ABSTRACT
Ulcerative colitis (UC) is the most prevalent form of chronic inflammatory bowel disease, the etiology of which is poorly understood. This study investigated the role of miR-151-5p on UC and explored the role of brain-derived neurotrophic factor (BDNF) in a UC mouse model and cell model. A UC mouse model was engineered by dextran sulfate sodium (DSS) induction. Primary mouse intestinal epithelial cells (IECs) were isolated. Colitis mice were intraperitoneally injected with miR-151-5p antagomir and antagomir negative control, and weight loss, disease activity index, and colon length of mice were measured. Colon tissues of mice were histologically analyzed. A UC cell model was constructed by treating MODE-K cells with DSS. miR-151-5p expression in the cell model was modulated by transfection. The exogenous BDNF effect on the UC cell model and intestinal cell apoptosis, viability and proliferation was detected by flow cytometry, CCK-8 and EdU experiment. The expression of miR-151-5p and apoptosis-related proteins was assessed through q-PCR and western blotting. miR-151-5p was upregulated in the colon tissues and primary IECs of colitis mice. miR-151-5p directly inhibited the expression of BNDF. miR-151-5p upregulation promoted apoptosis in UC MODE-K cells. miR-151-5p upregulation repressed the viability of UC MODE-K cells. Exogenous BNDF treatment reversed the effect of miR-151-5p on UC MODE-K cells. miR-151-5p knockdown improved UC symptoms in mice, including alleviating weight loss, reducing disease activity index and improving colon length and damaged colon tissues. miR-151-5p contributed to intestinal epithelial cells apoptosis in colitis mice via inhibiting BNDF expression.
Introduction
Inflammatory bowel disease (IBD) is a general term for gastrointestinal diseases caused by inflammatory disorders. The two most common forms of IBD are ulcerative colitis (UC) and Crohn’s disease (CD) [Citation1]. UC usually occurs in the colon, consistent with cryptitis and crypt abscesses. The manifestations of UC are ulcers and hemorrhage induced by digestive tract mucosa inflammation [Citation2]. The incidence of UC is reported to have increased over the past 20 y, and UC is recognized as a modern incurable disease by the World Health Organization [Citation3]. The current treatment of UC is still unsatisfactory and approximately one in five patients with severe UC requires surgical resection [Citation2]. Moreover, the severity and duration of colonic mucosal inflammation are largely related to the risk of cancer development in UC patients [Citation4]. The elucidation of the pathogenesis of UC will be conducive to finding more effective treatment strategies for UC.
miRNAs are small endogenous non-coding RNAs with a size of 17–25 nucleotides; they degrade mRNA or inhibit mRNA translation by binding to the 3’-UTR region of target mRNA [Citation5,Citation6]. Multiple miRNAs have been identified to be associated with the development and regulation of UC [Citation7]. miR-151-5p has recently been found to participate in the regulation of some human diseases. miR-151-5p has been shown to be unusually downregulated in breast cancer. miR-151-5p acts as a tumor suppressor in breast cancer by inhibiting breast cancer cell proliferation, colony formation, invasion, and migration in vivo [Citation8]. In contrast, miR-151-5p was reported to promote the progression of hepatocellular carcinoma, gastric carcinoma, and prostate cancer in vivo [Citation9–11]. Moreover, the systemic delivery of miR-151-5p has been shown to relieve bone marrow mesenchymal stem cell damage and immune disorders in mice with systemic sclerosis [Citation12]. Recently, studies have been identified that miR-151-5p was differentially expressed in UC [Citation5,Citation13]. Moreover, an increased level of miR-151-5p was observed in the blood samples of UC cases when relative to healthy controls [Citation14,Citation15]. These data indicated that miR-151-5p might be involved in the progression of UC. However, little is known about the expression levels and precise mechanism of miR-151-5p in regulating UC development. Therefore, we investigated the expression and mechanism of miR-151-5p in UC.
Brain-derived neurotrophic factor (BDNF) is an important member of the neurotrophin family of proteins essential for the growth, differentiation, and survival of neurons [Citation16]. BDNF has been found to be upregulated in the colon of patients with CD and UC [Citation17]. BNDF knockdown has been shown to have compromised intestinal lining integrity and excessive apoptosis of intestinal epithelial cells [Citation18]. Furthermore, the excessive apoptosis of colon cells in patients with IBD has been found to cause focal destruction of epithelial integrity [Citation19,Citation20]. Thus, this study speculated that BNDF might be involved in the progression of UC. More interestingly, our preliminary research discovered that BNDF possessed binding sites for miR-151-5p through TargetScan online prediction. Therefore, in this study, we investigated the role of miR-151-5p in regulating UC development by targeting BNDF. This study suggests miR-151-5p as a potential target for UC treatment.
Materials and Methods
Dextran sulfate sodium (DSS)-induced ulcerative colitis mouse model
C57BL/6 mice (n = 40, 7 wk old, 19–22 g) were purchased from Shanghai SLAC Laboratory Animals Co., Ltd (Shanghai, China). The initial body weight of each mouse was weighted and recorded. The animal study in this study has been approved by the animal ethics committee.
A total of 16 mice were randomly divided into the sham group (n = 8) and DSS group (n = 8), and the initial body weight of all mice was recorded. Mice were kept in a room with a day/night cycle of 12/12 h. DSS group mice were given drinking water containing 2% DSS, while sham group mice were given drinking water alone. All mice had free access to food and water for 7 consecutive days, and all mice were weighed every day. Weight loss was calculated as body weight/initial body weight × 100%. On the seventh day, mice were euthanized and the colon tissues of mice were excised [Citation21].
miR-151-5p antagomir treatment of mice
Another 24 mice were randomly divided into the sham group (n = 8), UC + miR-151-5p antagomir group (n = 8) and a negative control (NC) UC + miR-NC antagomir group (n = 8). Mice from the UC + miR-NC antagomir group and UC + miR-151-5p antagomir group were intraperitoneally injected miR-151-5p antagomir negative control (100 μL) and miR-151-5p antagomir (100 μL), respectively, diluted in phosphate buffered saline (PBS) to a concentration of 2 mg/mL three times a week for 2 wk. One week after injection, the mice were treated with 2% DSS for 7 consecutive days. The sham group mice were intraperitoneally injected with PBS three times a week for 2 wk. After 1 wk of injection, mice were given drinking water without DSS for 7 consecutive days. The bodyweight of the mice was recorded every day, and weight loss was calculated. Then, mice were euthanized to collect the colon tissues. The length of colon tissues was measured [Citation21].
Disease activity index
The disease activity index of mice was measured based on body weight, stool, and blood in the stool, and points corresponding to the following features were allotted 0 points, mice did not lose any weight and had normal stool without blood; 1 point, mice lost 1–5% weight and had loose stools with fecal occult blood; 2 points, mice lost 5–10% weight and had loose stools with fecal occult blood; 3 points, mice lost 10–15% weight and had watery bloody stools; 4 points, mice lost over 15% weight and had watery bloody stools [Citation22].
Histological analysis
The colon tissues were fixed in 10% formaldehyde. They were then dehydrated, permeabilized, and embedded in paraffin. The paraffinized tissues were cut into 5 μm thick sections. The sections were deparaffinized using xylene, and then xylene was removed using absolute ethanol. Hematoxylin and eosin (H&E) was used to stain the sections. The histological changes in the colon tissues were observed under a microscope.
Isolation of primary mouse intestinal epithelial cells (IECs)
A total of 10 C57BL/6 mice (7 wk old, 19–22 g) (Shanghai SLAC Laboratory Animals Co., Ltd, Shanghai, China) were given normal drinking water (sham group, n = 8) or drinking water containing 2% DSS (DSS group, n = 8) for 7 consecutive days. Then, mice were euthanized to collect the colon tissues. The isolation of mouse IECs was implemented as follows: The colon tissues of mice were chopped up, and the tissue pieces were incubated with Dulbecco’s Modified Eagle’s Medium (DMEM) suspended with 10% fetal bovine serum (FBS) and 1 mmol/L dithiothreitol (DTT) for 30 min at 37°C. Next, the remaining tissues were treated by PBS suspended with 1.5 mmol/L ethylenediamine tetraacetic acid (EDTA) for 10 min. The supernatant samples were then harvested, filtered and centrifuged at 400 × g for 5 min in order to collect the cell pellet. Cells were then dispersed into DMEM suspended with 10% FBS. The purification of primary mouse IECs suspension was performed by 30 min centrifugation at 600 × g via a 25%/40% discontinuous Percoll gradient. The expression of miR-151-5p in the primary mouse IECs was evaluated by q-PCR [Citation23].
Cell culture and transfection
Mouse intestine epithelial cell line MODE-K was purchased from the Shanghai Institute of Cell Biology (Shanghai, China). DMEM with 10% FBS was used to culture MODE-K cells at 37°C and 5% CO2. At 80–90% confluence, the cells were collected and a cell suspension using serum-free DMEM (1 × 106 cells/mL) was prepared; 1 mL of this cell suspension was seeded into six-well plates. miR-151-5p mimic, mimic negative control, miR-151-5p inhibitor, and inhibitor negative control (GenePharma, Shanghai, China) were transfected into the MODE-K cells of the miR-151-5p mimic group, NC mimic group, miR-151-5p inhibitor group, and NC inhibitor group, respectively, using the Lipofectamine 2000 reagent (Thermo Fisher Scientific, Waltham, MA, USA). The transfection procedure was implemented in line with the directions of Lipofectamine 2000. After 6 h of transfection, the cells were cultured in DMEM containing 10% FBS at 37°C and 5% CO2 for 48 h. MODE-K cells without treatment were used as control (Con group). The transfection efficiency was determined by quantitative (real-time) polymerase chain reaction (q-PCR).
Dual-luciferase reporter gene assay
According to TargetScan prediction (http://www.targetscan.org/vert_71/), BNDF has a binding site for miR-151-5p and, therefore, may be a target of miR-151-5p. Dual-luciferase reporter gene assay was used to verify this prediction. HEK293 cells were cultured in DMEM containing 10% FBS at 37°C and 5% CO2 and harvested at 80–90% confluence. Subsequently, the cells were suspended in serum-free DMEM (1 × 106 cells/mL) and 1 mL of this suspension was seeded into six-well plates. Groups of HEK293 cells were transfected with miR-151-5p mimic, mimic negative control, miR-151-5p inhibitor, or inhibitor negative control (GenePharma, Shanghai, China). The pGL3-BNDF-WT (wild type) and pGL3-BNDF-MUT (mutant type) type luciferase reporters (GenePharma, Shanghai, China) were then transfected into the HEK293 cells. All transfection operations were conducted using the Lipofectamine 2000 reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. After 48 h of transfection, luciferase activity was measured using a Dual-luciferase reporter assay kit (Promega, Southampton, UK). Renilla luciferase activity was used as the control.
MODE-K cells treatment by DSS and exogenous BNDF
A cell suspension of 1 × 106 MODE-K cells/mL was prepared using DMEM containing 10% FBS and 2% DSS, seeded into six-well plates and cultured for 48 h at 37°C and 5% CO2. This group of cells was called the DSS group. In addition, the MODE-K cells transfected by miR-151-5p mimic, mimic negative control, miR-151-5p inhibitor, or inhibitor negative control were also collected and cultured in DMEM containing 10% FBS and 2% DSS for 48 h at 37°C and 5% CO2 (miR-151-5p mimic + DSS group, NC mimic + DSS group, miR-151-5p inhibitor + DSS group, and NC inhibitor + DSS group, respectively). Furthermore, MODE-K cells transfected with miR-151-5p mimic and those with mimic negative control were further cultured in DMEM containing 10% FBS, 2% DSS and 50 ng/mL exogenous BNDF protein [Citation21] for 48 h at 37°C and 5% CO2 (miR-151-5p mimic + DSS + BNDF group and NC mimic + DSS + BNDF group, respectively).
Flow cytometry
The transfected MODE-K cells were harvested and washed with PBS twice, then suspended in PBS and centrifuged at room temperature for 5 min at 100 × g. The cell pellet obtained was collected and resuspended into the binding buffer (500 µL). The cell suspension was then filtered using a 400‑mesh filter. Annexin V-FITC (5 µL) and propidium iodide (PI, 5 µL) were then added to the single-cell suspension and incubated for 10 min in the dark at room temperature. The cells that underwent apoptosis were detected using flow cytometry. FlowJo software (version 7.6.1; Tree Star, Ashland, OR, USA) was used for flow cytometry data analysis.
Cell Counting Kit-8 (CCK-8) assay
MODE-K cells were collected at about 85% confluence and dispersed into DMEM with 10% FBS to a density of 1 × 105 cells/mL. Then, MODE-K cell suspension was fostered into 96-well plates with 1 mL per well. After being cultured by the relevant medium for 48 h, the 96-well plates were placed under a multiwell microplate reader (AllSheng instrument, Hangzhou, China) to read the absorbance values. The relative viability of MODE-K cells was calculated with the Con group as the control.
EdU experiment
MODE-K cell suspension (1 × 105 cells/mL) was inoculated into a six-well plate and cultured for 49 h with the relevant medium at 37°C, 5% CO2. The EdU kit (Beyotime, Shanghai, China) was employed for the detection of proliferation ability in line with the directions. The staining of MODE-K cells was observed under a fluorescence microscope with red fluorescence as the EdU-positive cells. The number of EdU-positive cells was then counted.
Q-PCR
Total miRNAs in tissues and cells were extracted using an miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the instructions. Reverse transcription reaction was carried out by using a TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). qRT-PCR was performed with 5 μg of samples in a Cycler (Bio-Rad) using SYBR-Green (Roche). The reaction procedure was as follows: 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 60 s. Primers used were listed as follows: miR-151-5p 5ʹ-TGATCTGACACTCGAGGAGCT-3ʹ and U6 5ʹ-CTCGCTTCGGCAGCACATATACT-3ʹ. U6 was used as the control and miR-151-5p expression was calculated by the 2−ΔΔCT method [Citation22].
Western blot
The colon tissues of mice were homogenized in lysis buffer, and the homogenate was centrifuged at 12000 × g at 4°C for 10 min. The total protein concentration in the supernatants was detected using BCA kits (Beyotime, Shanghai, China). In addition, MODE-K cells were lysed using lysis buffer, and the total protein concentration in the cells was determined using BCA kits. The protein extract was subjected to electrophoresis using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After separation, the protein bands were transferred onto a polyvinylidene fluoride (PVDF) membrane and the proteins were blocked using 5% skimmed milk for 2 h at room temperature. The proteins were then probed using primary antibodies for 12 h at 4°C, followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:2000, ab6721, Abcam, Cambridge, UK) for 2 h at room temperature. Primary antibodies used are as follows: rabbit anti-BNDF (1:1000, SC546, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-cleaved caspase 3 (1:1000, ab49822, Abcam, Cambridge, UK), rabbit anti-cleaved PARP (1:1000, ab4830, Abcam, Cambridge, UK), rabbit anti-claudin-1 (1:1000, ab211737, Abcam, Cambridge, UK), rabbit anti-claudin-2 (1:1000, ab53032, Abcam, Cambridge, UK) and rabbit anti-occludin (1:1000, ab31721, Abcam, Cambridge, UK). The enhanced chemiluminescent reagent (ECL, Boster, Wuhan, China) was used for the visualization of the protein bands. Quantity One software (Bio-Rad, Hercules, CA, USA) was used for quantifying the proteins. The expression of each protein was normalized to β-actin.
In situ hybridization (ISH) experiment
ISH was implemented on the colon tissues of mice in order to research the expression of miR-151-5p. The detection process was carried out in line with the MiRCURY LNA miRNA ISH Optimization kit (Exiqon, Vedbaek, Denmark) directions. The colon tissues of mice were embedded into paraffin and then prepared into sections (5 μm in thickness). After being treated for 20 min by 50 μg/mL proteinase K, the sections were probed by miR-151-5p nucleic acid probe for 1 h at 52°C. Thereafter, the sections were washed with saline sodium citrate 5 times, followed by being blocked for 15 min by blocking solution. The sections were then incubated for 2 h by alkaline phosphatase-conjugated anti-digoxigenin Fab fragments (1:500, Roche, Penzberg, Germany) at room temperature. After being stained with diaminobenzidine, the sections were dehydrated by alcohol and then sealed in neutral resin for observation under a microscope.
Statistical analysis
All experiments in this study were performed independently three times. Data are presented as mean ± standard deviation. SPSS 21.0 (SPSS Inc., Chicago, IL, USA) was utilized for data analysis. Two tailed paired Student's t-test was performed for the comparison between two different groups. One-way analysis of variance followed by post hoc Tukey’s test was carried out for the comparison among at least three different groups. P < 0.05 was considered a statistically significant difference.
Results
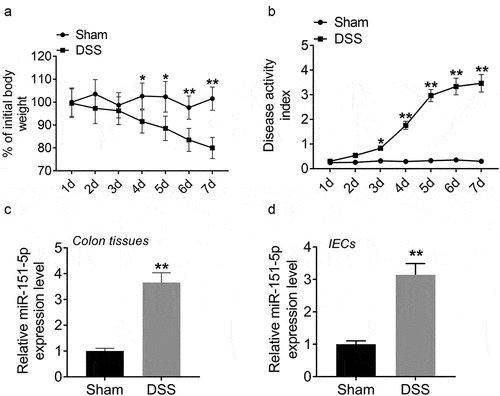
miR-151-5p was upregulated in the colon tissues of colitis mice
We engineered a UC mouse model by 2% DSS induction. We found that DSS group mice showed greater body weight loss on the fourth to seventh day, with 2% DSS induction than the sham group (P < 0.05 or P < 0.01) (). Besides, on the third to seventh days, DSS group mice scored higher on the disease activity index than the sham group mice (P < 0.05 or P < 0.01) (). The miR-151-5p expression in the colon tissues was investigated using qPCR. miR-151-5p expression was found to be significantly higher in the colon tissues of the DSS group mice than that of the sham group mice (P < 0.01) (). Mouse primary IECs were isolated to detect the expression of miR-151-5p. As a result, compared with IECs isolated from mice in the sham group, the expression of miR-151-5p was significantly increased in IECs collected from mice in the DSS group (P < 0.01) (). Thus, the UC mouse model was successfully constructed and miR-151-5p expression was increased in colitis mice.
Figure 1. miR-151-5p was upregulated in colon tissues of colitis mice.
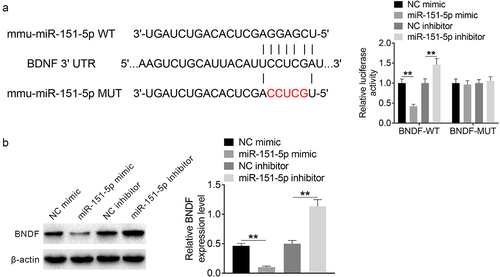
BNDF expression was directly inhibited by miR-151-5p
The WT and MUT sequences of BNDF to miR-151-5p were designed and synthesized. A dual-luciferase reporter gene assay was conducted using HEK293 cells to explore the relationship between miR-151-5p and BNDF. miR-151-5p mimic group HEK293 cells showed significantly lower (P < 0.01) relative Bnfd-WT luciferase reporter activity than the NC mimic group HEK293 cells. In contrast, miR-151-5p inhibitor group cells showed significantly higher (P < 0.01) relative Bnfd-WT luciferase reporter activity than the NC inhibitor group cells (). However, the difference in the relative activity of BNDF-MUT luciferase reporter among the NC mimic, miR-151-5p mimic, NC inhibitor, and miR-151-5p inhibitor groups was not obvious. BNDF expression in the transfected MODE-K cells was also assessed. BNDF expression was significantly lower (P < 0.01) in the miR-151-5p mimic group than in the NC mimic group but was significantly higher (P < 0.01) in the miR-151-5p inhibitor group than in the NC inhibitor group (). Therefore, BNDF was identified to be a target of miR-151-5p, which expression could be directly repressed by miR-151-5p.
Figure 2. BNDF expression was directly inhibited by miR-151-5p.
miR-151-5p promoted apoptosis in mouse intestinal epithelial cells induced by DSS
miR-151-5p expression in MODE-K cells treated with 2% DSS was assessed using qRT-PCR. Compared to the Con group, MODE-K cells of the DSS group showed increased miR-151-5p expression (P < 0.01) (). Flow cytometry revealed that the rate of apoptosis was higher in the NC mimic + DSS group MODE-K cells than in the Con group (P < 0.01) and that of the miR-151-5p mimic + DSS and miR-151-5p inhibitor + DSS group was higher than that of the NC mimic + DSS (P < 0.01) and NC inhibitor + DSS group, respectively (P < 0.01) ().
Figure 3. miR-151-5p promoted mouse intestinal epithelial cells apoptosis induced by DSS.
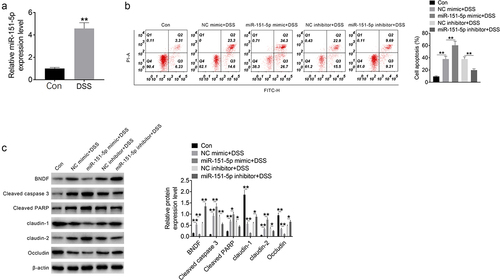
Western blot revealed that the expression of BNDF, cleaved caspase 3, cleaved PARP, and claudin-2 was higher and that of claudin-1 and occludin was lower in the NC mimic + DSS group than that in the Con group (P < 0.01). The expression of cleaved caspase 3, cleaved PARP, and claudin-2 was markedly higher and that of BNDF, claudin-1, and occludin was lower in the miR-151-5p mimic + DSS group than in the NC mimic + DSS group (P < 0.05 or P < 0.01). In contrast, the expression of cleaved caspase 3, cleaved PARP, and claudin-2 was lower and that of BNDF, claudin-1, and occludin was higher in the miR-151-5p inhibitor + DSS group than in the NC inhibitor + DSS group (P < 0.05 or P < 0.01) (). Thereby, miR-151-5p promoted the apoptosis of MODE-K cells induced by DSS.
miR-151-5p repressed the viability of mouse intestinal epithelial cells induced by DSS
This research further evaluated the influence of miR-151-5p on the viability and proliferation of MODE-K cells by using CCK-8 assay and EdU experiment, respectively. As shown in Figure S1 A and B, miR-151-5p overexpression (miR-151-5p group) did not obviously change the viability and proliferation of MODE-K cells when compared to normal MODE-K cells (Con group). However, matched to the miR-151-5p group, lower viability and proliferation were observed in MODE-K cells of the NC mimic + DSS group (P < 0.01). Moreover, MODE-K cells of the miR-151-5p mimic + DSS group showed lower viability than those of the NC mimic + DSS group (P < 0.05). The proliferation difference between the NC mimic + DSS group and the miR-151-5p mimic + DSS group had no statistical significance. Thus, DSS treatment could induce the weakened viability and proliferation of MODE-K cells. Meanwhile, miR-151-5p up-modulation further repressed the viability (rather than proliferation) of the DSS-treated MODE-K cells. These data suggested that miR-151-5p repressed the viability of MODE-K cells induced by DSS.
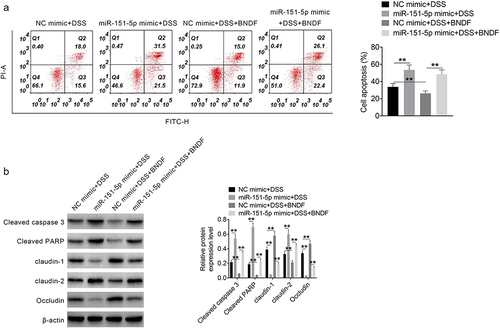
Exogenous BNDF reversed the apoptotic effects of miR-151-5p in intestinal epithelial cells
The effect of exogenous BNDF on apoptosis in MODE-K cells induced by 2% DSS was assessed by flow cytometry. The rate of apoptosis was much higher (P < 0.01) in the miR-151-5p mimic + DSS group than in the NC mimic + DSS group. In contrast, the rate of apoptosis was significantly lower in the NC mimic + DSS + BNDF group than in the NC mimic + DSS group (P < 0.01), while it was much higher in the miR-151-5p mimic + DSS + BNDF group than in the NC mimic + DSS + BNDF group (P < 0.01) (). Western blot revealed that the expression of cleaved caspase 3, cleaved PARP, and claudin-2 was much higher and that of claudin-1 and occludin was lower in the miR-151-5p mimic + DSS group than in the NC mimic + DSS group (P < 0.01) but that of cleaved caspase 3, cleaved PARP, and claudin-2 was higher and that of claudin-1 and occludin was lower in the NC mimic + DSS + BNDF group than in the NC mimic + DSS group (P < 0.01). Likewise, the expression of cleaved caspase 3, cleaved PARP, and claudin-2 was higher and that of claudin-1 and occludin was lower in the miR-151-5p mimic + DSS + BNDF group than in the NC mimic + DSS + BNDF group (P < 0.01) (). Hence, exogenous BNDF reversed the promotion of miR-151-5p on the apoptosis of MODE-K cells induced by DSS.
miR-151-5p knockdown improved UC symptoms in mice
In vivo studies were performed to assess the effect of miR-151-5p knockdown on DSS-induced colitis mice. The mice were intraperitoneally injected with miR-151-5p antagomir or antagomir negative control for 2 wk, and changes in body weight and disease activity index were detected in the following 7 consecutive days. As shown in , the UC + miR-NC antagomir group showed greater weight loss and higher disease activity index (P < 0.05 or P < 0.01) than the sham group. However, the UC + miR-NC antagomir group showed lesser weight loss and lower disease activity index (P < 0.05 or P < 0.01) than the UC + miR-151-5p antagomir group. After euthanization, the colon tissues of mice were collected to measure the colon length. The colon of the UC + miR-NC antagomir group was significantly shorter (P < 0.01) than that of the sham group, but that of UC + miR-151-5p antagomir group mice was longer than that of the UC + miR-NC antagomir group ().
Figure 5. miR-151-5p knockdown improved UC symptoms in mice.
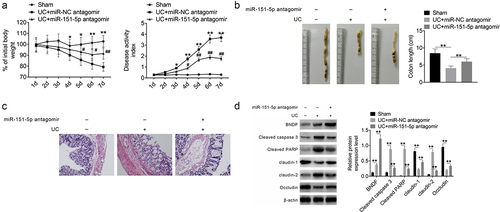
Furthermore, histological analysis of the colon sections revealed a clear colonic crypt structure without ulcers and inflammatory cell infiltration in the sham group, while disrupted colonic crypts, as well as ulcers and inflammatory cell infiltration, were seen in the UC + miR-NC antagomir group. However, the crypt structure was restored and ulcers and inflammatory cell infiltration decreased in the colon of the UC + miR-151-5p antagomir group mice (). Western blot revealed that the expression of BNDF, cleaved caspase 3, cleaved PARP and claudin-2 was higher and that of claudin-1 and occludin was lower (P < 0.01) in the UC + miR-NC antagomir group than in the sham group. The expression of cleaved caspase 3, cleaved PARP, and claudin-2 was lower and that of BNDF, claudin-1 and occludin was higher (P < 0.01) in the UC + miR-151-5p antagomir than in the UC + miR-NC antagomir group (). Moreover, ISH experiment was implemented on the colon tissues of mice in order to research the expression of miR-151-5p. As shown in , mice of the UC + miR-NC antagomir group showed higher miR-151-5p expression in colon tissues than the sham group. However, lower miR-151-5p expression was observed in colon tissues of mice in the UC + miR-151-5p antagomir group when relative to the UC + miR-NC antagomir group. These data indicated that miR-151-5p knockdown improved the UC symptoms in mice.
Discussion
UC is an important form of IBD characterized by chronic nonspecific intestinal inflammation [Citation3]. Several studies have suggested that the role of miRNAs in UC development and miRNAs has thus been considered as a potential target for UC treatment [Citation7,Citation24]. A previous study suggested that the inhibition of miR-21-5p may reduce inflammation apoptosis in colitis mice [Citation3]. In DSS-induced UC, the suppression of miR-330-3p was found to alleviate UC symptoms and apoptosis [Citation25]. miR-650 expression was found to be elevated in the inflamed mucosa of UC patients and DSS-induced colitis mice, aggravating intestinal epithelial cell apoptosis in colitis mice [Citation26]. Simultaneously, miR-223 was found to trigger inflammation and apoptosis in colitis mice [Citation27]. miR-495 could alleviate intestinal epithelial cell apoptosis in colitis mice, improving intestinal mucosal barrier function [Citation28]. Apoptosis of intestinal epithelial cells can promote the development of UC [Citation29] and extensive apoptosis of colon cells in patients with IBD may lead to focal destruction of epithelial integrity [Citation19,Citation20]. The epithelial barrier is the first line of defense as it prevents the entry of various harmful substances (such as intestinal bacteria). In UC, the mucosal cytokines and leukocytes infiltration can result in epithelial destruction, including intestinal epithelial cell apoptosis and subsequent crypt hyperplasia, leading to tissue ulcers and epithelial hyperplasia [Citation30]. Therefore, preventing the apoptosis of intestinal epithelial cells is crucial for UC treatment. In this study, miR-151-5p was abnormally upregulated in the UC mouse model and the cell line, resulting in colon tissues’ damage of colitis mice and the apoptosis of mouse intestinal epithelial cells, respectively. Interestingly, miR-151-5p up-modulation repressed the viability of the DSS-treated mouse intestinal epithelial cells. Moreover, miR-151-5p knockdown alleviated intestinal epithelial cell apoptosis and colon tissues damage in colitis mice.
In this study, we first identified that BNDF was a target of −151-5p and BNDF expression was directly suppressed by miR-151-5p. The dynamic balance between apoptosis and regeneration of intestinal epithelial cells is crucial in maintaining intestinal integrity. Under normal physiological conditions, new epithelial cells are derived from stem cells in the crypt and subsequently mature, replacing apoptotic cells [Citation18]. In this study, we found that miR-151-5p aggravated mouse intestinal epithelial cell apoptosis and caused colon tissue damage, including the loss of the crypt structure, while exogenous BNDF alleviated mouse intestinal epithelial cells apoptosis induced by DSS. In line with this finding, another study showed that BDNF can reduce the apoptosis of enteric glial cells induced by TNF-α [Citation17]. Zhao et al. [Citation18] suggested that BDNF may maintain the integrity of the intestinal barrier through its anti-apoptotic properties. Moreover, miR-151-5p promoted the expression of cleaved caspase 3, cleaved PARP, and claudin-2 but reduced that of claudin-1 and occludin in the UC mouse model and the cell line. However, the addition of exogenous BNDF revered this effect and improved UC symptoms. Caspase-3 is important in the terminal apoptosis pathway and its activated form, that is, cleaved caspase 3, promotes apoptosis [Citation31]. PPAR is also pro-apoptotic, and the inhibition of cleaved PARP has been found to reduce apoptosis in colitis mice induced by DSS [Citation25]. Occludin and claudin-1 proteins strengthen the intestinal barrier and reduce the permeability of epithelial cells, whereas claudin-2 is associated with the increased permeability of intestinal epithelial cells [Citation32,Citation33]. Therefore, we postulate that miR-151-5p likely promotes intestinal epithelial cells apoptosis and intestinal permeability to destroy the intestinal epithelial barrier by targeting BNDF. miR-151-5p was thus proposed to be a promising target for the treatment of UC. In clinical practice, the development of drugs that can target the expression of miR-151-5p will provide a more effective strategy for the treatment of UC. Further research is warranted to validate this hypothesis.
This study has limitations. First, this paper researched the expression of miR-151-5p in the colon tissues of mice. It should be better to research the expression of miR-151-5p in different kinds of intestinal epithelial cells, such as goblet cells, tuft cells and Paneth cells. Moreover, this study verified that miR-151-5p could directly regulate the expression of BNDF by binding to BNDF. It should be better to explore whether any other signaling pathways are involved in miR-151-5p regulate BNDF at mRNA level. Furthermore, it should be better to verify the miR-151-5p change between CD and UC. However, due to the laboratory limitations, these issues cannot currently be performed. The above interesting issues will be the focus of our future research.
Conclusion
We explored the expression pattern and role of miR-151-5p in colitis mice and cells induced by DSS. miR-151-5p was significantly upregulated in colitis mice, and DSS-induced cells and exacerbated UC symptoms and intestinal epithelial cells apoptosis in mice. Considering the effect of BNFD in improving UC symptoms and intestinal homeostasis, we postulate that miR-151-5p may contribute to intestinal epithelial cells apoptosis in colitis mice by directly inhibiting BNDF expression. All the findings in this paper indicated that miR-151-5p might be a promising target for the treatment of UC. Clinically, the development of drugs that can target the expression of miR-151-5p will provide a more effective strategy for the treatment of UC.
Highlights
miR-151-5p was upregulated in colon tissues of colitis mice.
BNDF expression was directly inhibited by miR-151-5p.
miR-151-5p promoted intestinal epithelial cells’ apoptosis induced by DSS.
BNDF reversed miR-151-5p effect on UC intestinal epithelial cells’ apoptosis.
miR-151-5p knockdown improved UC symptoms in mice.
Supplemental Material
Download TIFF Image (904.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2105905
Additional information
Funding
References
- Soroosh A, Rankin CR, Polytarchou C, et al. miR-24 is elevated in ulcerative colitis patients and regulates intestinal epithelial barrier function. Am J Pathol. 2019;189:1763–1774.
- Wang JP, Dong LN, Wang M, et al. MiR-146a regulates the development of ulcerative colitis via mediating the TLR4/MyD88/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:2151–2157.
- Lu X, Yu Y, Tan S. The role of the miR-21-5p-mediated inflammatory pathway in ulcerative colitis. Exp Ther Med. 2020;19:981–989.
- Gillen CD, Walmsley RS, Prior P, et al. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592.
- Coskun M, Bjerrum JT, Seidelin JB, et al. miR-20b, miR-98, miR-125b-1*, and let-7e* as new potential diagnostic biomarkers in ulcerative colitis. World J Gastroenterol. 2013;19:4289–4299.
- Modak JM, Roy-O’Reilly M, Zhu L, et al. Differential microribonucleic acid expression in cardioembolic stroke. J Stroke Cerebrovasc Dis. 2019;28:121–124.
- Qu S, Shen Y, Wang M, et al. Suppression of miR-21 and miR-155 of macrophage by cinnamaldehyde ameliorates ulcerative colitis. Int Immunopharmacol. 2019;67:22–34.
- Liu C, Li W, Zhang L, et al. Tumor-suppressor microRNA-151-5p regulates the growth, migration and invasion of human breast cancer cells by inhibiting SCOS5. Am J Transl Res. 2019;11:7376–7384.
- Hsu KW, Fang WL, Huang KH, et al. Notch1 pathway-mediated microRNA-151-5p promotes gastric cancer progression. Oncotarget. 2016;7:38036–38051.
- Ding J, Huang S, Wu S, et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol. 2010;12(4):390–399.
- Chiyomaru T, Yamamura S, Zaman MS, et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS One. 2012;7(8):e43812.
- Chen C, Wang D, Moshaverinia A, et al. Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 2017;27(4):559–577.
- Yi LI, Liu Y, Wang XP, et al. Preliminary study on microRNA differential expression in patients with peripheral blood of ulcerative colitis and its relation to biaoben-xushi syndrome. Guiding J Traditional Chinese Med Pharm. 2017;23(06):19–21.
- Paraskevi A, Theodoropoulos G, Papaconstantinou I, et al. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012;6(9):900–904.
- Fisher K. MicroRNA in inflammatory bowel disease: translational research and clinical implication. World J Gastroenterol. 2015;21(43):12274–12282.
- Han K, Jia N, Li J, et al. Chronic caffeine treatment reverses memory impairment and the expression of brain BNDF and TrkB in the PS1/APP double transgenic mouse model of Alzheimer’s disease. Mol Med Rep. 2013;8:737–740.
- Steinkamp M, Schulte N, Spaniol U, et al. Brain derived neurotrophic factor inhibits apoptosis in enteric glia during gut inflammation. Med Sci Monit. 2012;18:Br117–Br122.
- Zhao DY, Zhang WX, Qi QQ, et al. Brain-derived neurotrophic factor modulates intestinal barrier by inhibiting intestinal epithelial cells apoptosis in mice. Physiol Res. 2018;67:475–485.
- Di Sabatino A, Ciccocioppo R, Luinetti O, et al. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis Colon Rectum. 2003;46:1498–1507.
- Gitter AH, Wullstein F, Fromm M, et al. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–1328.
- Li M, Zhang S, Qiu Y, et al. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis. 2017;8:e2699.
- Bing X, Xuelei L, Wanwei D, et al. EGCG maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-κB signaling pathway in rats. Can J Gastroenterol Hepatol. 2017;2017:3057268.
- Shkoda A, Ruiz PA, Daniel H, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207.
- Liu S, Zhang S, Lv X, et al. Limonin ameliorates ulcerative colitis by regulating STAT3/miR-214 signaling pathway. Int Immunopharmacol. 2019;75:105768.
- Chen Q, Fang X, Yao N, et al. Suppression of miR-330-3p alleviates DSS-induced ulcerative colitis and apoptosis by upregulating the endoplasmic reticulum stress components XBP1. Hereditas. 2020;157:18.
- Xu X, Zhu X, Wang C, et al. microRNA-650 promotes inflammation induced apoptosis of intestinal epithelioid cells by targeting NLRP6. Biochem Biophys Res Commun. 2019;517:551–556.
- Jiang W, Han YP, Hu M, et al. A study on regulatory mechanism of miR-223 in ulcerative colitis through PI3K/Akt-mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:4865–4872.
- Chu XQ, Wang J, Chen GX, et al. Overexpression of microRNA-495 improves the intestinal mucosal barrier function by targeting STAT3 via inhibition of the JAK/STAT3 signaling pathway in a mouse model of ulcerative colitis. Pathol Res Pract. 2018;214:151–162.
- Zhang SQ, Ni WK, Xiao MB, et al. Actin related protein 3 (ARP3) promotes apoptosis of intestinal epithelial cells in ulcerative colitis. Pathol Res Pract. 2019;215:235–242.
- Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60.
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424.
- Amasheh S, Meiri N, Gitter AH. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976.
- Zhang YG, Wu S, Xia Y, et al. Salmonella infection upregulates the leaky protein claudin-2 in intestinal epithelial cells. PLoS One. 2013;8:e58606.