ABSTRACT
Excessive apoptosis of placental trophoblast cells is considered a major cause of pre-eclampsia (PE) pathogenesis. Phosphorylation of the widely expressed cAMP response element binding protein (CREB) regulates apoptosis and may be involved in PE incidence. Low-dose aspirin (LDA) is an effective approach for preventing PE with unclear mechanisms. Thus we examined whether LDA protects against PE by inhibiting trophoblast cell apoptosis through CREB. The effects of LDA on human PE placenta, PE model rat placenta, and hydrogen peroxide (H2O2)-induced HTR-8/SVneo cell apoptosis were analyzed. TUNEL assay, immunohistochemistry, Cell Counting Assay Kit-8 (CCK-8) assay, western blot, and flow cytometry assay were performed. In the placenta of human PE and rat PE models, the TUNEL index increased and was partially corrected with LDA pre-treatment. Meanwhile, decreased Bcl-2 and increased Bax expression were significantly reversed by LDA pre-treatment. In HTR-8/SVneo cells, H2O2 decreased cell viability, promoted apoptosis, reduced the Bcl-2/Bax ratio, aggravated loss of mitochondrial membrane potential (MMP), increased cytoplasmic cytochrome c release, and simultaneously activated caspase-9 and caspase-3. These effects were effectively restored by LDA pre-treatment in the cells. Moreover, LDA promoted CREB phosphorylation in trophoblast cells. CREB interference further promoted apoptosis, reduced the Bcl-2/Bax ratio, and increased MMP loss. CREB interference also reversed the inhibitory effect of LDA on H2O2-induced apoptosis in HTR-8/SVneo cells. Thus, LDA was shown to inhibit trophoblast cell mitochondrial apoptosis by activating the CREB/Bcl-2 pathway, providing novel evidence for the protective mechanism of LDA in PE.
Abbreviations; PE: Pre-eclampsia; LDA: low-dose aspirin; CREB: cAMP response element binding protein; ROS: reactive oxygen species; H2O2: hydrogen peroxide; PBS: Phosphate-buffered saline; Bcl-2: B-cell lymphoma-2; MMP: Mitochondrial membrane potential; Cyt-c: CytochromeC.
Introduction
Pre-eclampsia (PE) is an unpredictable multisystem disorder of pregnancy and a persistent hypertensive gestational disease with highly variable complications [Citation1]. It can cause an attendant increase in maternal and infant morbidity and mortality [Citation2]. However, the pathogenesis of PE remains unclear and multifactorial. Inflammation, oxidative stress, apoptosis, and endothelial dysfunction have been consistently associated with PE [Citation3,Citation4]. Recently, increasing attention has been focused on increased placental trophoblast cells apoptosis [Citation5].
Apoptosis can be induced via two pathways: the extrinsic (death receptor) pathway and the intrinsic (mitochondrial) pathway [Citation6]. Bcl-2, a pivotal member of the Bcl-2 protein family, attenuates mitochondria-dependent apoptosis [Citation7]. Accumulating evidence has demonstrated reduced Bcl-2 expression in PE [Citation8,Citation9]. Bcl-2 is a target of the cAMP response element binding protein (CREB) [Citation10], which plays an important role in the forskolin pathway [Citation11], and in the proliferation and invasion of trophoblast cells [Citation12]. The phosphorylation activation of CREB promotes Bcl-2 protein expression, thereby inhibiting cell apoptosis [Citation10,Citation13–16]. CREB has been suggested as a potential diagnostic and therapeutic target for PE [Citation17].
To the best of our knowledge, the most effective management for PE is prevention [Citation18]. Aspirin, a cyclooxygenase (COX) inhibitor, inhibits vasoconstriction and platelet aggregation [Citation19–21]. Since 1978, numerous studies have confirmed that low-dose aspirin (LDA) can reduce the incidence of preterm PE, especially in women with high risk factors [Citation22–25]. However, the underlying biological mechanisms remain elusive.
Considering that CREB/Bcl-2 affects apoptosis negatively and that apoptosis is involved in PE pathophysiology [Citation26]. We hypothesized that LDA inhibits trophoblast cell apoptosis via the CREB/Bcl-2 pathway, thus preventing PE. In this study, we assessed apoptosis and the expression of apoptosis-related proteins in human PE, hydrogen peroxide (H2O2)-induced trophoblast cells (HTR-8/Svneo cells), and in an NG-nitro-L-arginine methyl ester (L-NAME, a nonselective NOS inhibitor)-induced rat PE model, to explore the underlying mechanism of LDA in the prevention of PE via the CREB/Bcl-2 pathway.
Materials and methods
Reagents
The TUNEL assay kit was obtained from Elabscience. DMEM/F12 medium, fetal calf serum, and protease inhibitor cocktail were obtained from Gibco/Invitrogen (Carlsbad, CA, USA). Acetyl-salicylic acid (ASA) was obtained from Sigma-Aldrich (St. Louis, MO, USA), reconstituted in dimethyl sulfoxide (DMSO, Sigma-Aldrich), and filter-sterilized prior to use. For cell experiments, 0.1 mmol/L aspirin, a dose equivalent to the serum concentration of patients treated with LDA was used, whereas 2.5 mg/kg/d aspirin was used for intragastric administration in rats. Hydrogen peroxide (H2O2) was purchased from Merck. The Cell Counting Assay Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies (MD, Japan). The nonselective NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME) was obtained from Sigma-Aldrich (St. Louis, MO, USA) and reconstituted in dH2O. Anti-Bax, anti-cytochrome c (Cyt-c), anti-caspase-9, anti-cleaved caspase-9, anti-caspase-3, and anti-cleaved caspase-3 were purchased from Cell Signaling Technology (Boston, MA, USA). Anti-CREB, anti-p-CREB and Anti-Bcl-2 were obtained from Affinity Biosciences LTD. The mitochondrial membrane potential assay kit with JC-1 and Annexin V-PE/7-AAD Apoptosis Detection Kit was obtained from Keygen Biotech (Nanjing, China). The Mitochondria Isolation Kit was purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). The animal protocol was approved by the Guangzhou Women and Children’s Medical Center, Guangzhou Medical University Institutional Animal Care and Use Committee. Sprague Dawley (SD) wild-type (Wt) rats were obtained from Guangdong Medical Experimental Animal Center. The rats received 50 mg/kg/day L-NAME to generate the PE model.
Sample collection
The Ethical Committee of Guangzhou Medical University, Guangzhou Women and Children’s Medical Center approved the sample collection and experiments in this study (No.262B00). All participants signed an informed consent form, and placental tissues were obtained from PE pregnant women (treated with LDA or not), maternal age, and gestational age-matched control groups (14–16 cases, separately for each group). Aspirin (100 mg/d) was administered from 11–16 weeks of gestation for a high risk of PE.
Inclusion criteria
Pregnant women with preeclampsia, singleton, with (PE+LDA group) or without (PE group) aspirin treatment, or gestational age-matched pregnant women (control group), with normal blood pressure, and blood/urine examination. The causes of premature termination of pregnancy in the control group were premature rupture of membranes or premature labor, etc.
Exclusion criteria
Pregnancy with medical or surgical complications, other pregnancy complications such as intrauterine infection, gestational diabetes mellitus(GDM).
The placenta samples were stored at −80°C for western blotting or fixed in formalin for immunohistochemistry (IHC) and TUNEL staining.
Tunel assay kit for apoptosis
The TUNEL assay was used to detect apoptotic cells in placental tissues. The placental tissue sections were deparaffinized, rehydrated, stripped, permeated, and quenched. Next, 0.5% H2O2 was used to block endogenous peroxidase activity at room temperature for 20 min. The sections were then preincubated. After adding 100 μl of TdT equilibration working buffer to each slide and incubating at room temperature for 30 min, 50 μl of TdT Enzyme working solution was mixed on each slide and incubated at 37°C for 60 min. Subsequently, the paraffin-embedded placental sections were stained with DAPI and sealed. The expression of apoptotic nuclei was determined by green fluorescence. In a fluorescence microscope (confocal microscope), the selected excitation wavelength range was 450–500 nm, and the emission wavelength was 515–565 nm (green fluorescence). TUNEL-positive cells were counted in 10 digital images of random high-power fields and then analyzed.
Immunohistochemistry
Briefly, placental tissues were first immobilized with formaldehyde and then transferred to paraffin. After being serially sectioned to 4 µM thickness, paraffin-embedded tissues were deparaffinized in xylene and dehydrated in ethanol. Subsequently, the slides were incubated with primary antibodies (Anti-Bcl-2, Bax, precaspase-9,cleaved caspase-9, precaspase-9, cleaved caspase-3, p-CREB, and CREB antibodies at 1:200 dilution) overnight at 4°C, washed three times with PBS, and counterstained with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 30 min. Subsequently, the tissues were stained using a 0.5% diaminobenzidine kit with Mayer’s hematoxylin (Shanghai Genomics, Shanghai, China) and imaged.
Cell culture and drug treatment
In this study, HTR-8/SVneo trophoblast cells were purchased from American Type Culture Collection (ATCC). To explore the protective effect of LDA, HTR-8/SVneo cells pretreated with aspirin at 0.1 mmol/L for 30 min were exposed to 200 μmol/L H2O2, which was established as a PE model in cell experiments. After 24 h, protein lysates or cells were collected for western blotting or other experiments. All experiments were repeated five times.
CCK-8 assay for cell viability
HTR-8/SVneo cells were incubated for another 2 h after adding 10 μl CCK-8 solution to each well. Absorbance at 450 nm was measured using a microplate reader (Bio-Tek, Winooski, VT, USA).
Quantification of apoptosis by flow cytometry
Briefly, HTR-8/SVneo cells were digested, centrifuged, and washed three times, followed by staining with Annexin V-APC and 7-AAD. They were then placed in the dark at room temperature for 15 min and analyzed using flow cytometry (Coulter, Hialeah, FL, USA).
siRNA transfection
Sequences of human CREB siRNA were synthesized by RiboBio (Guangzhou, China). The sequences were as follows: 5’- GCACUUAAGGACCUUUACUTT −3’. Scrambled RNA was used as the negative control. HTR-8/SVneo cells were transfected with CREB siRNA and the negative control. Briefly, at room temperature, 5 μl of CREB siRNA was diluted in 88 μl of DMEM/F12 mixed with 7 μl of HiPerFect reagent (Proteintech, Chicago, U.S.) for 20 min, added to the cells, and incubated in DMEM/F12 without serum (the final siRNA concentration was 100 nmol/L). After 3 h, the culture medium was replaced with DMEM/F12 supplemented with 10% fetal calf serum for another 45 h.
Western blot
Cells were harvested and lysed in RIPA lysis buffer containing a 1% cocktail. After separation by SDS-PAGE, the protein was transferred to a PVDF membrane and blocked with 5% skim milk for 1 h at room temperature. The membrane was then incubated overnight at 4°C with the primary antibody, followed by incubation with the secondary antibody at room temperature for 1 h. The blots were detected and quantified using a gel imaging system (Bio-Rad, Hercules, CA, USA). β-actin or Cox IV was used as the control to verify equal protein loading.
Flow cytometric measurement of mitochondrial membrane potential (MMP)
The cells were digested, washed, and incubated with JC-1 dye for 15 min at 37°C in the dark. The excess staining solution was removed, and the cells were centrifuged and resuspended in JC-1 staining working solution. The fluorescence intensity ratio of monomeric JC-1 to aggregated JC-1 (green/red) represents MMP loss.
Isolation of mitochondria
Briefly, the cell suspension was discarded after centrifugation and the pellet was obtained. Mitochondrial isolation reagents A, B, and C were used. After centrifugation, the supernatant (cytosolic fraction) and pellet (containing isolated intact mitochondria) were separated and used for western blotting. Cox IV was used as a loading control for the mitochondrial fraction.
Animal studies
According to previously published procedures [Citation27–31], a PE rat model was established by treatment with L-NAME (a nonspecific nitric oxide inhibitor). After being approved and trained by the Guangzhou Medical University, researchers carried out the experiment in compliance with the institutional animal care regulations and guidelines for the care and use of laboratory animals. SD wild-type (Wt) rats were mated at a male: female ratio of 1:2 and exposed to a daily 12 h light–dark cycle in an SPF barrier system, with a temperature of 20–26°C and 50–60% humidity. Female nulliparous rats aged 8–10 weeks, weighing 240–270 g were bred and gestation day (GD) 0.5th was confirmed by sperm and estrum; L-NAME was used with subcutaneous injection in the back, dH2O or 2.5 mg/kg/d aspirin was administered intragastrically from GD7.5 to GD18.5.
Animal experimental design
The rats were randomly divided into five groups: SD WT, low-dose aspirin (LDA), L-NAME, and L-NAME+LDA groups(n = 5). The rats received dH2O or aspirin (2.5 mg/kg/d, Sigma Aldrich, USA) daily from GD7.5 to GD18.5; PE model pregnant rats received dH2O or L-NAME (50 mg/kg/day) from GD7.5 to GD18.5 for inducing preeclampsia. All animals were housed in individual metabolic cages and received chow ad libitum; urine output was collected on GD19.5. The systolic blood pressure (SBP) of pregnant rats was measured on GD0.5, GD6.5, GD12.5, and GD18.5, using a noninvasive tail-cuff BP-2000 Blood Pressure Analysis System (Visitech Systems, Inc., Apex, NC, USA). At the end of the experiment, the blood and placenta tissues were collected on GD19.5 after sacrifice, the placenta samples were stored at −80°C or fixed in formalin; after centrifugation, blood was stored at −20°C for further analysis.
Statistical analysis
All data were analyzed using GraphPad Prism ver. 8 (GraphPad Software, La Jolla, CA, USA). Measurement data are expressed as the mean ± standard deviation (SD); the n value represents the number of independent experiments. Among multiple groups One-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test was used for comparisons, and statistical significance was set at P < 0.05.
Results
Demographics and obstetrical characteristics of patients with PE, with and without LDA
lists the basic characteristics, blood pressure, and neonatal birth weight in the control, PE, and PE+LDA groups. No significant differences were observed among the three groups in maternal age or weight (P > 0.05). In contrast, the greatest amount of proteinuria and the highest systolic blood pressure (SBP) and diastolic blood pressure (DBP) were detected in LDA-free patients with PE compared to those treated with LDA (P < 0.01). Further, in the LDA group, women had a significantly higher neonatal birth weight than those in the LDA-free group (P < 0.01).
Table 1. Clinical characteristics of study populations.
LDA attenuates placental apoptosis in PE
To explore the effect of LDA on human PE or on the PE rat model, apoptosis was detected using the TUNEL assay kit. In human PE placenta, the ability of placental trophoblast cells to undergo apoptosis was increased, which was downregulated by LDA treatment ()). We then employed L-NAME to induce PE symptoms in rats, and measured the SBP of pregnant rats on GD0.5, GD6.5, GD12.5, and GD18.5 ()). On GD0.5 and GD6.5, no significant differences were observed in SBP in each group. After L-NAME administration on GD12.5 and GD18.5, SBP in female rats was significantly elevated and this was significantly decreased by LDA treatment. Moreover, the high level of urinary protein excretion induced by L-NAME was markedly downregulated by LDA treatment ()). Simultaneously, LDA reduced the TUNEL apoptosis index, which was increased in the L-NAME group ()).
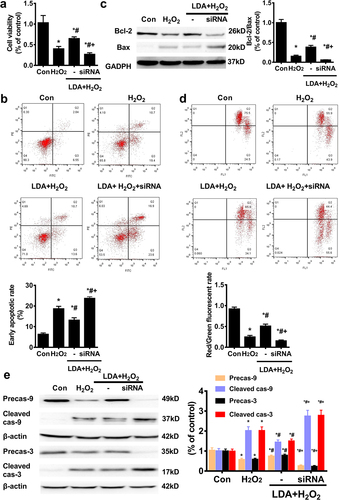
Figure 1. LDA inhibits apoptosis in the placenta of human PE or L-NAME-induced PE model rats and in H2O2 -induced HTR-8/SVneo cells. a. In human placenta tissue, the TUNEL apoptosis index was detected using a Tunel Assay Kit. Under a confocal microscope: the placenta tissue is shown with blue fluorescence (DAPI), and green fluorescence (Tunel positive cells), 10 high-power fields were randomly selected to calculate the rate of TUNEL-positive cells. Administration of LDA reduced the number of TUNEL-positive nuclei (n = 14-16, *P < 0.05 vs. con, #P < 0.05 vs. PE); b. The SBP of each group was measured using the noninvasive tail-cuff method on GD0.5, GD6.5, GD12.5, and GD18.5 (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. L-NAME); c. Proteinuria in each group was analyzed on GD0.5, GD6.5, GD12.5, and GD18.5 (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. L-NAME); d. In rat placenta tissue, the TUNEL apoptosis index was detected using the TUNEL Assay Kit, and the rate of TUNEL-positive cells was calculated. Administration of LDA reduced the number of TUNEL-positive nuclei compared to those in the L-NAME-induced PE model rats (n = 10,*P < 0.05 vs. con, #P < 0.05 vs. L-NAME); HTR/SVneo-8 cells were pretreated with aspirin (0.1 mmol/L) for 30 min and treated with H2O2 (200 μmol·L−1) for 24 h. Cell viability was measured using the CCK-8 assay (e), and apoptosis was measured by flow cytometry (FCM), the percentage of early apoptotic cells (lower right corner) and apoptotic cells (right quadrant) was analyzed (f) (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. H2O2).
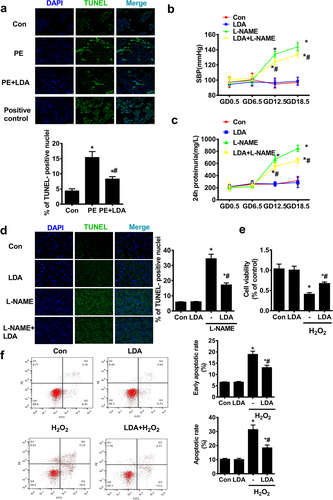
Further, in the HTR-8/SVneo trophoblast cells under treatment with H2O2 (200 μmol/L) for 24 h, the cell viability rate ()) was significantly decreased to 40.60% ± 3.97%, and when pretreated with 0.1 mmol/L LDA for 30 min, a protective effect against H2O2 was observed with a cell viability rate of 67.24% ± 3.79%. Furthermore, cell apoptosis ()) was assessed by flow cytometry and the cell apoptosis rate (control group, 6.57% ± 0.19%, for early apoptotic ratio) induced by H2O2 (18.92% ±1.46% for early apoptotic ratio) was found to be significantly decreased by LDA (13.04% ±1.06% for early apoptotic ratio).
LDA inhibits apoptosis in HTR-8/Svneo cells through the mitochondrial-dependent pathway
Two critical factors indicating apoptosis, the anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bax, regulate the mitochondrial pathway of apoptosis [Citation32]. The increase in the Bcl-2/Bax ratio represents increased survival under apoptotic stimulation, whereas a decrease indicates increased death.
In HTR-8/SVneo cells, H2O2 treatment decreased Bcl-2 protein expression and increased Bax protein expression as indicated by western blotting, leading to a decline in the Bcl-2/Bax ratio, which was partially upregulated when pretreated with LDA ()).
Figure 2. The effect of LDA on the mitochondrial apoptosis of HTR8/SVneo trophoblast cells. a. The expression of apoptosis markers Bcl-2 and Bax in HTR8/SVneo cells was detected by western blotting. The optical density analysis of Bcl-2/Bax is shown in the bar graph (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. H2O2); b. MMP loss was detected by JC-1 staining. The decrease in the red/green fluorescence ratio represents MMP loss. The optical density analysis of the red/green fluorescence intensity is displayed in the bar graph (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. H2O2); c. western blot analysis was used to detect the expression of Cyt-c in HTR8/SVneo cell cytoplasm and mitochondria to evaluate the release of Cyt-c, the optical density analysis of protein expression is shown in the bar graph (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. H2O2); d. The expression and activity of caspase-9 (cas-9) and caspase-3 (cas-3) in HTR8/SVneo cells were detected by western blot analysis. The optical density analysis of protein expression is shown in the bar graph (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. H2O2).
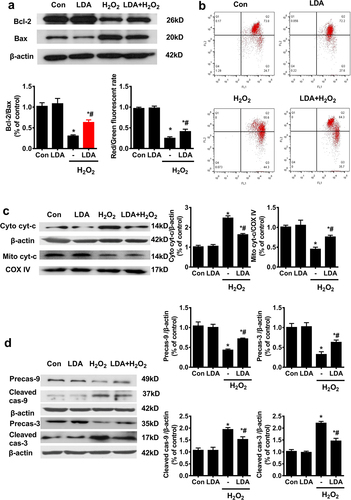
Loss of mitochondrial membrane potential (MMP) is a sign of early apoptosis and might be a consequence of increased Bax expression [Citation33]. A decreased red/green fluorescence ratio reflects loss of MMP. The x- and y-axes in the flow cytometry plot represent the green and red fluorescence intensities, respectively. H2O2 decreased the red/green fluorescence ratio and promoted MMP loss, which was alleviated when cells were pretreated with LDA ()). MMP loss could also lead to the release of Cyt-c in the mitochondria into the cytosol. H2O2 induced Cyt-c release from the mitochondria into the cytosol, which was counteracted by LDA treatment ()). Caspase-9 and caspase-3 cleavages were significantly increased upon H2O2 treatment and were partially downregulated upon pretreatment with LDA ()).
LDA inhibits apoptosis in PE placenta via the mitochondria-dependent pathway
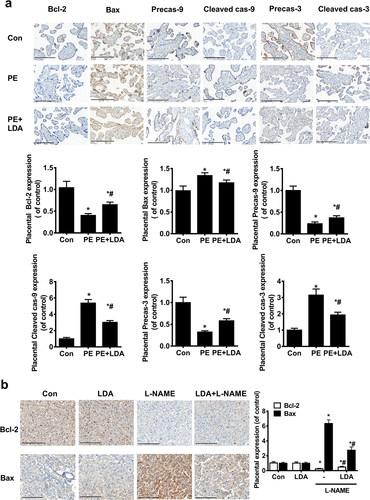
Immunohistochemistry was used to determine the expression of apoptosis-related proteins Bcl-2, Bax, Precaspase-9, Precaspase-3, cleaved caspase-9, and cleaved caspase-3 ()) in the human placenta. The expression of Bcl-2, precaspase-9, and precaspase-3 was decreased whereas the expression of Bax, cleaved caspase-9, and cleaved caspase-3 was increased in the PE placentas; these were restored upon LDA administration. In rat placenta ()), Bcl-2 decreased and Bax increased in the L-NAME-induced PE model placenta, which was partially reversed when administered LDA.
Figure 3. LDA inhibits mitochondrial apoptosis in PE placenta or in the L-NAME-induced PE rat model. a. Expression of Bcl-2, Bax, Precaspase-9, Precaspase-3, cleaved caspase-9, and cleaved caspase-3 in human placental specimens by immunohistochemistry; brown-yellow particles are shown with a staining intensity score of 0 (none), 1 (weak), 2 (medium), or 3 (strong). Scale bar = 200 µm, the number of positive cells was counted for statistical analysis (n = 14-16, *P < 0.05 vs. con, #P < 0.05 vs. PE); b. The expression of Bcl-2 and Bax was detected in rat model placental specimens by immunohistochemical methods. Scale bar = 200 µm, the number of positive cells was counted for statistical analysis (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. L-NAME).
LDA promotes CREB phosphorylation in the PE placenta
Immunohistochemistry assays showed that in human ()) and rat PE ()) placenta, the expression of p-CREB was significantly decreased, and was significantly increased with the pretreatment of LDA. However, no significant difference was observed for the expression of CREB in PE placentas.
Figure 4. LDA increases the CREB phosphorylation level which is reduced in PE placental trophoblasts and H2O2-induced HTR8/SVneo trophoblast cells. The expression of CREB and the phosphorylated CREB in human placental tissues was detected by immunohistochemistry (a) (n = 14-16, *P < 0.05, vs. con, #P < 0.05 vs. PE) and in PE model rat placenta tissues (b) (n = 5, *P < 0.05 vs. con, #P < 0.05 vs. L-NAME); c. HTR8/SVneo trophoblast cells were treated with 200 µmol/L H2O2 for 24 h and pretreated with LDA for 30 min; the effects of LDA on CREB activation were evaluated by detecting the expression and activation of CREB and p-CREB using western blotting. Densitometric analysis of the expression of CREB and p-CREB is shown in the bar graphs (n = 5, *P < 0.05, vs. con, #P < 0.05 vs. H2O2).
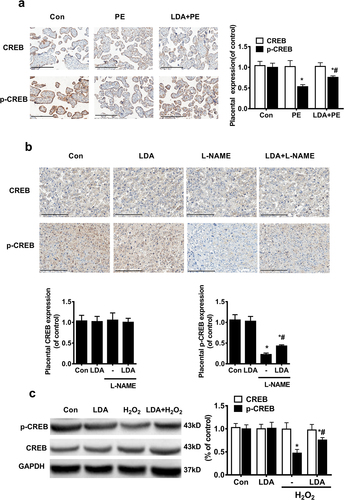
In HTR-8/SVneo cells, p-CREB significantly decreased when treated with H2O2 and was partially upregulated when pretreated with LDA ()); however, no difference was seen in CREB expression when pretreated with H2O2 or LDA.
CREB interference further promotes H2O2-induced apoptosis in HTR-8/SVneo cells
We then assessed the impact of CREB on apoptosis levels upon H2O2-induced HTR-8/SVneo cell death. After identifying the effectiveness of CREB interference ()), the results indicated that CREB interference further decreased cell viability ()) from 42.96% ±3.61% to 26.60% ±3.65% and increased the early apoptosis rate ()) from 17.90% ± 1.21% to 28.58% ±1.64%; CREB interference further reduced the decrease in Bcl-2 and aggravated the increase of Bax induced by H2O2 in HTR8/SVneo cells ()). CREB interference also decreased the red/green fluorescence ratio and promoted the loss of MMP induced by H2O2 in HTR8/SVneo cells ()).
Figure 5. The effect of CREB interference on mitochondrial apoptosis of HTR8/SVneo trophoblast cells. a. In HTR8/Svneo cells, the CREB siRNA was transfected for 48 hours, and the control was defined as neg; western blot was used to detect interference efficiency. Subsequent experiments were performed using CREB siRNA (100 nM) for 48 h (n = 5, *P < 0.05 vs. con). After being treated with CREB siRNA for 24 hours, H2O2 was added to the cells for another 24 hours; the cell survival rate was then evaluated using the CCK-8 assay (b), the apoptosis rate (c) measured by flow cytometry (FCM), expression of Bcl-2 and Bax (d) detected by WB, and MMP loss (e) were analyzed (n = 5, *P < 0.05 vs. neg, #P < 0.05 vs. neg, + P < 0.05 vs. H2O2).
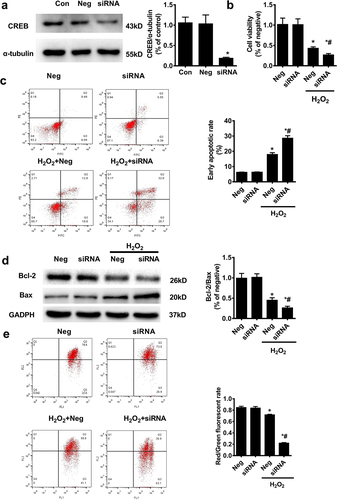
LDA inhibits apoptosis by promoting CREB activation in trophoblast cells
Next, we examined whether the protective effect of LDA on trophoblast cell apoptosis was mediated by CREB. The protective effect of LDA on cell viability was counterbalanced from 64.90% ± 4.13% to 26.80% ± 3.70% upon treatment with CREB siRNA in HTR-8/SVneo cells ()). In the next step, by flow cytometry, CREB interference was found to significantly cancel out the protective role of LDA on apoptosis with an increase in the apoptotic rate from 13.16% ± 1.16% to 23.74% ± 0.75% (for the early apoptotic ratio) ()). The expression of Bcl-2 and Bax proteins influenced by LDA treatment was attenuated by CREB knockdown, leading to a decline in the Bcl-2/Bax ratio ()). Further, the decreased loss of MMP by LDA pretreatment in HTR8/SVneo cells was blocked by CREB interference ()). CREB interference reversed the effect of LDA on the expression of caspase-9 and caspase-3 cleavage in HTR8/SVneo cells()).
Figure 6. LDA could not correct the effect of CREB on the mitochondrial apoptosis of HTR8/SVneo cells. After treatment with CREB siRNA for 24 h, LDA was used 30 min ahead of H2O2 treatment for another 24 h, and the cell survival rate was evaluated using the CCK-8 assay (a); the apoptosis rate was measured by flow cytometry (FCM) (b), the expression of Bcl-2 and Bax was detected by western blotting (c), and MMP loss (d) was analyzed. Meanwhile, the expression and activation of caspase-9 (cas-9) and caspase-3 (cas-3) were detected by western blotting(e). Densitometric analysis of the cas-9/3 expression is shown in the bar graphs (n = 5, *P < 0.05, vs. con, #P < 0.05, vs. H2O2, +P < 0.05 vs. LDA + H2O2).
Discussion
LDA is currently considered the most effective prophylactic drug for preventing PE [Citation23,Citation24]. However, its underlying mechanisms of action are still being explored. Panagodage et al. reported that LDA attenuates the increase in apoptotic marker and caspase 3 activity in BeWo cells induced with PE serum [Citation34]. Qing et al. [Citation35] indicated that LDA decreases the expression of Bax and cleaved caspase-3, which are increased in the sFlt-1-induced mouse PE model. However, these studies failed to explore the apoptotic pathway and the underlying mechanism in detail. It is well known that the Bcl-2 gene family affects MMP [Citation32,Citation36] and alters cytochrome c (Cyt-c) release from the inner mitochondrial membrane into the cytoplasm [Citation37]. Subsequently, Cyt-c in the cytoplasm activates caspase-9 and caspase-3, which ultimately modulates apoptosis [Citation38].
Lower Bcl-2 expression and higher Bax were observed in PE [Citation39] or in cytotrophoblasts cultured under hypoxic conditions [Citation40], suggesting that they contribute to placental dysfunction. In this study, we examined mitochondrial apoptosis-related factors including Bcl-2, Bax, caspase-9, and caspase-3 in LDA (defined as 75–150 mg/d) pre-treated human PE placenta. In PE placenta, the expression of Bcl-2, precaspase-9 and precaspase-3 decreased together with an increase in Bax, cleaved caspase-9, and cleaved caspase-3. Moreover, pre-treatment with LDA partially reversed the expression of these proteins compared to that in the PE group. Further, treatment with L-NAME increased the apoptotic rate [Citation41]. In the present study, L-NAME was used to induce the symptoms and pathophysiological changes similar to PE symptoms in rats; further, the expression of Bcl-2 decreased with an increase in Bax in the L-NAME-induced PE rat model. However, LDA administration partially restored Bcl-2 and Bax expression. Therefore, we confirmed LDA as a promising treatment in human PE and L-NAME-induced PE rat models by inhibiting mitochondrial apoptosis.
As one of the major reactive oxygen species (ROS), it is well established that excessive H2O2 triggers mitochondrial pathway apoptosis [Citation42,Citation43]. H2O2 (200 μmol/L, in vitro) is widely used to induce rapid and sensitive apoptosis [Citation44,Citation45]. We thus hypothesized that exposure to H2O2 could induce apoptosis in trophoblast cells. In this study, H2O2-induced HTR-8/SVneo cells showed an increased apoptotic rate, decreased Bcl-2/Bax ratio, and increased MMP loss. However, LDA administration partially alleviated the effect of H2O2 in HTR-8/SVneo cells. The results showed that LDA attenuated mitochondrial apoptosis in H2O2-induced trophoblast cells.
As a member of the CREB/ATF family, CREB is phosphorylated and activated [Citation46], and binds to the CRE [Citation16,Citation47], the homo- and hetero-dimerize then regulates cell proliferation, differentiation, and survival or apoptosis [Citation15]. Few reports have described the relationship between CREB and PE or trophoblast cells. Depoix et al. have reported that cAMP/CREB signaling regulates PIGF gene expression, which participates in PE pathogenesis [Citation48]. Meanwhile, in cytotrophoblast cells, CREB is considered to modulate human chorionic gonadotropin (hCG) gene expression, whose functional modifications might contribute to PE pathogenesis [Citation49]. In the current study, CREB activation was found to be differentially decreased in the placenta of human PE, L-NAME-induced PE rat model, and H2O2-induced HTR-8/SVneo cells. CREB interference further reduced the Bcl-2/Bax ratio and increased the apoptotic rate induced by H2O2. Similarly, Zhang et al. demonstrated that Bcl-2 expression was down-regulated [Citation50], whereas, the expression of Bax and cleaved caspase-3 was upregulated in CREB1 knock-down rats. Thus, CREB negatively affected mitochondrial apoptosis in trophoblast cells and might be related to the occurrence of PE. We thus propose that promoting CREB activation might be a potential treatment for PE.
Further, LDA was found to promote CREB phosphorylation in the placenta of human PE and L-NAME-induced PE rat model and HTR-8/SVneo cells. Therefore, we hypothesized that LDA might prevent PE by activating CREB. In the present study, the effect of LDA on trophoblast cell apoptosis was reversed by interference using CREB siRNA. Compared to the LDA+H2O2 group, CREB interference significantly increased the apoptotic rate, decreased the ratio of Bcl-2/Bax, exacerbated MMP loss, and activated caspase-9/3. Thus, we proposed that LDA inhibits mitochondrial apoptosis by promoting CREB/Bcl-2 in trophoblast cells.
However, further in-depth exploration is needed. LDA plays a protective role in PE mainly by inhibiting cyclooxygenase-2 (COX-2) [Citation19]. By consulting the GEO database, the upstream pathways of CREB including CaMKII, Akt, AMPK, and MAPKs, have been analyzed in COX-2 knockout rats [Citation51]. The results indicated that the expression of CaMKII delta mRNA was increased, whereas the mRNA expression of Akt and MAPKs was not significantly changed; further, the mRNA expression of AMPK was not detected. LDA also plays a key role in vasodilation and inhibits apoptosis by mediating an increase in NO production [Citation52,Citation53]. Simultaneously, Martinez et al. identified that COX-2 reduced the activity and bioavailability of NO, resulting in vascular dysfunction and elevation of blood pressure [Citation54,Citation55]. Zhang et al. showed that CREB phosphorylation decreased after endodermal nitric oxide synthase (eNOS) was blocked [Citation56]. We thus hypothesized that LDA might activate CaMKII or eNOS by inhibiting COX-2, thereby promoting CREB phosphorylation and inhibiting trophoblast mitochondrial pathway apoptosis.
Moreover, by analyzing CREB KO rats, CREB was found to affect ion channel remodeling and to regulate heart failure [Citation57]. Sampurno et al. illustrated that CREB activation is elevated in gastrointestinal malignancy [Citation58] and in other cancers. Furthermore, CREB single-nucleotide polymorphisms (SNPs) have been found to influence several psychiatric disorders such as major depressive disorder [Citation59], bipolar disorder (BD) [Citation60], and developmental disorders such as Rubinstein-Taybi syndrome (RSTS) [Citation61]. Therefore, the present study provides new insights into the treatment of these diseases with LDA via the CREB pathway, which need to be further studied.
In conclusion, our results suggest that LDA inhibits mitochondrial apoptosis in trophoblast cells by activating the CREB/Bcl-2 pathway. Our findings also indicate that the preventive effect of LDA on PE might be related to the regulation of mitochondrial function via the CREB/Bcl-2 pathway. Most importantly, our study provides potential opportunities for therapeutic intervention via activation of CREB in several apoptosis-related diseases in obstetrics and gynecology such as fetal growth restriction (FGR) and gestational diabetes mellitus (GDM).
Author contribution statement
KM G performed the cell culture, conceived the study and drafted the manuscript. W L performed the flow cytometry and western blotting. ZH W performed animal experiment and immunohistochemistry. LC H carried out the data analysis. Y F carried out human placenta collection and cell experiment. KM G and HS L conceived the study and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank Qi Wu at Guangzhou Women and Children’s Medical Center in China for kindly guiding flow cytometry experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Dachew BA, Mamun A, Maravilla JC, et al. Association between hypertensive disorders of pregnancy and the development of offspring mental and behavioural problems: a systematic review and meta-analysis. Psychiatry Res. 2018;260:458–467.
- Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016;387(10022):999–1011.
- Rana S, Karumanchi SA, Lindheimer MD. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension. 2014;63(2):198–202.
- Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29(7–8):518–522.
- Leung DN, Smith SC, To KF, et al. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184(6):1249–1250.
- Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498.
- Akgul C, Moulding DA, Edwards SW. Alternative splicing of Bcl-2-related genes: functional consequences and potential therapeutic applications. Cell Mol Life Sci. 2004;61(17):2189–2199.
- Aban M, Cinel L, Arslan M, et al. Expression of nuclear factor-kappa B and placental apoptosis in pregnancies complicated with intrauterine growth restriction and preeclampsia: an immunohistochemical study. Tohoku J Exp Med. 2004;204(3):195–202.
- Tong J, Niu Y, Chen ZJ, et al. Comparison of the transcriptional profile in the decidua of early-onset and late-onset pre-eclampsia. J Obstet Gynaecol Res. 2020;46(7):1055–1066.
- Cai X, Fu H, Wang Y, et al. Depletion of GPSM1 enhances ovarian granulosa cell apoptosis via cAMP-PKA-CREB pathway in vitro. J Ovarian Res. 2020;13(1):136.
- Schubert SW, Abendroth A, Kilian K, et al. bZIP-type transcription factors CREB and OASIS bind and stimulate the promoter of the mammalian transcription factor GCMa/Gcm1 in trophoblast cells. Nucleic Acids Res. 2008;36(11):3834–3846.
- Darashchonak N, Koepsell B, Bogdanova N, et al. Adenosine A2B receptors induce proliferation, invasion and activation of cAMP response element binding protein (CREB) in trophoblast cells. BMC Pregnancy Childbirth. 2014;14(1):2.
- Shi Y, Ye D, Huang R, et al. Down syndrome critical region 1 reduces oxidative stress-induced retinal ganglion cells apoptosis via CREB-Bcl-2 pathway. Invest Ophthalmol Vis Sci. 2020;61(12):23.
- Gu M, Zheng W, Zhang M, et al. LncRNA NONHSAT141924 promotes paclitaxel chemotherapy resistance through p-CREB/Bcl-2 apoptosis signaling pathway in breast cancer. J Cancer. 2020;11(12):3645–3654.
- Cho EC, Mitton B, Sakamoto KM. CREB and leukemogenesis. Crit Rev Oncog. 2011;16(1–2):37–46.
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116(1):1–9.
- Perschbacher KJ, Deng G, Sandgren JA, et al. Reduced mRNA expression of RGS2 (regulator of G protein signaling-2) in the placenta is associated with human preeclampsia and sufficient to cause features of the disorder in mice. Hypertension. 2020;75(2):569–579.
- Roberts JM, Speer P. Antioxidant therapy to prevent preeclampsia. Semin Nephrol. 2004;24(6):557–564.
- Patrono C. The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J Am Coll Cardiol. 2015;66(1):74–85.
- Atallah A, Lecarpentier E, Goffinet F, et al. Aspirin for prevention of preeclampsia. Drugs. 2017;77(17):1819–1831.
- Cadavid AP. Aspirin: the mechanism of action revisited in the context of pregnancy complications. Front Immunol. 2017;8:261.
- Goodlin RC, Haesslein HO, Fleming J. Aspirin for the treatment of recurrent toxaemia. Lancet. 1978;2(8079):51.
- Poon LC, Wright D, Rolnik DL, et al. Aspirin for evidence-based preeclampsia prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol. 2017;217(5):585 e581–585 e585.
- Wright D, Poon LC, Rolnik DL, et al. Aspirin for evidence-based preeclampsia prevention trial: influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. Am J Obstet Gynecol. 2017;217(6):685 e681–685 e685.
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613–622.
- Buemi M, Allegra A, D’Anna R, et al. Is apoptosis cause of pre-eclampsia? Eur Rev Med Pharmacol Sci. 1998;2(5–6):185–188.
- Bilanda DC, Dzeufiet PDD, Kouakep L, et al. Bidens pilosa ethylene acetate extract can protect against L-NAME-induced hypertension on rats. BMC Complement Altern Med. 2017;17(1):479.
- Zou Y, Li S, Wu D, et al. Resveratrol promotes trophoblast invasion in pre-eclampsia by inducing epithelial-mesenchymal transition. J Cell Mol Med. 2019;23(4):2702–2710.
- Shin YY, An SM, Jeong JS, et al. Comparison of steroid hormones in three different preeclamptic models. Mol Med Rep. 2021;23. doi:10.3892/mmr.2021.11891.
- ShamsEldeen AM, Mehesen MN, Aboulhoda BE, et al. Prenatal intake of omega-3 promotes Wnt/beta-catenin signaling pathway, and preserves integrity of the blood-brain barrier in preeclamptic rats. Physiol Rep. 2021;9(12):e14925.
- Ma XP, Liu CD, Cao GM, et al. Transthyretin increases migration and invasion of rat placental trophoblast cells. FEBS Open Bio. 2020;10(8):1568–1576.
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18(4):157–164.
- Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43(1):95–118.
- Panagodage S, Yong HE, Da Silva Costa F, et al. Low-dose acetylsalicylic acid treatment modulates the production of cytokines and improves trophoblast function in an in vitro model of early-onset preeclampsia. Am J Pathol. 2016;186(12):3217–3224.
- Zuo Q, Zou Y, Huang S, et al. Aspirin reduces sFlt-1-mediated apoptosis of trophoblast cells in preeclampsia. Mol Hum Reprod. 2021;27. doi:10.1093/molehr/gaaa089.
- Garcia-Saez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19(11):1733–1740.
- Sharp AN, Heazell AE, Baczyk D, et al. Preeclampsia is associated with alterations in the p53-pathway in villous trophoblast. PLoS One. 2014;9(1):e87621.
- Sharp AN, Heazell AE, Crocker IP, et al. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64(3):159–169.
- Levy R, Smith SD, Yusuf K, et al. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186(5):1056–1061.
- Hu R, Zhou S, Li X. Altered Bcl-2 and Bax expression is associated with cultured first trimester human cytotrophoblasts apoptosis induced by hypoxia. Life Sci. 2006;79(4):351–355.
- Dash PR, Cartwright JE, Baker PN, et al. Nitric oxide protects human extravillous trophoblast cells from apoptosis by a cyclic GMP-dependent mechanism and independently of caspase 3 nitrosylation. Exp Cell Res. 2003;287(2):314–324.
- Xu T, Ding W, Ao X, et al. ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol. 2019;20:414–426.
- Di Meo F, Cuciniello R, Margarucci S, et al. Ginkgo biloba prevents oxidative stress-induced apoptosis blocking p53 activation in neuroblastoma cells. Antioxidants (Basel). 2020;9(4):279.
- Qian Y, Du YH, Tang YB, et al. ClC-3 chloride channel prevents apoptosis induced by hydrogen peroxide in basilar artery smooth muscle cells through mitochondria dependent pathway. Apoptosis. 2011;16(5):468–477.
- Jiang L, Liu Y, Ma MM, et al. Mitochondria dependent pathway is involved in the protective effect of bestrophin-3 on hydrogen peroxide-induced apoptosis in basilar artery smooth muscle cells. Apoptosis. 2013;18(5):556–565.
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16(11):1211–1227.
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68(1):821–861.
- Depoix C, Tee MK, Taylor RN. Molecular regulation of human placental growth factor (PlGF) gene expression in placental villi and trophoblast cells is mediated via the protein kinase a pathway. Reprod Sci. 2011;18(3):219–228.
- Norris W, Nevers T, Sharma S, et al. Review: hCG, preeclampsia and regulatory T cells. Placenta. 2011;32 Suppl 2:S182–185.
- Zhang P, Wang J, Lang H, et al. Knockdown of CREB1 promotes apoptosis and decreases estradiol synthesis in mouse granulosa cells. Biomed Pharmacother. 2018;105:1141–1146.
- Toscano CD, Prabhu VV, Langenbach R, et al. Differential gene expression patterns in cyclooxygenase-1 and cyclooxygenase-2 deficient mouse brain. Genome Biol. 2007;8(1):R14.
- Taubert D, Berkels R, Grosser N, et al. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143(1):159–165.
- Chen J, Wang L, Liu WH, et al. Aspirin protects human coronary artery endothelial cells by inducing autophagy. Physiol Int. 2020;107(2):294–305.
- Kirkby NS, Tesfai A, Ahmetaj-Shala B, et al. Ibuprofen arginate retains eNOS substrate activity and reverses endothelial dysfunction: implications for the COX-2/ADMA axis. FASEB J. 2016;30(12):4172–4179.
- Martinez CS, Piagette JT, Escobar AG, et al. Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: a concerted action of NAD(P)H oxidase and COX-2. Toxicology. 2017;390:10–21.
- Zhang QH, Zu XY, Cao RX, et al. An involvement of SR-B1 mediated PI3K-Akt-eNOS signaling in HDL-induced cyclooxygenase 2 expression and prostacyclin production in endothelial cells. Biochem Biophys Res Commun. 2012;420(1):17–23.
- Schulte JS, Seidl MD, Nunes F, et al. CREB critically regulates action potential shape and duration in the adult mouse ventricle. Am J Physiol Heart Circ Physiol. 2012;302(10):H1998–2007.
- Sampurno S, Bijenhof A, Cheasley D, et al. The Myb-p300-CREB axis modulates intestine homeostasis, radiosensitivity and tumorigenesis. Cell Death Dis. 2013;4(4):e605.
- Crisafulli C, Shim DS, Andrisano C, et al. Case-control association study of 14 variants of CREB1, CREBBP and CREM on diagnosis and treatment outcome in major depressive disorder and bipolar disorder. Psychiatry Res. 2012;198(1):39–46.
- Mamdani F, Alda M, Grof P, et al. Lithium response and genetic variation in the CREB family of genes. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):500–504.
- Sharma N, Mali AM, Bapat SA. Spectrum of CREBBP mutations in Indian patients with Rubinstein-Taybi syndrome. J Biosci. 2010;35(2):187–202.
