ABSTRACT
Accumulating evidence has unfolded the significance of extracellular vesicles (EVs) in diseases and cancers. Here, we attempted to discuss the role of cancer-associated fibroblasts (CAFs)-derived EVs containing miR-199a-5p in gastric tumorigenesis. Upregulated miR-199a-5p was first identified in cancer cells. Then, we selected CAFs for isolation of EVs which were co-cultured with AGS cells. We observed successful delivery of miR-199a-5p via CAF-derived EVs. Besides, miR-199a-5p promoted malignant properties of AGS cells. Moreover, miR-199a-5p downregulated FKBP5, leading to upregulated phosphorylation level of AKT1, which promoted the malignant phenotypes of AGS cells by activating mammalian target of rapamycin complex 1(mTORC1). Exosomal miR-199a-5p from CAFs promoted gastric tumorigenesis in vivo. Our findings point toward the critical role of CAFs-derived EVs carrying miR-199a-5p in gastric cancer progression.
Introduction
Gastric cancer, responsible for a majority of cancer-related mortality on a global scale, can be categorized into true gastric and gastroesophageal-junction adenocarcinomas [Citation1]. Gastric cancer is regarded to be heterogeneous both phenotypically and genotypically [Citation2]. Risk factors for gastric cancer consist of Helicobacter pylori infection, age, high salt consumption, and diets lacking fruit and vegetables [Citation3]. Gastric cancer at early stage may be asymptomatic but may manifest obvious weight loss, dysphagia, abdominal pain, vomiting in advanced stage, in addition to serious upper gastrointestinal bleeding [Citation4]. It has been reported that surgery is the only chance for curing gastric cancer, but most patients would suffer recurrence, even with complete resection [Citation5].
Cancer-associated fibroblasts (CAFs) are main constituent parts of tumor microenvironment and participate in tumor progression [Citation6]. Moreover, the correlation of CAFs with the invasion and metastasis of multiple cancers, including gastric cancer, has been pronounced [Citation7]. Extracellular vesicles (EVs) which are enclosed in lipid bilayers play an important role in intracellular communication by containing as well as delivering a variety of bioactive molecules including microRNAs (miRs) and proteins to recipient cells [Citation8]. Of note, the effect of EVs on gastric cancer progression, microenvironment, drug resistance, as well as therapy has been highlighted [Citation9]. According to a previous study, CAFs-derived CD9-positive EVs induce the migration of scirrhous-type gastric cancer cells [Citation10]. Intriguingly, upregulation of miR-199a-5p facilitates gastric tumorigenesis [Citation11]. Noteworthy, the regulatory relationship between miR-199a-5p and FKBP5 was seen in paroxysmal atrial fibrillation [Citation12]. FKBP5 is identified as a co-chaperone of heat shock protein 90 and can affect glucocorticoid receptor sensitivity [Citation13]. FKBP5 polymorphisms are implicated in anxiety and depression of patients with gastric cancer in advanced stage [Citation14]. It has been found that FKBP5 can function as a scaffolding protein to regulate AKT1 activation [Citation15]. There is evidence that AKT regulated by PI3K signaling can upregulate mTORC1 [Citation16]. Interestingly, the feedback involving AKT1 and mTOR was found to induce proliferation of gastric cancer cells [Citation17]. Thus, we hypothesized in this study that CAFs-derived EVs may transfer miR-199a-5p to affect gastric cancer, with the implication of FKBP5, AKT1 and mTORC1.
Materials and methods
Ethical approval
The study was consented by the ethics committee of The First Affiliated Hospital of Soochow University and Tumor Hospital Affiliated to Nantong University & Nantong Tumor Hospital. All procedures regarding animals followed the guidelines for the care and use of laboratory animals issued by the National Institutes of Health.
Bioinformatics analysis
The bioinformatics website GEPIA was utilized to identify the expression of FKBP5 in gastric cancer with the TCGA database. The bioinformatics website was searched to gain binding sites between miR-199a-5p 3ʹUTR and FKBP5. KEGG enrichment analysis of FKBP5-related genes was performed based on the bioinformatics website.
Extraction of CAFs-EVs
CAFs (Cat NO.: CP-H154, Procell, Wuhan, Hubei, China) were cultured for 2–3 d for EV isolation through differential centrifugation [Citation18]. The collected precipitates were EVs which were identified through transmission electron microscopy. The concentrations of CAFs-EVs were estimated through absorbance at 562 nm with a BCA protein assay kit (Pierce, Rockford, USA) [Citation19].
Cell culture and transfection
Human gastric cancer cell lines HGC-27 (Cat NO.: TCHu 22), NCI-N87 (Cat NO.: TCHu130) and AGS (Cat NO.: TCHu232) were bought from the cell bank of Shanghai Academy of Sciences, Shanghai, China). SNU-5 (Cat NO.: CL-0444) and human normal gastric epithelial cell line GES-1 (Cat NO.: CL-0563) were bought from Procell. All cells were cultured in RPMI-1640 (Gibco, Carlsbad, CA) containing 10% FBS, 100 IU/mL penicillin and 100 mg/mL streptomycin (Sigma-Aldrich, St Louis, MO) at 37°C with 5% CO2 under saturated humidity. Then, gastric cancer cells or CAFs were submitted to 0.25% trypsin detachment (Invitrogen, Carlsbad, CA) with concentration adjusted to 3.0 × 106 cells/mL in complete medium. Next, 200 μL cell suspension was seeded into a Corning 6-well plate for 24 h of culture. Cell transfection was implemented by Lipofectamine 2000 reagent (11668–019, Invitrogen). GenePharma (Shanghai, China) provided the sequences and plasmids. In addition, gastric cancer cells were cultured with AKT1 phosphorylation inhibitor MK-2206 (10 μm, HY-108232, MCE, USA) [Citation20] and mTORC1 inhibitor temsirolimus (15 μm, HY-50910, MCE) [Citation21].
RT-qPCR
PureLink® RNA Mini Kit (Invitrogen) was used for total RNA extraction. Then, the total RNA concentration was determined by a spectrophotometer, and the purity was determined according to the ratio at 260 nm/280 nm. The total volume of 10 μL reaction system was prepared by Mir-X miRNA First-Strand Synthesis Kits (Clontech Laboratories, CA). cDNA was synthesized by RT utilizing Bio-Rad S1000 Thermal Cyclers. Next, the cDNA of the sample to be tested was extracted, and Takara TB Green® Premix Ex Taq ™ II (Takara, Dalian, China) was used to prepare a reaction system of 25 μL. RT-qPCR was implemented on LightCycler® 480 (Roche Diagnostics GmbH, Mannheim, Germany). U6 was used as the internal reference gene and the relative transcription expression was calculated by means of the 2−ΔΔCT method. Primer sequences are displayed in Table S1.
Western blot analysis
Total protein was extracted, electrophoresed and then electroblotted to polyvinylidene fluoride membranes. Primary antibodies against TSG101 (ab125011, 1:1000, Abcam, Cambridge, UK), CD9 (ab92726, 1:1000, Abcam), CD63 (ab217345, 1:1000, Abcam), AKT1 (4685, 1:2000, Cell Signaling Technologies [CST], Beverly, MA, USA), phosphorylated (p-)AKT1 (Ser473) (4060, 1:1000, CST), FKBP5 (ab126715, 1:1000, Abcam), mTOR (2983, 1:1000, CST), p-mTOR (Ser2448) (5536, 1:500, CST), p-mTOR (Ser2481) (2974, 1:500, CST), and GAPDH (ab128915, 1:10000, Abcam) as well as horseradish peroxidase-labeled secondary antibody (7074, 1:10000, CST) were utilized here. Enhanced chemiluminescence reaction solution was added to the proteins to develop the protein bands. Band exposure imaging was performed on the ChemiDocTM XRS + System (Bio-Rad Laboratories, Hercules, CA). Using GAPDH antibody as internal reference, the gray value of each band was analyzed by Bio-Rad Image LabTM.
Co-immunoprecipitation (Co-IP)
Protein A/G agarose magnetic beads (sc-2003, Santa Cruz, TX, USA) were cleaned, and centrifuged for 3 min at 3000 × g at 4°C for further use. AGS cells were lysed with RIPA lysis (P0013D, Beyotime) to collect the supernatant, and a part was chosen as the input sample. After that, the supernatant was incubated with antibodies against FKBP5 (12210, 1:500, CST) and immunoglobulin G (IgG) (ab197767, 1:500, Abcam), and subjected to binding with the cleaned magnetic beads on ice for 2 h. IgG was used as a control. Then, the content of AKT1 was tested utilizing anti-AKT1 (4685, 1:2000, CST).
EdU staining
Cell proliferation was detected by EdU staining [Citation22]. The obtained images were quantitatively analyzed using Apollo® 567.
Transwell assay
Analysis of cell migration and invasion were implemented with the help of Transwell chambers (8 μm pore; 3422, Corning Glass Works, Corning, NY) with or without Matrigel [Citation23]. Under a microscope (IXplore Pro, Olympus, Japan), five visual fields were randomly taken for photographing, followed by calculation of invaded cells or migrative cells.
Microvessel formation experiment
Microvessel formation experiment was implemented with diluted BD Matrigel ™ Matrix (20 μL, BD Biosciences, Franklin Lakes, NJ) [Citation24]. HUVECs (CRL-4053, ATCC) were cultured in EGM-2 medium containing 10% FBS. The images were gained under a microscope (IXplore Pro, Olympus). The number of capillary lumens surrounded by cells was tested by means of Image Pro Plus (version 6.0). At least five visual fields were counted in each group.
Flow cytometry
AGS cells were collected after 48-h transfection, fixed in 70% ethanol and re-suspended in 400 μL PBS. The suspension was submitted to 30-min of incubation with 100 μg/mL RNase A (Takara) and 50 μg/mL propidium iodide (Sigma-Aldrich). DNA content in cells was measured using FACSCalibur system (BD Biosciences), followed by analysis using ModFit_LT software.
Luciferase assay
The constructed FKBP5-WT and FKBP5-MUT were manipulated with miR-199a-5p-mimic, mimic negative control (NC), miR-199a-5p-inhibitor, or inhibitor-NC into gastric cancer cells. Detection of luciferase activity was implemented with the help of dual-luciferase reporter gene detection kits (Beyotime, RG027).
Establishment of gastric cancer xenograft tumor model
Totally 60 BALB/c nude mice (age: 3–5 wk; weight: 10–12 g) were raised under specific-pathogen-free environment. AGS cell suspension (50 μL, 1 × 106 cells/mL) was subcutaneously injected into the right side of the studied animals (once a week at the same time point for three consecutive times). One week later, every 10 mice were injected with (1) PBS of equal volume, (2) 25 μg untreated CAFs-EVs, (3) 25 μg CAFs-EVs harboring inhibitor NC via tail vein, (4) 25 μg CAFs-EVs harboring miR-199a-5p inhibitor via tail vein, (5) AGS cells transfected with NC for gene overexpression (oe-NC) and 25 μg untreated CAFs-EVs via tail vein, or (6) AGS cells transfected with overexpressed FKBP5 (oe-FKBP5) and 25 μg untreated CAFs-EVs via tail vein. The tumor size of nude mice was tested every 5 d. After 30 d, anesthesia was implemented utilizing pentobarbital sodium (100 mg/kg, P3761, Sigma-Aldrich). The tumors were dissected and separated with their volume calculated.
Immunohistochemistry
The subcutaneous tumor tissues of nude mice were embedded in paraffin, sectioned, xylene-dewaxed, and hydrated with gradient alcohol. Anti-Ki-67 (ab15580, 1:500, Abcam) or anti-KFBP5 (ab126715, 1:500, Abcam) was added to each section. Observation of positive cells was implemented under the microscope (IXplore Pro, Olympus).
Immunohistochemistry for measurement of microvessel density (MVD)
CD34 antibody (1:3000, ab81289, Abcam) was utilized to detect the MVD of subcutaneous tumor tissues. Weidner microvessel counting method [Citation25] was used to select the most abundant microvessels in the middle of the slices under the low-power microscope, and then three visual fields were randomly examined under a high-power microscope. The number of CD34-positive microvessels in each field was counted three times with the average value taken as the MVD. When the average value was greater than or equal to the MVD threshold, it was regarded as MVD positive; otherwise, it was MVD negative.
Statistical analysis
Data analysis was implemented by means of SPSS 21.0 (IBM Corp., Armonk, NY, USA). Measurement data were demonstrated as the mean ± standard deviation. Unpaired t test was used for comparison between groups, and data among multiple groups were compared utilizing one-way analysis of variance (ANOVA) and a Tukey’s post hoc test. Data among multiple groups at different time points were compared using repeated measures ANOVA, in combination with Bonferroni post hoc test. A p less than 0.05 was concluded as statistically significant.
Results
CAFs-EVs promoted the progression of gastric cancer through miR-199a-5p
Elevation of miR-199a-5p has been noted in gastric cancer [Citation11]. Analysis from Starbase clarified that patients with highly expressed miR-199a-5p had poor prognosis (Fig. S1). Moreover, miR-199a-5p wasenriched in EVs derived from myofibroblasts [Citation26]. We therefore speculated that CAFs-EVs may promote gastric cancer through miR-199a-5p. To verify this hypothesis, we first used differential centrifugation to extract CAFs-EVs. Under the transmission electron microscope, the extracted CAFs-EVs were round vesicles with a diameter of about 40–100 nm ( left), and there were statistically significant differences in CAFs-EVs concentrations between CAFs-EVs and PBS groups ( right). We discovered that TSG101, CD63 and CD9 were highly expressed in EVs (Fig. S2A), which suggested that CAFs-EVs were successfully isolated. We further co-cultured CAFs-EVs with HGC-27, NCI-N87, AGS and SNU-5 cells. RT-qPCR results showed elevated miR-199a-5p in all cancer cells (), which indicated that CAFs-EVs increased miR-199a-5p expression in gastric cancer cells. AGS cells presented with the highest miR-199a-5p expression were chosen for follow-up experiments.
Figure 1. CAFs-EVs promoted the occurrence and development of gastric cancer through miR-199a-5p. (a) Morphology of CAFs-EVs was observed by transmission electron microscope (left), and the concentrations of CAFs-EVs were estimated with a BCA protein assay kit (right). (b) After co-cultured with CAFs, RT-qPCR was used to detect the expression of miR-199a-5p in human normal gastric epithelial cell line GES-1 and gastric cancer cell lines HGC-27, NCI-N87, AGS and SNU-5. (c) The expression of miR-199a-5p was determined in AGS cells co-cultured with CAFs or CAFs-EVs by RT-qPCR. (d) The expression of miR-199a-5p was detected by RT-qPCR after AGS cells were co-cultured with CAFs-EVs carrying miR-199a-5p inhibitor or mimic. (e) CD63-labeled CAFs-EVs were co-cultured with AGS cells after transfection with Cy3-miR-199a-5p-mimic, and co-localization of CD63 and Cy3 in AGS cells was detected by confocal microscopy (1000 ×). (f) AGS cells were co-cultured with CAFs-EVs after transfection with miR-199a-5p inhibitor, and then the proliferation of AGS cells was detected by EdU staining. (g) AGS cells were co-cultured with CAFs-EVs after transfection with miR-199a-5p inhibitor, and the migration of AGS cells was detected by Transwell assay. (h) AGS cells were co-cultured with CAFs-EVs after transfection with miR-199a-5p inhibitor, and the invasion of AGS cells was detected by Transwell assay. (i) AGS cells were co-cultured with CAFs-EVs after transfected with miR-199a-5p inhibitor, and the effect of AGS cells on microtubule formation of HUVECs was detected by microtubule formation experiment. *p < 0.05. The experiment was repeated three times independently.
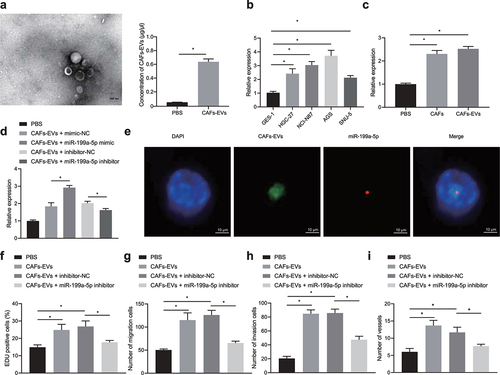
Next, we co-cultured AGS cells with CAFs, and CAFs-EVs, respectively. The results revealed that co-culture of AGS cells with CAFs or CAFs-EVs caused an elevation in miR-199a-5p expression in AGS cells (). We then discovered an enhancement in miR-199a-5p expression in AGS cells upon CAFs-EVs overexpressing miR-199a-5p but decrease upon CAFs-EVs treated with loss-of-function of miR-199a-5p (). Besides, we observed that Cy3-miR-199a-5p-mimic- and CD63-labeled CAFs-EVs were co-located in the cytoplasm of AGS cells (), suggesting that CAFs transmitted miR-199a-5p to cancer cells through EVs.
Finally, co-culture with CAFs-EVs notably promoted the proliferationve, migrationve, invasionve and angiogenesis characteristics of AGS cells, while transfection of miR-199a-5p inhibitor after co-culture resulted in opposite changing tendency (, Fig. S3A, 3B, and Fig. S4A).
miR-199a-5p activated AKT1 phosphorylation through inhibition of FKBP5
We first discovered a binding site between miR-199a-5p and 3ʹUTR of FKBP5 through bioinformatics analysis (). GEPIA database showed that FKBP5 was downregulated in gastric cancer (). Analysis from Starbase revealed that low expression of FKBP5 corresponded to poor prognosis of patients with gastric cancer (Fig. S5). Luciferase assay revealed that gain-of-function of miR-199a-5p decreased the luciferase activity of FKBP5-WT reporter vector in AGS cells, while loss-of-function of miR-199a-5p brought about contrary result. At the same time, we also found that neither gain-of-function of miR-199a-5p nor loss-of-function of miR-199a-5p affected the luciferase activity of FKBP5-MUT reporter vector ().
Figure 2. miR-199a-5p inhibits FKBP5 and upregulates AKT1 phosphorylation. (a) Bioinformatics website (http://www.microrna.org/) was used to predict the binding site of miR-199a-5p with 3ʹUTR of FKBP5. (b) GEPIA database was used to analyze FKBP5 expression in gastric cancer. (c) Dual luciferase reporter gene assay was performed to verify the targeted binding of miR-199a-5p with FKBP5. (d) KEGG enrichment analysis of FKBP5 related genes. (e) Determination of binding of FKBP5 and AKT1 by Co-IP. (f) After transfection with miR-199a-5p mimic or inhibitor, the phosphorylation of FKBP5 and AKT1 was detected by Western blot. *p < 0.05. The experiment was repeated three times independently.
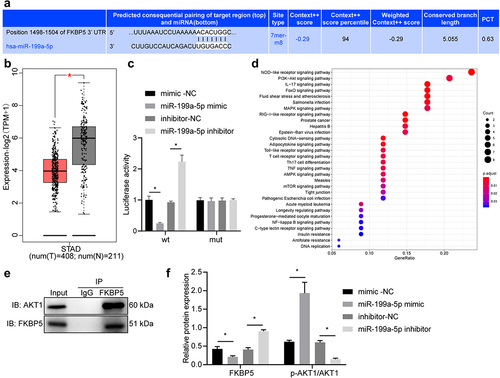
FKBP5-related genes were mainly enriched in PI3K-AKT and other signal pathways (). Co-IP unfolded that compared with IgG, FKBP5 antibody precipitated more AKT1 (). Therefore, FKBP5 was deciphered to combine with AKT1 to form a physical complex.
Finally, we verified whether miR-199a-5p, FKBP5 and AKT1 had a direct regulatory relationship. Western blot results showed that after transfection of AGS cells overexpressing miR-199a-5p, reductions in FKBP5 and AKT1 expression, and an increase in the phosphorylation level of AKT1-Ser473 was seen, while miR-199a-5p inhibitor treatment brought about opposite results (, Fig. S2B). Overall, miR-199a-5p transferred by CAFs-EVs in gastric cancer cells can target FKBP5 to upregulate AKT1 phosphorylation.
miR-199a-5p upregulated AKT1 phosphorylation by inhibiting FKBP5, thus promoting the malignant characteristics of cancer cells
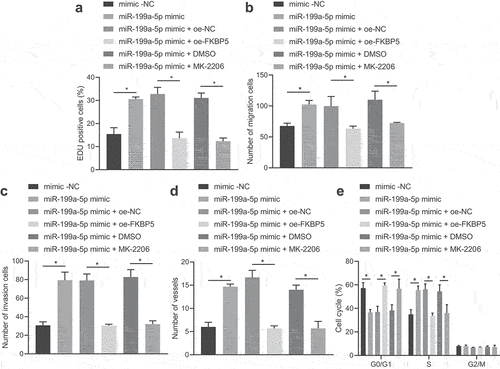
It was evident that transfection of gain-of-function of miR-199a-5p promoted the proliferative, migrative, invasive properties as well as angiogenesis of AGS cells, and then transfection with oe-FKBP5 or AKT1 phosphorylation inhibitor MK-2206 eliminated the promotion effect of miR-199a-5p mimic on AGS cells (, Fig. S3C, 3D, and Fig. S4B). Flow cytometry revealed that gain-of-function of miR-199a-5p led to fewer AGS cells arrested in G1 phase and more in S phase. The transition from G1 to S phase was slowed down by further treatment of oe-FKBP5 or MK-2206 (, Fig. S6).
Figure 3. miR-199a-5p upregulates AKT1 phosphorylation by inhibiting FKBP5 to induce malignant phenotypes of gastric cancer cells. (a) AGS cells were transfected with miR-199a-5p-mimic, oe-FKBP5 or MK-2206, and the proliferation of AGS cells was detected by EdU staining (200 ×). (b) AGS cells were transfected with miR-199a-5p-mimic, oe-FKBP5 or MK-2206, and Transwell assay was used to detect the migration of AGS cells. (c) AGS cells were transfected with miR-199a-5p-mimic, oe-FKBP5 or MK-2206, and the invasion of AGS cells was detected by Transwell assay. (d) After AGS cells were transfected with miR-199a-5p-mimic, oe-FKBP5 or MK-2206, the effect of AGS cells on microtubule formation of HUVECs was detected by microtubule formation experiment. (e) After AGS cells were transfected with miR-199a-5p-mimic, oe-FKBP5 or MK-2206, cell cycle distribution was detected by flow cytometry. *p < 0.05. The experiment was repeated three times independently.
miR-199a-5p activated the AKT1/mTORC1 signaling pathway to facilitate biological functions of gastric cancer cells
Our findings pointed out that transfection of gain-of-function of miR-199a-5p increased the phosphorylation of mTORC1 in AGS cells, and then AKT1 phosphorylation inhibitor MK-2206 led to a reduction (, Fig. S2C).
Figure 4. miR-199a-5p activates the AKT1/mTORC1 signaling pathway by upregulating AKT1 phosphorylation. (a) The phosphorylation of mTOR in AGS cells treated with miR-199a-5p-mimic or MK-2206 was detected by Western blot. (b) AGS cells were transfected with miR-199a-5p-mimic or temsirolimus, and their proliferation was detected by EdU staining. (c) AGS cells were transfected with miR-199a-5p-mimic or temsirolimus, and their migration was detected by Transwell assay. (d) After AGS cells were transfected with miR-199a-5p-mimic or temsirolimus, the invasion was detected by Transwell assay. (e) After AGS cells were transfected with miR-199a-5p-mimic or temsirolimus, the effect of AGS cells on microtubule formation of HUVECs was detected by microtubule formation experiment. *p < 0.05. The experiment was repeated three times independently.
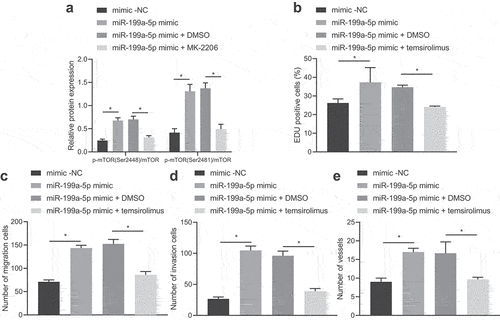
We also discovered that gain-of-function of miR-199a-5p markedly promoted the proliferative, migrative, invasive and angiogenesis of AGS cells, while the subsequent treatment with temsirolimus, an inhibitor of mTORC1, counteracted the above effect (, Fig. S3E, 3 F, and Fig. S4C).
CAFs-EVs transferred miR-199a-5p to promote the tumorigenicity of gastric cancer cells in nude mice
A xenograft mouse model of gastric cancer model was developed, followed by injection of CAFs-EVs via tail vein. As displayed in ), co-culture with CAFs-EVs increased the tumor volume, weight and growth rate in nude mice injected with treated AGS cells. After further loo-of-function of miR-199a-5p or oe-FKBP5, the tumor volume, weight and tumor growth rate were significantly decreased.
Figure 5. CAFs-EVs transfer miR-199a-5p to promote the tumorigenicity of gastric cancer cells in nude mice. (a) The tumor weight of nude mice injected with treated AGS cells. (b) Volume of the subcutaneous tumor in nude mice injected with treated AGS cells. (c) Immunohistochemical detection of Ki67 protein expression in the subcutaneous tumor tissues in nude mice injected with treated AGS cells. (d) The MVD in the subcutaneous tumor tissues in nude mice injected with treated AGS cells. (e) RT-qPCR detection of the expression of miR-199a-5p in the subcutaneous tumor tissues in nude mice injected with treated AGS cells. (f) Immunohistochemical detection of FKBP5 protein expression in the subcutaneous tumor tissues in nude mice injected with treated AGS cells. (g) The protein expression of FKBP5 and the phosphorylation level of AKT1 and mTORC1 were detected by Western blot. *p < 0.05. n = 10. The experiment was repeated three times independently.
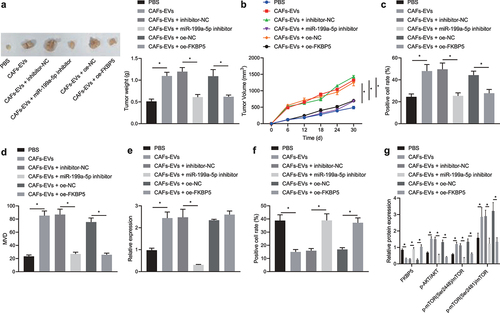
Moreover, co-culture with CAFs-EVs elevated the positive protein expression of Ki-67 in the subcutaneous tumor tissues in nude mice with treated AGS cells, while miR-199a-5p-inhibitor or oe-FKBP5 treatment caused contrary results ( and Fig. S7A). Moreover, co-culture with CAFs-EVs upregulated the MVD in nude mice injected with treated AGS cells, while treatment of loss-of-function of miR-199a-5p or overexpressed FKBP5 downregulated it (). In addition, co-culture with CAFs-EVs led to an enhancement in miR-199a-5p (). The protein expression of FKBP5 was reduced and the phosphorylation of AKT1 and mTORC1 were enhanced by co-culture with CAFs-EVs in nude mice, while transfection of loss-of-function of miR-199a-5p or overexpressed FKBP5 brought about opposite trends (), and Fig. S7B). These data in vivo were consistent with those in vitro, demonstrating that CAFs-EVs transferred miR-199a-5p to participate in the gastric tumorigenesis by inhibiting FKBP5 to upregulate the AKT1/mTORC1 axis.
Discussion
Gastric cancer is still a main unsolved clinical problem with more than 1 million new cases globally [Citation27]. As reported, CAFs acted as critical roles in solid tumor microenvironments, including that of gastric cancer [Citation28]. Our study clarified the significance of CAFs-derived EVs containing miR-199a-5p in gastric tumorigenesis and found that CAFs-derived EVs carrying miR-199a-5p played an oncogenic role by regulating the FKBP5-AKT1-mTORC1 axis.
In the first place, we found that CAFs-derived EVs transferred miR-199a-5p into gastric cancer cells, thereby promoting the malignant properties of gastric cancer cells. To our knowledge, CAFs exert an essential role in modulating tumor progression by delivering EVs to neighboring cells [Citation29]. As previously reported, CD9-positive exosomes secreted from CAFs led to enhanced scirrhous-type gastric cancer cell migration [Citation10]. Moreover, it was unfolded that the inhibition of released miR-carrying exosomes in gastric cancer by proton-pump inhibitor might function as a putative anticancer mechanism [Citation30]. Intriguingly, elevated miR-199a-5p was seen in exosomes derived from Duchenne muscular dystrophy fibroblasts, which aggravated skeletal muscle fibrosis [Citation26]. Consistently, evidence has unveiled the participation of miR-199a-5p in gastric tumorigenesis. For instance, upregulated miR-199a-5p possesses tumor-promoting function in gastric cancer [Citation31]. In addition, miR-199a-5p can facilitate the metastasis as well as EMT in gastric cancer [Citation11]. Taken together, the aforementioned findings can support our finding in regard to the promoting effect of CAFs-derived EVs containing miR-199a-5p on gastric cancer development.
Importantly, we demonstrated that miR-199a-5p specifically inhibited the expression of FKBP5, which upregulated the phosphorylation level of AKT1, leading to activated mTORC1. Notably, a previous study demonstrated the inter-correlation between miR-199a-5p and FKBP5 in paroxysmal atrial fibrillation [Citation12]. PPIase-FKBP was reported to induce the tumorigenic transformation of gastric epithelial cells [Citation32], and FKBP5 polymorphisms have been revealed to affect the anxiety and depression in patients with gastric cancer in the advanced stage [Citation14], indicating an oncogenic role of FKBP5 in gastric cancer. In fact, the regulatory relationship between FKBP5 and AKT has been previously reported. For instance, FKBP5 has been revealed as a scaffolding protein to modulate the activation of AKT1 [Citation15]. In addition, Qi et al. found the interaction between FKBP5 and AKT signaling in pediatric acute lymphoblastic leukemia [Citation33]. Further, the involvement of the mTOR signaling pathway has been confirmed in promoting malignant potentials of human gastric cancer cells [Citation34], and the inhibited mTORC1 signaling pathway due to downregulation of NUSAP1 inhibited the metastatic phenotypes of gastric cancer cells [Citation35]. Intriguingly, the inactivated PI3K/AKT/matrix metalloprotein-9 pathway by Fentanyl exerted inhibitory effect on the cell metastatic phenotypes in gastric cancer [Citation36]. Additionally, PI3K signaling-regulated AKT can induce upregulation of mTORC1 [Citation16]. Strikingly, suppression of the PI3K/AKT1/mTOR pathway was also demonstrated to limit gastric cancer [Citation37]. From above, the effect of AKT1/mTORC1 axis on gastric cancer progression has been partially validated. Overall, miR-199a-5p promoted malignant properties of gastric cancer cells by limiting FKBP5 to upregulate the AKT1/mTORC1 axis.
Conclusion
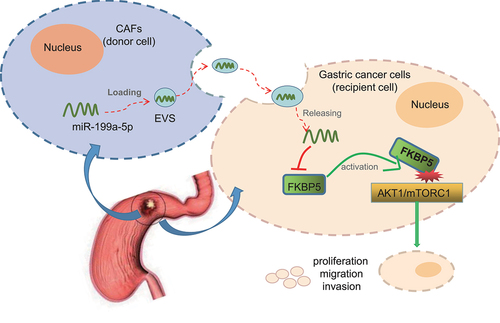
We concluded that CAFs-derived EVs transmitted miR-199a-5p into gastric cancer cells, which inhibited FKBP5 to activate the AKT1/mTORC1 signaling pathway, ultimately leading to gastric cancer development (). Nevertheless, the incorporation mechanism of miR-199a-5p into CAFs-EVs remains largely unknown and more targets are expected to curb the metastasis of CAFs-EVs for inhibition on the occurrence of gastric cancer. Also, the clinical feasibility warrants further exploration regarding the indicative potential of EVs-miR-199a-5p for diagnosis and treatment of gastric cancer.
Figure 6. The molecular mechanism of CAFs-EVs containing miR-199a-5p in gastric cancer via regulation of the AKT1/mTORC1 signaling pathway mediated by FKBP5. CAFs-derived EVs transfer miR-199a-5p into gastric cancer cells, which downregulate FKBP5 to activate the AKT1/mTORC1 signaling pathway, thereby promoting the proliferation, invasion, migration and angiogenesis of gastric cancer cells and ultimately leading to the occurrence and development of gastric cancer.
Author contributions
Yan Wang, Tao Li, and Zhenxin Wang designed the study. Xunlei Zhang, Xiaoli Wang, Xiaoqin Su and Congfei Ji performed the experiments. Lei Yang, Zhenxin Wang collated and analyzed the data. Yan Wang, Tao Li contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Supplemental Material
Download JPEG Image (226 KB)Supplemental Material
Download Zip (24.2 MB)Data availability statement
The datasets generated/analyzed during the current study are available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2105092
Additional information
Funding
References
- Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–2664.
- Ajani JA, Lee J, Sano T, et al. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036.
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet. 2020;396(10251):635–648.
- Marghalani AM, Bin Salman TO, Faqeeh FJ, et al. Gastric carcinoma: insights into risk factors, methods of diagnosis, possible lines of management, and the role of primary care. J Family Med Prim Care. 2020;9(6):2659–2663.
- Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep. 2019;21(8):67.
- Naito Y, Yamamoto Y, Sakamoto N, et al. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene. 2019;38(28):5566–5579.
- Izumi D, Ishimoto T, Miyake K, et al. CXCL12/CXCR4 activation by cancer-associated fibroblasts promotes integrin beta1 clustering and invasiveness in gastric cancer. Int J Cancer. 2016;138(5):1207–1219.
- Urabe F, Kosaka N, Ito K, et al. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am J Physiol Cell Physiol. 2020;318(1):C29–C39.
- Huang T, Song C, Zheng L, et al. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer. 2019;18(1):62.
- Miki Y, Yashiro M, Okuno T, et al. CD9-positive exosomes from cancer-associated fibroblasts stimulate the migration ability of scirrhous-type gastric cancer cells. Br J Cancer. 2018;118(6):867–877.
- Zhao X, He L, Li T, et al. SRF expedites metastasis and modulates the epithelial to mesenchymal transition by regulating miR-199a-5p expression in human gastric cancer. Cell Death Differ. 2014;21(12):1900–1913.
- Chiang DY, Zhang M, Voigt N, et al. Identification of microRNA-mRNA dysregulations in paroxysmal atrial fibrillation. Int J Cardiol. 2015;184:190–197.
- Zoladz PR, Dailey AM, Nagle HE, et al. FKBP5 polymorphisms influence pre-learning stress-induced alterations of learning and memory. Eur J Neurosci. 2017;45(5):648–659.
- Kang JI, Chung HC, Jeung HC, et al. FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: a controlled and prospective study. Psychoneuroendocrinology. 2012;37(9):1569–1576.
- Wang L. FKBP51 regulation of AKT/protein kinase B phosphorylation. Curr Opin Pharmacol. 2011;11(4):360–364.
- Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25(9):545–555.
- Pan C, Liu Q, Wu X. Feedback promotes the proliferation and glycolysis of gastric cancer cells. Cancer Manag Res. 2019;11:10145–10156. HIF1alpha/miR-520a-3p/AKT1/mTOR.
- Ren J, Ding L, Zhang D, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8(14):3932–3948.
- Wang J, Guan X, Zhang Y, et al. Exosomal miR-27a derived from gastric cancer cells regulates the transformation of fibroblasts into cancer-associated fibroblasts. Cell Physiol Biochem. 2018;49(3):869–883.
- Sangai T, Akcakanat A, Chen H, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res. 2012;18(20):5816–5828.
- Nishikawa T, Takaoka M, Ohara T, et al. Antiproliferative effect of a novel mTOR inhibitor temsirolimus contributes to the prolonged survival of orthotopic esophageal cancer-bearing mice. Cancer Biol Ther. 2013;14(3):230–236.
- Jiang B, Li Z, Zhang W, et al. miR-874 Inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J Gastroenterol. 2014;49(6):1011–1025.
- Cai X, Nie J, Chen L, et al. Circ_0000267 promotes gastric cancer progression via sponging MiR-503-5p and regulating HMGA2 expression. Mol Genet Genomic Med. 2020;8(2):e1093.
- Xu T, Wu K, Zhang L, et al. Long non-coding RNA LINC00858 exerts a tumor-promoting role in colon cancer via HNF4alpha and WNK2 regulation. Cell Oncol (Dordr). 2020;43(2):297–310.
- Takagi K, Takada T, Amano H, et al. Analysis of microvessels in pancreatic cancer: by light microscopy, confocal laser scan microscopy, and electron microscopy. J Hepatobiliary Pancreat Surg. 2008;15(4):384–390.
- Zanotti S, Gibertini S, Blasevich F, et al. Exosomes and exosomal miRNAs from muscle-derived fibroblasts promote skeletal muscle fibrosis. Matrix Biol. 2018;74:77–100.
- Sexton RE, Al Hallak MN, Diab M, et al. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39(4):1179–1203.
- Ma Z, Chen M, Yang X, et al. The role of cancer-associated fibroblasts in tumorigenesis of gastric cancer. Curr Pharm Des. 2018;24(28):3297–3302.
- Li YY, Tao YW, Gao S, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209–220.
- Guan XW, Zhao F, Wang JY, et al. Tumor microenvironment interruption: a novel anti-cancer mechanism of Proton-pump inhibitor in gastric cancer by suppressing the release of microRNA-carrying exosomes. Am J Cancer Res. 2017;7(9):1913–1925.
- He XJ, Ma YY, Yu S, et al. Up-regulated miR-199a-5p in gastric cancer functions as an oncogene and targets klotho. BMC Cancer. 2014;14:218.
- Zhu Y, Gong Y, Li A, et al. Differential proteomic analysis reveals protein networks and pathways that may contribute to Helicobacter pylori FKBP-type ppiase-associated gastric diseases. Proteomics Clin Appl. 2018;12(3):e1700127.
- Qi HX, Cao Q, Sun XZ, et al. MiR-410 regulates malignant biological behavior of pediatric acute lymphoblastic leukemia through targeting FKBP5 and Akt signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(24):8797–8804.
- Hibdon ES, Razumilava N, Keeley TM, et al. Notch and mTOR signaling pathways promote human gastric cancer cell proliferation. Neoplasia. 2019;21(7):702–712.
- Ge Y, Li Q, Lin L, et al. Downregulation of NUSAP1 suppresses cell proliferation, migration, and invasion via inhibiting mTORC1 signalling pathway in gastric cancer. Cell Biochem Funct. 2020;38(1):28–37.
- Li C, Qin Y, Zhong Y, et al. Fentanyl inhibits the progression of gastric cancer through the suppression of MMP-9 via the PI3K/Akt signaling pathway. Ann Transl Med. 2020;8(4):118.
- Wang LL, Zhang L, Cui XF. Downregulation of long noncoding RNA LINC01419 inhibits cell migration, invasion, and tumor growth and promotes autophagy via inactivation of the PI3K/Akt1/mTOR pathway in gastric cancer. Ther Adv Med Oncol. 2019;11:1758835919874651.
