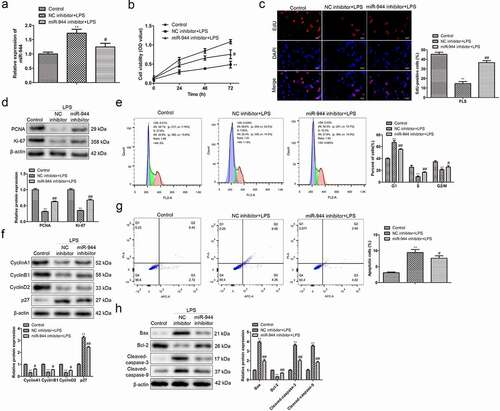
3.5 Down-regulation of miR-944 promotes proliferation, inhibits apoptosis, and reduces cell cycle arrest of chondrocytes treated with LPS
To assess the functional role of miR-944, miR-944 inhibitor was transfected in chondrocytes treated with LPS, and qRT-PCR was performed. As shown in ), miR-944 inhibitor remarkably decreased the expression of miR-944 in chondrocytes treated with LPS. Functionally, CCK-8 and EdU showed that miR-944 inhibitor obviously alleviated the inhibitory effects of LPS on viability and proliferation of chondrocytes ()). Additionally, the results of ) showed that miR-944 inhibitor significantly reverted the effects of LPS on the levels of proliferation-related proteins in chondrocytes. Additionally, flow cytometry analysis indicated that miR-944 inhibitor remarkably reverted the effects of LPS on cell apoptosis promotion, cell cycle distribution of chondrocytes, as well as the protein expression levels of the cell apoptosis- and cell cycle-related proteins in chondrocytes ()). These data suggested that down-regulation of miR-944 promoted proliferation, inhibited apoptosis, and reduced cell cycle arrest of chondrocytes treated with LPS.
Figure 5. Down-regulation of miR-944 promotes proliferation, inhibits apoptosis, and reduces cell cycle arrest of chondrocytes treated with LPS. (a) qRT-PCR was performed to evaluate the miR-944 expression in LPS-treated chondrocytes transfected with miR-944 inhibitor. (b) The viability of LPS-treated chondrocytes transfected with miR-944 inhibitor was assessed by CCK-8 assay at indicated times. (c) The proliferation of LPS-treated chondrocytes transfected with miR-944 inhibitor was evaluated by EdU assay. (d) The expression levels of proliferation-related proteins in LPS-treated chondrocytes transfected with miR-944 inhibitor were evaluated by a Western Blot assay. (e) The cell cycle distribution of LPS-treated chondrocytes transfected with miR-944 inhibitor was evaluated by flow cytometry analysis. (f) The expression levels of cell cycle-related proteins in LPS-treated chondrocytes transfected with miR-944 inhibitor were evaluated by a Western Blot assay. (g) The cell apoptosis of LPS-treated chondrocytes transfected with miR-944 inhibitor was evaluated by flow cytometry analysis. (h) The expression levels of cell apoptosis-related proteins in LPS-treated chondrocytes transfected with miR-944 inhibitor were evaluated by a Western Blot assay. **P< 0.01 vs. control group, ##P< 0.01 vs. NC inhibitor + LPS group. All data were presented as mean ± SD. n = 3.
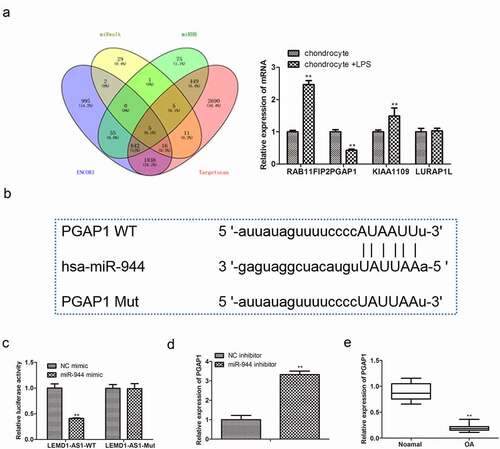
3.6 PGAP1 acts as a target of miR-944
To investigate the possible downstream targets of miR-944, bioinformatics analysis was performed. As displayed in ), a complementary sequence of miR-944 was identified in the 3’-UTR of PGAP1, and PGAP1 was lowly expressed in LPS-treated chondrocytes. Then, a dual-luciferase assay ()) was performed to confirm that PGAP1 was a direct target of miR-944. Moreover, qRT-PCR was adopted to detect the mRNA expression of PGAP1 in chondrocytes transfected with miR-944 inhibitor. The data of ) displayed that PGAP1 was high-expressed in chondrocytes transfected with miR-944 inhibitor. Furthermore, the expression of PGAP1 in cartilage tissues of OA patients was also detected by qRT-PCR. As shown in ), PGAP1 was notably down-regulated in OA tissues. These data suggested that PGAP1 was a direct target of miR-944 in OA.
Figure 6. PGAP1 acts as a target of miR-944. (a) The possible downstream targets of miR-944 were predicted by bioinformatics tools. (b) Putative binding sites between miR-944 and PGAP1. (c) Dual-luciferase reporter analysis was employed to validate the interactions between miR-944 and PGAP1. **P< 0.01 vs. NC mimic group. (d) The expression of PGAP1 in chondrocytes with miR-944 inhibitor was determined by qRT-PCR. **P< 0.01 vs. NC inhibitor group. (e) The expression of PGAP1 in cartilage tissues of OA patients was detected by qRT-PCR. **P< 0.01 vs.normal group.All data were presented as mean ± SD. n = 3.
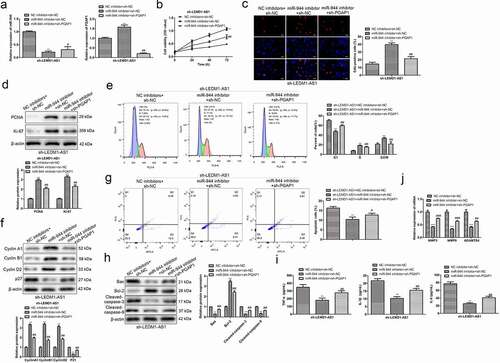
3.7 LEMD1-AS1 regulates proliferation, cell apoptosis and cycle, and inflammatory responses of LPS-treated chondrocytes by modulating miR-944/PGAP1 axis
To further explore whether LEMD1-AS1 exhibited its role by regulating miR-944/PGAP1 pathway, a series of rescue assays were performed. Firstly, chondrocytes were transfected with sh-LEMD1-AS1, and then transfected with miR-944 inhibitor or sh-PGAP1 ()). CCK-8 and EdU assays showed that compared with sh-LEMD1-AS1 + NC inhibitor group, miR-944 inhibitor obviously promoted the viability and proliferation of chondrocytes, while sh-PGAP1 partially restored the effects of miR-944 inhibitor ()). Similar results were shown in ). Moreover, flow cytometry analysis showed that relative to sh-LEMD1-AS1 + NC inhibitor group, miR-944 inhibitor notably suppressed cell cycle arrest at G1 phase, and inhibited cell apoptosis of chondrocytes, while sh-PGAP1 partially reverted the effects of miR-944 inhibitor ()). Furthermore, a Western Blot assay was also carried out to evaluate the levels of proteins related to cell apoptosis and cell cycle, and the results were consistent with the above ()). Finally, the results of ELISA showed that compared with sh-LEMD1-AS1 + NC inhibitor group, miR-944 inhibitor notably decreased the expressions of TNF-α, IL-1β and IL-6 in chondrocytes, while sh-PGAP1 partially reverted the effects of miR-944 inhibitor ()). Western blot was also carried out to evaluate the levels of proteins related to other key aspects in OA such as the expression of proteolytic enzymes like MMP3, MMP9 and ADAMTS4, and promoted expression of proteolytic enzymes, while sh-PGAP1 partially reverted the effects of miR-944 inhibitor ()). These data suggested that LEMD1-AS1 regulated proliferation, cell apoptosis and cycle, and inflammatory responses of LPS-treated chondrocytes by modulating miR-944/PGAP1 pathway.
Figure 7. LEMD1-AS1 regulates proliferation, cell apoptosis and cycle, and inflammatory responses of LPS-treated chondrocytes by modulating miR-944/PGAP1 axis. (a) Transfection efficiency of LPS-treated chondrocytes was evaluated by qRT-PCR. (b) The viability of LPS-treated chondrocytes after transfection was assessed by a CCK-8 assay. (c) The proliferation of LPS-treated chondrocytes after transfection was evaluated by EdU assay. (d) The expressions of proliferation-related proteins in LPS-treated chondrocytes after transfection were evaluated by a Western Blot assay. (e) The cell cycle of LPS-treated chondrocytes after transfection was evaluated by flow cytometry analysis assay. (f) The expressions of cell cycle-related proteins in LPS-treated chondrocytes after transfection were evaluated by a Western Blot assay. (g) The cell apoptosis of LPS-treated chondrocytes after transfection was evaluated by flow cytometry analysis assay. (h) The expressions of cell apoptosis-related proteins in LPS-treated chondrocytes after transfection were evaluated by a Western Blot assay. (i) The secreted levels of TNF-α, IL-1β and IL-6 in LPS-treated chondrocytes after transfection were determined by an ELISA assay. (j) The cells expression of proteolytic enzymes like MMPs, and ADAMTS after transfection were evaluated by a Western Blot assay when the LEDM1-AS1 treated. **P< 0.01 vs. NC inhibitor + sh-NC group, #P< 0.05, ##P< 0.01 vs. miR-944 inhibitor + sh-NC group. All data were presented as mean ± SD. n = 3.
4. Discussion
OA is a degenerative disease of articular cartilage characterized by cartilage destruction [Citation22]. For a long time, scholars in the field of bone and joint have carried out a lot of research, but little breakthrough have been achieved, and there is no basic and effective treatment method. Recently, more and more studies have shown that lncRNAs play an important role in the development of OA. For example, MALAT1 is up-regulated in OA, and MALAT1 knockdown inhibits cell proliferation, promotes apoptosis of IL-1β-treated chondrocytes [Citation23]. Additionally, MFI2-AS1 expression is increased in OA tissues and LPS-treated C28/I2 cells. Silence of MFI2-AS1 attenuates LPS-induced viability suppression, apoptosis production, inflammatory response and extracellular matrix degradation [Citation24]. Moreover, PVT1 is up-regulated in OA chondrocytes. Silencing PVT1 inhibits the apoptosis of OA chondrocytes, and over-expression of PVT1 promotes the apoptosis of normal chondrocytes [Citation25]. These findings suggest that lncRNAs may be considered new therapeutic targets for the treatment of OA. Recently, studies have shown that LEMD1-AS1 acts as a suppressor in tumor progression [Citation26]. For example, there are low levels of LEMD1-AS1 in ovarian cancer (OC) tissues and cell lines. In the preliminary study, LEMD1-AS1 presented low expression in OA based on the GSE82107 dataset. It was one of the top 10 up- and down-regulated lncRNAs on the dataset. In our study, LEMD1-AS1 was lowly expressed in OA tissues and in LPS-treated chondrocytes. Over-expression of LEMD1-AS1 significantly promoted viability, proliferation and inhibited cell apoptosis, cell cycle arrest and inflammatory responses of chondrocytes treated with LPS. These data indicated that LEMD1-AS1 might play an important role in the regulation of chondrocyte progression.
It is commonly acknowledged that lncRNAs serve as ceRNAs to boost the expressions of target mRNAs by sequestering miRNAs in various cellular processes including OA. For example, MCM3AP-AS1 is up-regulated in OA, and down-regulation of MCM3AP-AS1 notably inhibits apoptosis of chondrocytes treated with LPS via regulating miR-142-3p/HMGB1 axis [Citation27]. In addition, MEG3 is significantly down-regulated in chondrocytes treated with IL-1β, whereas miR-93 is up-regulated concomitantly. Over-expression of MEG3 induces the proliferation, suppresses the apoptosis, and relieves the degradation of ECM in IL-1β-induced chondrocytes through negatively regulating miR-93 [Citation28]. Moreover, PART-1 expression level is down-regulated in the OA cartilages. Silence of PART-1 decreases the cell viability and promotes chondrocyte apoptosis by acting as a sponge for miR-590-3p [Citation29]. Thus, in our study, online bioinformatics were used to retrieve miRNAs interacting with LEMD1-AS1. Fortunately, miR-944 was chosen for further study. MiR-944 is located in 3q28 region of human chromosome, and has been reported to possess multiple functions regulated by lncRNAs [Citation30]. For instance, miR-944 inhibitor partially abrogates silenced SNHG14-mediated inhibition on proliferation, migration and invasion, as well as promotion on apoptosis in colorectal cancer (CRC) cells [Citation31]. In addition, lncRNA JPX is discovered as highly expressed in oral squamous cell carcinoma (OSCC) cells. Silencing JPX restrains OSCC cell proliferation, migration and invasion through binding with miR-944 [Citation32]. Based on these findings, we proposed that LEMD1-AS1 might regulate chondrocyte progression by targeting miR-944. In the present study, we found that miR-944, negatively associated with LEMD1-AS1, was highly expressed in OA tissues and in LPS-treated chondrocytes. Suppression of miR-944 significantly promoted viability, proliferation and inhibited cell apoptosis, cell cycle arrest and inflammatory responses of chondrocytes treated with LPS. These data indicated that LEMD1-AS1 exhibited functional role in OA partially via regulating miR-944.
MiRNAs exert essential role in progression of OA through regulating downstream target genes. For example, the expression of miR-107 in OA chondrocytes was obviously lower. Over-expression of miR-107 inhibits apoptosis and promotes autophagy in OA chondrocytes by targeting TRAF3 [Citation33]. In addition, miR-29b-3p was up-regulated in cartilage tissues from patients with OA. MiR-29b-3p mimic notably induces apoptosis, inhibits proliferation and scratch wound closure of chondrocytes via targeting PGRN [Citation34]. In this regard, we conducted search and validation of targets that were regulated by miR-944 based on bioinformatic analysis, and found that PGAP1 was negatively regulated by miR-944 in chondrocytes. PGAP1, a protein localized in the endoplasmic reticulum responsible for the first step of the remodeling of glycosylphosphatidylinositol, is linked to a disorder characterized by psychomotor retardation and facial dysmorphism [Citation35–37]. However, whether LEMD1-AS1 regulated PGAP1 expression by targeting miR-944 in chondrocytes remained unknown. In the present study, PGAP1 acted as a target of miR-944 in chondrocytes. PGAP1 was positively associated with LEMD1-AS1, and negatively associated with miR-944. Moreover, the mechanisms of LEMD1-AS1/miR-944/PGAP1 were further explored through rescue experiments. As expected, over-expression of PGAP1 partially restored the effects of miR-944 inhibitor on viability, proliferation, cell apoptosis, cell cycle distribution and inflammatory responses of LPS-treated chondrocytes transfected with sh-LEMD1-AS1.
To conclude, the expression of LEMD1-AS1 was obviously down-regulated both in cartilage tissues of OA patients and in LPS-treated chondrocytes. Up-regulation of LEMD1-AS1 remarkably promoted proliferation, inhibited cell apoptosis, reduced cell arrest, and ameliorated inflammatory responses of LPS-treated chondrocytes via regulating miR-944/PGAP1axis. Taken together, LEMD1-AS1/miR-944/PGAP1 might be a novel therapeutic candidate target in OA treatment.
Authors’ contributions
Haitao Li and Jianguo Zang conceived and designed the study. Kaihua Lian and Haitao Li and Jianguo Zang performed the literature search and data extraction. Jianguang Mao and Fuguo Huang and Chunli Zhang drafted the manuscript. All authors read and approved the final manuscript.
Availability of data and material
All data generated or analysed during this study are included in this published article.
Consent for publication
The authors agree to publication in the Journal.
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Animal Ethics Committee of Beicheng New District Hospital of Linyi people’s hospital.
Supplemental Material
Download TIFF Image (5.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2084294
Additional information
Funding
References
- Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27(3):359–364.
- Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311.
- Sacitharan PK. Ageing and Osteoarthritis. Subcell Biochem. 2019;91:123–159.
- Varela-Eirin M, Loureiro J, Fonseca E, et al. Cartilage regeneration and ageing: targeting cellular plasticity in osteoarthritis. Ageing Res Rev. 2018;42:56–71.
- Sun X, Zhen X, Hu X, et al. Osteoarthritis in the middle-aged and elderly in china: prevalence and influencing factors. Int J Environ Res Public Health. 2019;16(23):4701.
- Skou ST, Roos EM. Physical therapy for patients with knee and Hip osteoarthritis: supervised, active treatment is current best practice. Clin Exp Rheumatol. 2019;120:112–117.
- Saccomano SJ. Osteoarthritis treatment: decreasing pain, improving mobility. Nurse Pract. 2018;43(9):49–55.
- Lindahl A. From gristle to chondrocyte transplantation: treatment of cartilage injuries. Philos Trans R Soc Lond B Biol Sci. 2015;370(1680):20140369.
- Harrell CR, Markovic BS, Fellabaum C, et al. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–2326.
- Schinhan M, Gruber M, Dorotka R, et al. Matrix-associated autologous chondrocyte transplantation in a compartmentalized early stage of osteoarthritis. Osteoarthritis Cartilage. 2013;21(1):217–225.
- Salem HS, Parvizi J, Ehiorobo JO, et al. The safety and efficacy of a novel cell-based gene therapy for knee osteoarthritis. Surg Technol Int. 2019;35:370–376.
- Zhao L, Huang J, Fan Y, et al. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann Rheum Dis. 2019;78(5):676–682.
- Chen WK, Yu X-H, Yang W, et al. lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Prolif. 2017;50(9):e12313.
- Huynh NP, Anderson BA, Guilak F, et al. Emerging roles for long noncoding RNAs in skeletal biology and disease. Connect Tissue Res. 2017;58(1):116–141.
- Zhang H, Li J, Shao W, et al. LncRNA SNHG9 is downregulated in osteoarthritis and inhibits chondrocyte apoptosis by downregulating miR-34a through methylation. BMC Musculoskelet Disord. 2020;21(1):020–03497.
- Ji Y, Fang Q-Y, Wang S-N, et al. Lnc-RNA BLACAT1 regulates differentiation of bone marrow stromal stem cells by targeting miR-142-5p in osteoarthritis. Eur Rev Med Pharmacol Sci. 2020;24(6):2893–2901.
- Chen L, Zhang Y, Rao Z, et al. Integrated analysis of key mRNAs and lncRNAs in osteoarthritis. Exp Ther Med. 2018;16(3):1841–1849.
- Guo R, Qin Y. LEMD1-AS1 suppresses ovarian cancer progression through regulating miR-183-5p/TP53 axis. Onco Targets Ther. 2020;13:7387–7398.
- Rheumatology C. Guidelines for the diagnosis and treatment of osteoarthritis. Zhong Hua Feng Shi Bing Xue Za Zhi. 2010;14:416–419.
- Yu Y, Zhao J. Modulated autophagy by microRNAs in osteoarthritis chondrocytes. Biomed Res Int. 2019;8:1484152.
- Cai Q, Wang Z, Wang S, et al. Long non-coding RNA LINC00152 promotes gallbladder cancer metastasis and epithelial-mesenchymal transition by regulating HIF-1α via miR-138. Open Biol. 2017;7(1):160247.
- Choi MC, Jo J, Park Y, et al. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8(7):734.
- Li H, Xie S, Zhang R, et al. LncRNA MALAT1 mediates proliferation of LPS treated-articular chondrocytes by targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sci. 2020;254:28.
- Luo X, Wang J, Wei X, et al. Knockdown of lncRNA MFI2-AS1 inhibits lipopolysaccharide-induced osteoarthritis progression by miR-130a-3p/TCF4. Life Sci. 2020;240:31.
- Lu X, Yu Y, Yin F, et al. Knockdown of PVT1 inhibits IL-1β-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int Immunopharmacol. 2020;79:18.
- Li N, Zhan X. Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes. Epma J. 2020;11(2):289–309.
- Gao Y, Zhao H, Li Y. LncRNA MCM3AP-AS1 regulates miR-142-3p/HMGB1 to promote LPS-induced chondrocyte apoptosis. BMC Musculoskelet Disord. 2019;20(1):019–2967.
- Chen K, Zhu H, Zheng MQ, et al. LncRNA MEG3 inhibits the degradation of the extracellular matrix of chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 axis. Cartilage. 2019;28:1947603519855759.
- Lu C, Li Z, Hu S, et al. LncRNA PART-1 targets TGFBR2/Smad3 to regulate cell viability and apoptosis of chondrocytes via acting as miR-590-3p sponge in osteoarthritis. J Cell Mol Med. 2019;23(12):8196–8205.
- Kim KH, Cho E-G, Yu SJ, et al. ΔNp63 intronic miR-944 is implicated in the ΔNp63-mediated induction of epidermal differentiation. Nucleic Acids Res. 2015;43(15):7462–7479.
- Pei Q, Liu G-S, Li H-P, et al. Long noncoding RNA SNHG14 accelerates cell proliferation, migration, invasion and suppresses apoptosis in colorectal cancer cells by targeting miR-944/KRAS axis through PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23(22):9871–9881.
- Yao Y, Chen S, Lu N, et al. LncRNA JPX overexpressed in oral squamous cell carcinoma drives malignancy via miR-944/CDH2 axis. Oral Dis. 2020;3:13626.
- Zhao X, Li H, Wang L. MicroRNA-107 regulates autophagy and apoptosis of osteoarthritis chondrocytes by targeting TRAF3. Int Immunopharmacol. 2019;71:181–187.
- Chen L, Li Q, Wang J, et al. MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J Cell Mol Med. 2017;21(12):3347–3359.
- Kettwig M, Elpeleg O, Wegener E, et al. Compound heterozygous variants in PGAP1 causing severe psychomotor retardation, brain atrophy, recurrent apneas and delayed myelination: a case report and literature review. BMC Neurol. 2016;16(1):016–0602.
- Murakami Y, Tawamie H, Maeda Y, et al. Null mutation in PGAP1 impairing Gpi-anchor maturation in patients with intellectual disability and encephalopathy. PLoS Genet. 2014;10(5):e1004320.
- Ueda Y, Yamaguchi R, Ikawa M, et al. PGAP1 knock-out mice show otocephaly and male infertility. J Biol Chem. 2007;282(42):30373–30380.
