ABSTRACT
Esophageal cancer (EC) remains a primary cause of cancer-associated fatality worldwide and is characterized by poor prognosis. HOXA10-AS is reported to be relevant with the development of different human cancers. However, its role and regulatory mechanism in EC are still obscure. Our study targeted at investigating the functional and mechanical roles of HOXA10-AS in EC. We confirmed by RT-qPCR that HOXA10-AS presented a remarkably high expression in EC cells. Functional experiments demonstrated that knocking down HOXA10-AS weakened proliferation, invasion and migration in vitro and impeded tumorigenesis in vivo. Further, we found that HOXA10-AS positively regulated its neighbor gene HOXA10 and influenced EC cell biological activities depending on HOXA10. Mechanistically, we showed that HOXA10-AS combined with FMR1 to target and stabilize HOXA10 mRNA. Moreover, HOXA10 served as a transcriptional factor to stimulate the transcription of its target gene CHDH. Finally, rescue assays confirmed that HOXA10 influenced EC cell growth through modulating CHDH. In conclusion, our study first determines the function of HOXA10-AS in EC and demonstrates its mechanism relating to HOXA10/CHDH, suggesting HOXA10-AS as a potential novel target for EC treatment.

Introduction
Esophageal cancer (EC) is among the most aggressive gastrointestinal cancers, featured with poor prognosis and a high fatality rate [Citation1,Citation2]. EC is mainly divided into two groups, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) [Citation3]. Commonly, treatment for EC is limited, including surgical or endoscopic resection, radiotherapy and chemotherapy [Citation4]. In spite of progress in the treatment of metastatic EC with auxiliary targeted therapy, the prognosis of EC is still comparatively poor [Citation5]. Therefore, it is quite urgent and significant to explore new effective targets for EC treatment.
Long non-coding RNAs (lncRNAs), a relatively novel type of noncoding RNAs, are pervasively classified as RNA molecules with a length of more than 200 nucleotides [Citation6]. In addition, lncRNAs are one of the predominant transcripts of mammals that emerge as key participants modulating various cellular activities [Citation7], and are involved in diverse pathological conditions, particularly in cancer [Citation8]. For example, lncRNA SNHG7 has been reported to promote the proliferation of EC cells while inhibiting their apoptosis [Citation9]. LncRNA MIAT has been confirmed to display a high expression in EC cells and enhance cell invasive and migratory abilities in EC [Citation10]. In addition, HOXA10-AS has been uncovered to be relevant with cell proliferation in oral squamous cell carcinoma and HOXA10-AS knockdown leads to restrained cell proliferation [Citation11]. ELK1-elevated HOXA10-AS has been pointed out to push lung adenocarcinoma progression via actuating Wnt/β-catenin signaling [Citation12]. Nevertheless, the role of HOXA10-AS in EC remains unexplored.
Referring to literature, lncRNAs make a difference in tumorigenesis through the modulation of neighboring genes. For instance, lncRNA-CDC6 has been found to contribute to breast cancer progression via targeting miRNA-215 to regulate CDC6 [Citation13]. LncRNA FAM83A-AS1 has been identified to promote hepatocellular carcinoma development by combining with NOP58 to stabilize FAM83A mRNA [Citation14]. Moreover, it has been proven that TRPM2-AS improves proliferative capability of colorectal cancer cells by increasing TAF15-mediated stability of TRPM2 mRNA [Citation15]. Therefore, it would be a good subject to investigate the mechanism involving HOXA10-AS and its nearby gene in EC cells.
Accumulating lncRNAs have been discovered to recruit regulatory complexes through RNA–protein interactions, which can impact the expression of neighboring genes, and it has also been demonstrated that a great deal of lncRNAs can serve as local modulators [Citation16]. As a common group of proteins in mammalian cells, nearly half of RNA-binding proteins (RBPs) employ unknown modes of RNA binding [Citation17]. Aside from that, a large body of studies have confirmed RNAs could interact with RBPs at post-transcriptional level, forming complexes that could influence cellular process like RNA synthesis and decay [Citation18,Citation19]. Taking a former research, for example, LINC00337 is revealed to stimulate autophagy and chemo-resistance to cisplatin in ESCC cells via recruiting E2F4 to up-regulate TPX2 [Citation20]. As a result, the RBP mechanism linked with HOXA10-AS in EC cells is worthy to be studied.
The present study focuses on the role of a novel lncRNA, HOXA10-AS, in EC cells. The mechanism in which HOXA10-AS could have an impact on EC cell malignant behaviors will also be well explored. With a new perspective of research, our study might become a useful material for researchers to develop potential therapeutic targets for EC.
Materials and methods
Cell culture
Human EC cell lines (TE-1, Eca-109 and Kyse-150) and human endometrial epithelial cell line (HEEC) were bought from the American Type Culture Collection (ATCC). All cells were cultured in RPMI-1640 medium (61,870,127, Gibco, USA) containing 10% fetal bovine serum (FBS; 10,270–106, Gibco) and 100× Penicillin–Streptomycin solution (SV30010, PERFEMIKER, Shanghai, China). After the cells reached 80–90% confluence, they were passaged and digested with 0.25% trypsin in an environment with 5% CO2 at 37°C.
Cell transfection
EC cells in the logarithmic growth phase were collected and planted into 96-well plates. The cells were then cultured overnight. After that, short hairpin RNA (sh-RNA) against HOXA10-AS or HOXA10, the corresponding negative control (sh-NC), pcDNA3.1-HOXA10, pcDNA3.1-FMR1, pcDNA3.1-CHDH as well as the empty control pcDNA3.1 were separate transfected into EC cells in line with the instruction of Lipofectamine 2000 (11,668,019, Invitrogen, USA). After transfection for 48 h, the medium was replenished.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA in EC cells was extracted by means of TRIzol reagent (9108–1, Thermo Fisher, USA). Next, the extracted RNAs were reversely transcribed into complementary DNA (cDNA). Finally, the collected RNAs were quantified with the help of qRT-PCR Kit (QR0100-1KT, Sigma-Aldrich, USA). The relative expression level was calculated by 2−∆∆Ct method. GAPDH and U6 served as the internal controls of cytoplasm and nucleus, respectively.
Western blot
Total proteins were extracted by protein lysis buffer (ZD409, ZOMANBIO, Beijing, China) and Total Protein Extraction Kit (BC3711, Solarbio, Beijing, China). Afterward, proteins were separated by SDS-PAGE (P1200, Solarbio) and then transferred onto PVDF membranes (T2234, Thermo Fisher). After being blocked with 5% nonfat milk at room temperature, the membranes were co-cultured with primary antibody (diluted at a certain ratio) including anti-FMR1 (ab17722, Abcam, UK), anti-HOXA10 (ab191470, Abcam), anti-CHDH (ab68815, Abcam) and the internal control anti-GAPDH (ab181602, Abcam) at 4°C for a whole night. Later, a secondary antibody was added for 1-hour incubation. In the end, western blots were exposed with the assistance of enhanced chemiluminescence (ECL).
Colony formation assay
The transfected EC cells were seeded into 6-well plates for 14 days of incubation. After being rinsed with phosphate buffered saline (PBS; C10010500BT, Gibco), formed colonies were fixed with 4% paraformaldehyde (PFA) for 30 minutes and stained with 0.5% crystal violet for 5 minutes. The quantity of formed clones (more than 50 cells in each clone) was measured manually.
Cell counting kit-8 (CCK-8) assay
CCK-8 assay was performed as per user guide of CCK-8 kit (M4839, ABMOLE, USA). Before the assay, transfected EC cells were harvested from experimental group and control group, severally. The cells were then seeded into 96-well plates. After cultivation for 24, 48 and 72 h, respectively, 10 μL CCK-8 solution was added to each independent well, followed by cultivation in darkness. Optical density (OD) value at 450 nm was detected by a microplate reader (51119770DP, Thermo Fisher).
Transwell assay
For transwell invasion assay, the top chamber was pre-coated with Matrigel. Next, the cells were added culturing in a serum-free medium. In the meantime, RPMI-1640 medium with 10% FBS was put in the lower chamber. After that, cells that remained in the upper chamber were removed. Invaded cells were finally stained with crystal violet and observed. For migration assay, the upper chamber was not coated with Matrigel, and the remaining procedures were similar to those in the invasion assay.
Terminal-deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL)
TUNEL Apoptosis Assay Kit (T2190, Solarbio) was applied to detect cell apoptosis in line with the manufacturer’s guideline. At first, EC cells were fixed with 4% PFA and then permeabilized with 0.5% Triton X-100. Later, TdT reaction mix was added for incubation. After the cell nuclei were stained by DAPI (D9542, Sigma-Aldrich), cells were observed by fluorescence microscopy.
Fluorescence in situ hybridization (FISH)
FISH assay was carried out in tune with the instruction of RiboTM Fluorescent In Situ Hybridization Kit (C10910, RiboBio, Guangzhou, China). Firstly, EC cells were fixed with 4% PFA, and then permeabilized by 0.5% TritonX-100 for 15 min at 4°C. After being washed with PBS, cells were co-cultured with the FISH probe targeted HOXA10-AS. Finally, cells dyed with DAPI were observed under a laser scanning confocal microscope (Smart zoom 5, ZEISS).
Subcellular fraction assay
After cell cytoplasm and nucleus were separated by centrifugation, the expression of HOXA10-AS in different fragments was detected by RT-qPCR. U6 and GAPDH were regarded as nuclear control and cytoplasmic control, respectively.
RNA binding protein immunoprecipitation (RIP) assay
Imprint® RNA Immunoprecipitation Kit (RIP-12RXN, Sigma-Aldrich) was utilized to complete RIP assay as guided. In brief, cells were cultured with negative control anti-IgG (ab6789, Abcam), anti-ELAVL1 (ab200342, Abcam), anti-FMR1, anti-U2AF2 (ab37530, Abcam) or anti-HNRNPC (111,503, NovoPro, Shanghai, China) conjugated with magnetic beads in RIP buffer. The enriched RNA was then purified and subject to RT-qPCR analyses.
Luciferase reporter assay
PmirGLO-HOXA10 3ʹUTR was constructed by inserting HOXA10 3ʹUTR into pmirGLO vector. Similarly, pGL3-HOXA10 promoter was produced by sub-cloning HOXA10 promoter into pGL3 vector. Later, the abovementioned plasmids along with their negative controls were co-transfected with pcDNA3.1 or pcDNA3.1-FMR1 into EC cells. As for wild-type pGL3-CHDH promoter (pGL3-CHDH promoter WT) and mutant pGL3-CHDH promoter-Site1/2/3/4/5 (pGL3-CHDH promoter-Site1/2/3/4/5 mut), they were co-transfected into cells with pcDNA3.1 or pcDNA3.1-HOXA10. After transfection for 24 hours, Dual Luciferase Reporter Assay Kit (DL101-01, Vazyme, Nanjing, China) was employed to detect the relative luciferase activities.
RNA pull-down assay
Biotinylated HOXA10-AS/HOXA10 sense probes (Bio-HOXA10-AS/HOXA10 sense) and Bio-HOXA10-AS/HOXA10 anti-sense were all synthesized by means of Pierce™ RNA 3’-End Desthiobiotinylation Kit (20,160, Thermo Fisher). These biotin-labeled probes were then co-cultured with cell lysates and Pierce™ Streptavidin Magnetic Beads overnight. In the end, proteins pulled down by biotin probes were purified and detected by western blot.
DNA pull-down assay
CHDH promoter was first biotin labeled by Pierce™ Biotin 3’ End DNA Labeling Kit (89818, Thermo Fisher). Bio-NC acted as a negative control. Next, DNA pull-down kit (KT104-01, gzscbio, Guangzhou, China) was utilized to evaluate the interaction between DNA and protein. Proteins precipitated with Bio-CHDH promoter were analyzed by western blot.
Chromatin immunoprecipitation (ChIP)
ChIP procedures were accomplished by using ChIP Kit (KT101-01, gzscbio) as per supplier’s instructions. The cross-linked chromatin was first sectioned into small fragments for the immunoprecipitation with anti-HOXA10 or anti-IgG, as well as magnetic beads. Enriched DNA was measured by qPCR after purification.
In-vivo assay
Mice used in this experiment were acquired from Qilu Hospital of Shandong University (Qingdao) and randomly divided into four groups. EC cells were first transfected with sh-NC or sh1-HOXA10-AS for incubation. After 48 hours, 2 × 106 cells were subcutaneously injected into BALB/c nude mice (6–8 week-old) to build a xenograft model. The tumor volume was measured by a vernier caliper every 3 days. Twenty-eight days later, mice were sacrificed and the tumors were cautiously excised and weighed. Tumor volume was measured with the formula: length × width [Citation2]/2. Animal experiments were approved by Qilu Hospital of Shandong University (Qingdao).
Immunohistochemistry (IHC)
IHC was used to analyze the expression of proliferation-linked proteins in xenograft tissues. Briefly speaking, tumor tissues were fixed and then embedded in paraffin. After that, the samples were cut into small sections. Later, these fragments were blocked and co-cultured with primary antibodies targeting Ki-67 (ab15580, Abcam) at 4°C for a whole night. After PBS washing, secondary antibodies were added for 1-hour incubation. Eventually, the sections were dyed with DAB and then subjected to fluorescence microscopy observation.
Statistical analysis
SPSS 22.0 software was applied for all data analysis. Each independent assay was repeated for at least three times. Obtained data were presented as mean ± standard deviation (SD). Differences between two or more groups were analyzed by means of Student’s t-test or one-way analysis of variance (ANOVA). P < 0.05 was deemed to have statistical significance.
Results
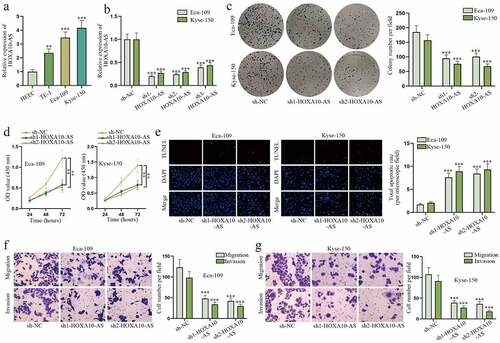
HOXA10-AS promotes EC cell malignant behaviors
To explore the role of HOXA10-AS in EC cells, UALCAN (http://ualcan.path.uab.edu) database was applied to determine HOXA10-AS expression in EC tissues. The results demonstrated HOXA10-AS was dramatically up-regulated in EC tissues versus normal tissues (Figure S1A). Meanwhile, HOXA10-AS was also confirmed to display a higher expression in EC cells, particularly in Eca-109 and Kyse-150, relative to normal HEEC cells (). After HOXA10-AS was uncovered to be down-regulated by sh1/2/3-HOXA10-AS, especially by sh1/2-HOXA10-AS, in RT-qPCR (), a series of functional assays were carried out. In colony formation and CCK-8 assays, cell proliferative abilities were remarkably weakened after HOXA10-AS knockdown (). Based on TUNEL assay, the amount of apoptotic EC cells dramatically rose after sh1/2-HOXA10-AS transfection (). Moreover, transwell assays revealed cell migratory and invasive capabilities were suppressed by HOXA10-AS depletion (). In brief, HOXA10-AS exacerbates malignant behaviors of EC cells.
Figure 1. HOXA10-AS promotes EC cell malignant behaviors.
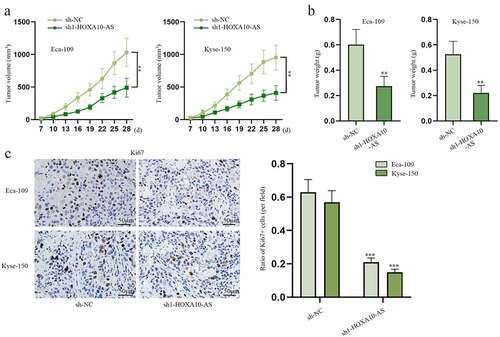
HOXA10-AS contributes to EC tumor growth
In this section, in vivo assays were designed and implemented to verify the influence of HOXA10-AS on EC tumor growth. As shown in , tumor volume and weight in sh1-HOXA10-AS groups were much smaller than those in sh-NC groups. In addition, the outcomes of IHC manifested that Ki67, a marker of cell growth, was obviously reduced by HOXA10-AS knockdown (). To sum up, in vivo assays suggest HOXA10-AS could facilitate EC tumor growth.
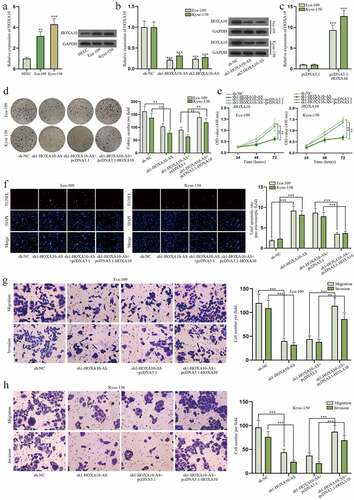
HOXA10-AS boosts EC cell growth via regulating HOXA10
Numerous studies have pointed out lncRNA could exert its effect on cancers via modulating neighboring genes [Citation13]. Hence, the mechanism of HOXA10-AS in EC cells involving its neighboring gene was investigated. HOXA10 was revealed to be the neighboring gene of HOXA10-AS based on NCBI (https://pubmed.ncbi.nlm.nih.gov/) website (Figure S1B). Search results on GEPIA 2 (http://gepia2.cancer-pku.cn/) database demonstrated that the expression of HOXA10-AS was positively correlated with that of HOXA10 in EC tissues (Figure S1C). And data on lncATLAS (http://lncatlas.crg.eu/) proved HOXA10-AS and HOXA10 co-existed in the nucleus and cytoplasm of cells (Figure S1D). These data implied the potent relation between HOXA10-AS and HOXA10, so the relationship between HOXA10-AS and HOXA10 was further explored. At first, the expression of HOXA10 was discovered to be higher in Eca-109 and Kyse-150 cells compared to normal HEEC cells according to RT-qPCR and western blot (). And then HOXA10 was found to be down-regulated in cells transfected with sh1/2-HOXA10-AS (). After the overexpression of HOXA10 was proved to be effective in EC cells (), functional assays with a rescuing group were performed. The results of colony formation and CCK-8 assays reflected cell proliferation was repressed by HOXA10-AS reduction but was then restored by elevating HOXA10 expression (). Cell apoptosis was evaluated in TUNEL assay, and it turned out the promoting effect of sh1-HOXA10-AS on cell apoptosis was offset by pcDNA3.1-HOXA10 (). As for the results of transwell assays, HOXA10-AS knockdown caused inhibition on cell migration and invasion was counteracted by increased HOXA10 (). To sum up, HOXA10-AS could aggravate biological behaviors of EC cells via up-regulating HOXA10.
Figure 3. HOXA10-AS boosts EC cell growth via regulating HOXA10.
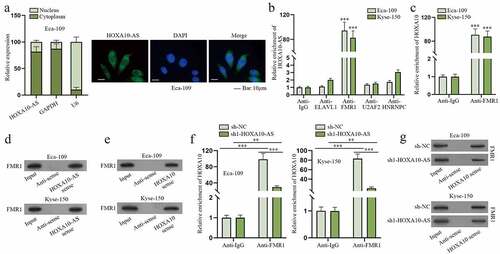
FMR1 is the common RBP for HOXA10-AS and HOXA10
As indicated in Figure S1D, HOXA10-AS was mainly amassed in the cytoplasm. Consistent with this result, subcellular fractionation and FISH assays also revealed HOXA10-AS was primarily located in the cytoplasm of Eca-109 (). So we presumed HOXA10-AS might modulate HOXA10 expression via certain RBP. After searching on starBase (http://starbase.sysu.edu.cn/), ELAVL1, FMR1, U2AF2 and HNRNPC were screened out to be the potentially common RBPs for HOXA10-AS and HOXA10 (Figure S2A-S2B). Based on the results of RIP assays, FMR1 was determined to be the only RBP that could substantially combine with HOXA10-AS and HOXA10 (), and Hum-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/Hum-mPLoc3/) predicted that FMR1 existed both in the nuclei and cytoplasm of cells (Figure S2C). Therefore, FMR1 was selected. The following RNA pull-down assay validated FMR1 could bind to HOXA10-AS and HOXA10 (). In addition, the affinity between FMR1 and HOXA10 was verified to be weakened due to decreased HOXA10-AS level according to RIP and RNA pull-down assays (). To conclude, FMR1 is the shared RBP for HOXA10-AS and HOXA10, and the combination of FMR1 and HOXA10 could be disrupted by HOXA10-AS inhibition.
Figure 4. FMR1 is the common RBP for HOXA10-AS and HOXA10.
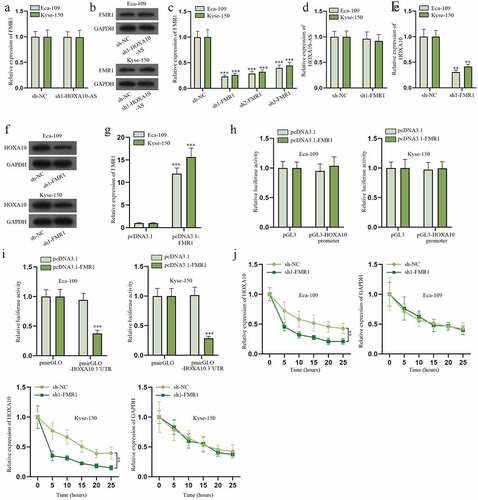
HOXA10-AS recruits FMR1 to stabilize HOXA10
Next, we further delved into the relationship among HOXA10-AS, HOXA10 and FMR1 in Eca-109 and Kyse-150. The results of RT-qPCR and western blot signified that reducing HOXA10 level had little influence on FMR1 expression (). Later, the efficiency of sh1/2/3-FMR1 was assessed. And sh1-FMR1 was chosen because it was the most effective in reducing FMR1 expression (). The results of RT-qPCR indicated that the expression of HOXA10-AS was hardly affected by FMR1 deficiency (). Additionally, data from RT-qPCR and western blot demonstrated FMR1 depletion conspicuously lessened the expression of HOXA10 (). Afterward, the overexpression efficiency of pcDNA3.1-FMR1 was confirmed (). In subsequent luciferase reporter assays, it was found that FMR1 elevation could affect the luciferase activity of pmirGLO-HOXA10 3ʹUTR rather than that of pGL3-HOXA10 promoter (), which indicated FMR1 regulated HOXA10 at the post-transcriptional level. RBP was reported to enhance the stability of downstream RNA [Citation21], so we deduced that FMR1 might also stabilize HOXA10 mRNA. RT-qPCR was carried out in EC cells treated with 50 mM α-amanitin. The outcomes presented that FMR1 inhibition distinctly shortened the half-life of HOXA10 mRNA and had no impact on that of GAPDH (). In brief, HOXA10-AS could combine with FMR1 to stabilize HOXA10 mRNA in EC cells.
Figure 5. HOXA10-AS recruits FMR1 to stabilize HOXA10.
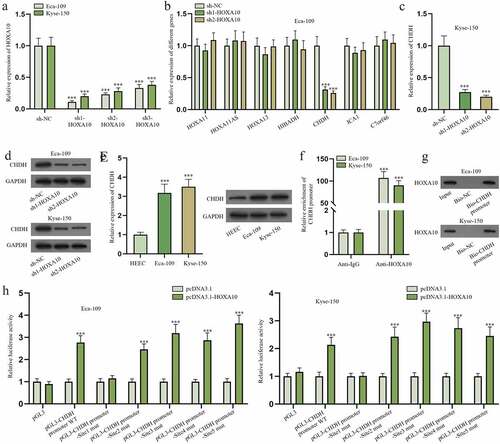
HOXA10 stimulates the transcription of CHDH
As shown in Figure S3A, UALCAN was utilized to predict genes that were positively correlated with HOXA10 in ESCA (short for esophageal carcinoma, also known as EC). HOXA11, HOXA11AS, HOXA13, HIBADH, CHDH, ICA1 and C7orf46 were screened out (Pearson CC ≥ 0.6). RT-qPCR was applied to detect the expression of HOXA10 after transfection of sh1/2/3-HOXA10 (). Sh1-HOXA10 and sh2-HOXA10 were chosen for their higher efficiency. Later, the expression of candidate genes in Eca-109 cells was quantified via RT-qPCR when HOXA10 level was cut down, and only CHDH level was noticeably lessened (). Accordingly, the expression of CHDH was checked in Kyse-150 cells after HOXA10 was down-regulated. The outcomes represented that CHDH was down-regulated when HOXA10 was knocked down (). The following western blot further confirmed HOXA10 knockdown could reduce the protein level of CHDH (). Next, the expression of CHDH was tested in Eca-109, Kyse-150 and HEEC cells by means of RT-qPCR and western blot. It was revealed that CHDH was highly expressed in EC cells compared to HEEC cells (). Considering HOXA10 is an important transcription factor [Citation22], we reckoned HOXA10 might target CHDH promoter to modulate CHDH mRNA expression. As projected in JASPAR (http://jaspar.genereg.net/), there were five binding sites (Score >7) in CHDH promoter for HOXA10 (Figure S3B). ChIP and DNA pull-down assays were then done to verify the combination of CHDH promoter and HOXA10 (). It turned out that CHDH promoter could bind to HOXA10. Later, the five predicted binding sites in CHDH promoter were mutated, respectively. And pGL3 vectors with different mutated sequences as well as wide-type CHDH promoter were constructed (pGL3-CHDH promoter WT, pGL3-CHDH promoter-Site1 mut, pGL3-CHDH promoter-Site2 mut, pGL3-CHDH promoter-Site3 mut, pGL3-CHDH promoter-Site4 mut, pGL3-CHDH promoter-Site5 mut). The results of luciferase reporter assay manifested Site1 was the binding site of HOXA10 and CHDH in EC cells as only the luciferase activity of pGL3-CHDH promoter-Site1 mut had no obvious change after HOXA10 overexpression (). In summary, HOXA10 is the transcription activator of CHDH.
Figure 6. HOXA10 stimulates the transcription of CHDH.
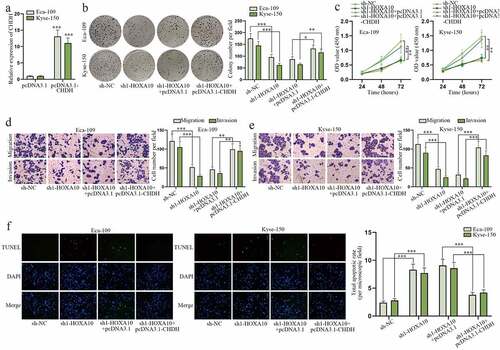
HOXA10 aggravates EC cell malignant process via regulating CHDH
To figure out the impact of HOXA10/CHDH axis on EC cell growth, rescue assays were conducted. First, the high transfection efficiency of pcDNA3.1-CHDH was validated based on the data of RT-qPCR assay (). Afterward, colony formation and CCK-8 assays were performed to detect cell proliferation. Weakened cell proliferative capability caused by sh1-HOXA10 was found to be recovered by pcDNA3.1-CHDH (). Furthermore, the suppressive effect of sh1-HOXA10 on cell migration and invasion was discovered to be counteracted by pcDNA3.1-CHDH in transwell assays (). And TUNEL assays uncovered strengthened cell apoptotic ability resulting from HOXA10 down-regulation was restricted again by augmenting CHDH expression (). Taken together, HOXA10 accelerates EC cell growth via modulating CHDH.
Figure 7. HOXA10 aggravates EC cell malignant process via regulating CHDH.
Discussion
Esophageal cancer (EC) is identified to be one of the most fatal human malignancies and a global health concern due to belated diagnosis and deficiency of effective treatment [Citation23]. Moreover, owing to some tricky risk factors such as smoking, red meat consumption and low economic status, the global morbidity of EC is comparatively high and continues to rise every year [Citation24,Citation25]. LncRNAs have sprung up as critical players at multiple levels of gene function and modulation [Citation26], and dysregulated lncRNA could exert enormous effects on a variety of cancer types [Citation27]. For instance, Chen et al. have confirmed lncRNA H19 is up-regulated in EC and could promote cell epithelial mesenchymal transition (EMT) and metastasis in EC via targeting STAT3/EZH2 axis [Citation28]. Zhao et al. have pointed out lncRNA NBAT-1 up-regulation notably hampered cell growth and tumor glycolysis in EC by regulating PKM2 [Citation29]. Herein, lncRNA HOXA10-AS was verified to present a high expression in EC cells and tissues by means of RT-qPCR and bioinformatics analyses. And HOXA10-AS depletion dramatically hindered proliferative, migratory and invasive capabilities but stimulated the apoptotic ability of EC cells. In addition, data of in vivo assays reflected HOXA10-AS could also boost EC tumor growth. A piece of former research has proven that HOXA10-AS is highly expressed in acute myeloid leukemia (AML), and down-regulated HOXA10-AS greatly impairs tumor growth in AML [Citation30]. Consistent with this literature, our study also demonstrated HOXA10-AS was an oncogene in EC and could accelerate EC cell growth.
Emerging lncRNAs have been reported to take part in the activities of cancers by working with their nearby genes. For instance, Wu et al. argue that lncRNA FOXP4-AS1 is transcriptionally elevated by PAX5 and advances the growth of prostate cancer by competitively binding with miR-3184-5p to up-regulate FOXP4 [Citation31]. Wu et al. reveal that IRF4-induced increase of lncRNA SOX2-OT contributes to cell proliferation and metastasis in cholangiocarcinoma via the regulation of SOX2 and PI3K/AKT signaling [Citation32]. In the current study, HOXA10-AS was determined to be positively correlated with its nearby gene HOXA10. A series of functional assays further validated HOXA10 overexpression could countervail the inhibitory effect of HOXA10-AS deficiency on EC cell malignant process. A previous study depicts that HOXA10‑AS facilitates cell growth and survival via lifting HOXA10 expression in glioma [Citation33]. In line with the published essay, our study unraveled the positive relationship between HOXA10-AS and HOXA10, along with the promoting role of HOXA10 in EC cells.
LncRNAs are linked to diverse cellular activities including gene expression regulation, most of which request the interaction with RBP [Citation34,Citation35]. Hereby, FMR1 was discovered to be the common RBP for HOXA10-AS and HOXA10 in EC cells based on database screening and a set of mechanism assays. Down-regulation of FMR1 had no impact on HOXA10-AS expression but lessened HOXA10 expression. Furthermore, FMR1 was verified to combine with HOXA10 3’ UTR and stabilize HOXA10. As reported before, lncRNAs could function as sponges for RBPs, thus affecting the expression of target genes of the corresponding RBP [Citation36]. In this research, HOXA10-AS was confirmed to recruit FMR1 for HOXA10 stabilization. Subsequently, CHDH was selected to be the gene that could be regulated by HOXA10 after database prediction and experiment verification. HOXA10 has been demonstrated to be a transcription factor [Citation37]. Here, we discovered HOXA10 could activate the transcription of CHDH, and CHDH was highly expressed in EC cells. In addition, rescue assays uncovered that CHDH increase could restore the suppressed EC cell growth resulting from diminished HOXA10.
Our study first ascertained HOXA10-AS played a tumor promoter role in EC cells and tumors via recruiting FMR1 and thereby stabilizing HOXA10 mRNA. Additionally, HOXA10 was also first unveiled to exacerbate EC cell malignant behaviors by raising CHDH expression at transcription level. Notwithstanding the lack of clinical samples, this study might provide useful knowledge for understanding EC development at the molecular level.
Supplemental Material
Download Zip (10.1 MB)Acknowledgments
We appreciate the support of our experimenters.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2108633
Additional information
Funding
References
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50(1):12–20.
- Short MW, Burgers KG, Fry VT. Esophageal Cancer. Am Fam Physician. 2017;95(1):22–28.
- Liu K, Zhao T, Wang J, et al. Etiology, cancer stem cells and potential diagnostic biomarkers for esophageal cancer. Cancer Lett. 2019;458:21–28.
- Rustgi A, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2015;372(15):1472–1473.
- Huang TX, Fu L. The immune landscape of esophageal cancer. In: Cancer communications. Vol. 39. London England: Cancer Commun (Lond); 2019. p. 79.
- Wang J, Su Z, Lu S, et al. LncRNA HOXA-AS2 and its molecular mechanisms in human cancer. Clin Chim Acta. 2018;485:229–233.
- Renganathan A, Felley-Bosco E. Long noncoding RNAs in cancer and therapeutic potential. Adv Exp Med Biol. 2017;1008:199–222.
- Chan JJ, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310.
- Xu LJ, Yu XJ, Wei B, et al. LncRNA SNHG7 promotes the proliferation of esophageal cancer cells and inhibits its apoptosis. Eur Rev Med Pharmacol Sci. 2018;22(9):2653–2661.
- Zhang W, Chen Q, Lei C. lncRNA MIAT promotes cell invasion and migration in esophageal cancer. Exp Ther Med. 2020;19(5):3267–3274.
- Yan X, Cong B, Chen Q, et al. Silencing lncRNA HOXA10-AS decreases cell proliferation of oral cancer and HOXA10-antisense RNA can serve as a novel prognostic predictor. J Int Med Res. 2020;48(8):300060520934254.
- Sheng K, Lu J, Zhao H. ELK1-induced upregulation of lncRNA HOXA10-AS promotes lung adenocarcinoma progression by increasing Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2018;501(3):612–618.
- Kong X, Duan Y, Sang Y, et al. LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J Cell Physiol. 2019;234(6):9105–9117.
- He J, Yu J. Long noncoding RNA FAM83A-AS1 facilitates hepatocellular carcinoma progression by binding with NOP58 to enhance the mRNA stability of FAM83A. Biosci Rep. 2019;39(11).
- Pan L, Li Y, Jin L, et al. TRPM2-AS promotes cancer cell proliferation through control of TAF15. Int J Biochem Cell Biol. 2020;120:105683.
- Engreitz JM, Haines JE, Perez EM, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539(7629):452–455.
- Castello A, Fischer B, Frese CK, et al. Comprehensive identification of RNA-binding domains in human cells. Mol Cell. 2016;63(4):696–710.
- Muthuramalingam M, Wang Y, Li Y, et al. Interacting protein partners of Arabidopsis RNA-binding protein AtRBP45b. Vol. 19. Germany): Plant biology (Stuttgart; 2017. p. 327–334.
- Gopalakrishna S, Pearce SF, Dinan AM, et al. C6orf203 is an RNA-binding protein involved in mitochondrial protein synthesis. Nucleic Acids Res. 2019;47(17):9386–9399.
- Yang C, Shen S, Zheng X, et al. Long non-coding RNA LINC00337 induces autophagy and chemoresistance to cisplatin in esophageal squamous cell carcinoma cells via upregulation of TPX2 by recruiting E2F4. FASEB J. 2020;34(5):6055–6069.
- Hu YP, Jin YP, Wu XS, et al. LncRNA-HGBC stabilized by HuR promotes gallbladder cancer progression by regulating miR-502-3p/SET/AKT axis. Mol Cancer. 2019;18(1):167.
- Guo C, Ju QQ, Zhang CX, et al. Overexpression of HOXA10 is associated with unfavorable prognosis of acute myeloid leukemia. BMC Cancer. 2020;20(1):586.
- Talukdar FR, Di Pietro M, Secrier M, et al. Molecular landscape of esophageal cancer: implications for early detection and personalized therapy. Ann N Y Acad Sci. 2018;1434(1):342–359.
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19(34):5598–5606.
- Hou X, Wen J, Ren Z, et al. Non-coding RNAs: new biomarkers and therapeutic targets for esophageal cancer. Oncotarget. 2017;8(26):43571–43578.
- Qian X, Zhao J, Yeung PY, et al. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci. 2019;44(1):33–52.
- Liang Y, Chen X, Wu Y, et al. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 2018;25(11):1980–1995.
- Chen MJ, Deng J, Chen C, et al. LncRNA H19 promotes epithelial mesenchymal transition and metastasis of esophageal cancer via STAT3/EZH2 axis. Int J Biochem Cell Biol. 2019;113:27–36.
- Zhao B, Cao P, Hu S, et al. LncRNA-NBAT-1 modulates esophageal cancer proliferation via PKM2. Am J Transl Res. 2019;11:5978–5987.
- Al-Kershi S, Bhayadia R, Ng M, et al. The stem cell-specific long noncoding RNA HOXA10-AS in the pathogenesis of KMT2A-rearranged leukemia. Blood Adv. 2019;3(24):4252–4263.
- Wu X, Xiao Y, Zhou Y, et al. LncRNA FOXP4-AS1 is activated by PAX5 and promotes the growth of prostate cancer by sequestering miR-3184-5p to upregulate FOXP4. Cell Death Dis. 2019;10(7):472.
- Wei CX, Wong H, Xu F, et al. IRF4-induced upregulation of lncRNA SOX2-OT promotes cell proliferation and metastasis in cholangiocarcinoma by regulating SOX2 and PI3K/AKT signaling. Eur Rev Med Pharmacol Sci. 2018;22(23):8169–8178.
- Dong CY, Cui J, Li DH, et al. HOXA10‑AS: a novel oncogenic long non‑coding RNA in glioma. Oncol Rep. 2018;40:2573–2583.
- Ferrè F, Colantoni A, Helmer-Citterich M. Revealing protein-lncRNA interaction. Brief Bioinform. 2016;17(1):106–116.
- Zhang Q, Wei Y, Yan Z, et al. The characteristic landscape of lncRNAs classified by RBP-lncRNA interactions across 10 cancers. Mol Biosyst. 2017;13(6):1142–1151.
- HafezQorani S, Houdjedj A, Arici M, et al. RBPSponge: genome-wide identification of lncRNAs that sponge RBPs. Bioinformatics. 2019;35(22):4760–4763.
- Long Z, Li Y, Gan Y, et al. Roles of the HOXA10 gene during castrate-resistant prostate cancer progression. Endocr Relat Cancer. 2019;26(3):279–292.