ABSTRACT
Octamer-binding transcription factor 4 (Oct4) is closely related to the occurrence and development of cancer. In the present study, we paid a special interest in exploring the effect of Oct4 on colon cancer (CC) proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) and its molecular mechanism. Immunohistochemistry (IHC) was used to detect the expression level of Oct4 in colon tissue of patients with colon cancer. Oct4 overexpression vector pcDNA-Oct4 was used to stably express Oct4 in human colon cancer cells HT29 and SW480. Cell counting kit-8 (CCK-8) assay was used to detect the cell proliferation. The invasion and migration abilities were observed by transwell and wound healing assays. The expression of EMT relate genes were observed by Western blot. We found that Oct4 was up-regulated in human colon cancer tissues than that in paracancerous tissues. The proliferation, migration, and invasion of HT29 and SW480 cells was significantly induced by transfection of pcDNA-Oct4. Furthermore, Oct4 overexpression enhanced EMT of CC cells, characterized by the increased expression of vimentin, Twist, and Snail, as well as decreased expression of E-cadherin. Mechanistically, Oct4 overexpression activated stem cell factor (SCF)/c-Kit signaling pathway in CC cells, and the SCF/c-Kit signaling inhibitor imatinib reversed pro-oncogenic effects of Oct4. These finding provide an insight into the potential of Oct4 for CC diagnosis and therapy.
Introduction
Colon cancer (CC) is a common malignant tumor of the digestive tract and its morbidity and mortality are among the top malignant tumors in the world [Citation1,Citation2]. There are 1.8 million new cases and 0.9 million deaths are reported each year [Citation3]. In recent years, with the improved changes in living standards and diet, the incidence of CC is tending to be younger [Citation4]. Lifestyle risk factors such as unhealthy diet, obesity, alcohol consumption, and smoking are major contributing factors to CC [Citation5,Citation6]. At present, the main treatment for CC is a multidisciplinary comprehensive treatment based on surgical treatment [Citation7]. However, there is still a considerable number of patients that have occurred in diagnosed with lymph node metastasis or distant metastasis [Citation8,Citation9]. Thus, there is a desperate need to identify sensitive biomarkers or drug targets of CC for developing improved diagnosis and clinical management.
Octamer-binding transcription factor 4 (Oct4), also known as POU class 5 homeobox 1 (POU5F1), is one of the key transcription factors of the POU family [Citation10,Citation11]. Oct4 played an important role in the regulation and maintenance of self-renewal and multi-directional differentiation of embryonic stem cells [Citation10,Citation12]. Recent studies showed that Oct4 was widely expressed in human solid tumors, including hepatocellular carcinoma [Citation13], lung cancer [Citation14], breast cancer [Citation15], and gastric cancer [Citation16], which was closely related to tumor cell proliferation, metastasis, and prognosis. Patients with Oct4 overexpression were associated with faster growth, easier metastasis, and poorer prognosis [Citation17,Citation18]. Therefore, Oct4 has been regarded as a potential treatment target for malignant tumors. However, its potential role and mechanisms in CC progression are poorly understood.
Abnormal expression of receptor tyrosine kinases (RTKs) plays an important role in the development and progression of cancers [Citation19]. c-Kit is a type III RTK and its ligand is a stem cell factor (SCF). Upon binding to SCF, c-kit will phosphorylate and activate multiple downstream signaling pathways, ultimately promoting cell survival, proliferation, apoptosis, migration, and so on [Citation20]. In recent years studies have confirmed that co-expression of c-kit and SCF played a central role in the occurrence and development of gastric cancer [Citation21], cutaneous melanoma [Citation22], and ovarian cancers [Citation23]. However to our best knowledge no report on SCF/c-Kit signaling and the development of CC.
In this study, the expression levels of Oct4 in CC tissue samples from CC patients were explored. Furthermore, we investigated the biological functions of Oct4 in CC progression, including the effects of Oct4 on the proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) of human colon carcinoma cell lines HT29 and SW480. Meanwhile, we probed whether Oct4 participates in CC progression through regulating the SCF/c-Kit signaling pathway in vitro.
Material and methods
Collection of clinical samples
Right-sided colon cancer (RCC, n = 11), left-sided colon cancer (LCC, n = 9), and paracancerous tissues (n = 5) were collected from clinical patients who underwent radical resection of colon cancer at Shanxi Bethune Hospital. Those who received preoperative therapy (including radiotherapy, chemotherapy, radiochemotherapy, or molecular biological treatment) were excluded. All the patients agreed to the research. This experiment was also approved by the ethics review committee of Shanxi Bethune Hospital (SBQKL-2022-102). The colon cancer tissues and adjacent normal tissues were rapidly snap-frozen in liquid nitrogen and stored at − 80°C for further analysis.
Immunohistochemistry (IHC) stain
Paraffin blocks of human colon cancer tissues and adjacent tissues were sectioned at 4 µm. IHC stain was performed to detect the Oct4 expression according to instructions of a biotin-streptavidin-peroxidase (SP) kit (No.SP9002, Zhongshan Jinqiao Biotechnology Co. Ltd.). Oct4 antibody was purchased from Abcam (No. ab184665, Abcam, Cambridge, MA, USA; 1:100). IHC images were captured using a digital trinocular camera microscope camera system (BA400Digital, Motic Instruments, Inc., Baltimore, MD, USA). Image quantification was performed using Halo software (Indica Labs; Albuquerque, NM).
Cell culture
The human colon carcinoma cell lines HT29 (No.CL-0118) and SW480 (No.CL-0223) were obtained from ProCell (Wuhan, China). The cells were cultured (37°C, 5% CO2) in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Hyclone, South Logan, UT, USA), 1% nonessential amino acids, and 100 U/mL penicillin and 100 μg/mL streptomycin (No. 15,070,063; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell transfection
The expression plasmid pcDNA-Oct4 was constructed for the expression of the Oct4 gene in cultured cells. To construct pcDNA-Oct4, the coding sequence of Oct4 was amplified by PCR and cloned into pGEX-Oct4. After restriction enzyme digestion, the PCR products were ligated with a pcDNA3.1(+) eukaryotic expression vector (Invitrogen, Carlsbad, CA, USA). HT29 and SW480 cells were grown for 24 h and transfected with pcDNA-Oct4 using Lipofectamine®2000 (Invitrogen, Grand Island, NY) according to the manufacturer’s instruction. For some experiments, c-Kit inhibitor imatinib (imatinib mesylate; No. S1026, Selleckchem Inc., Houston, TX, USA) was treated with HT29 and SW480 cells at 0, 25, 50, 100 nM concentrations for 24 h.
Cell proliferation assay
HT29 and SW480 cells were transfected with plasmids or treated with Imatinib before cell proliferation assays. Cell proliferation was measured by cell counting kit-8 (CCK-8) assay (CCK-8, Dojindo, Japan) following the manufacturer’s instructions. Briefly, 2.0 × 103 cells/well were seeded into 96-well plates and 10 μL of CCK-8 solution was added to each well containing 100 μL of RPMI-1640 medium and kept in an incubator (37°C with 5% CO2) for 3 h. After 0 h, 24 h, 48 h, and 72 h incubation, absorbance at 450 nm was recorded by a microplate reader (Spectra Max Plus 384, Molecular Devices, Sunnyvale, CA, USA).
Wound healing assay
HT29 and SW480 cells were transfected with plasmids or treated with Imatinib before cell migration assays. The cells were seeded into 6-well plates for 24 h to reach 80–90% confluence. A single wound was created in the center of the cell monolayer by scratching across the surface of the well with a 10 µl pipette. Cells were subsequently cultured in serum-free RPMI-1640 medium at 37°C for 24 h. The wound healing distance was measured under an Olympus X51 inverted microscope (Olympus, Tokyo, Japan). Relative wound recovery (%) = (initial wound width – wound width at 24 h)/initial wound width × 100.
Transwell assay
Before transwell assays for cell invasion assay, HT29 and SW480 cells were transfected with plasmids or treated with Imatinib. Briefly, transwell filters (BD Biosciences, San Jose, CA, USA) were filled with the 4°C pre-chilled serum‐free medium to form the chamber. HT29 and SW480 cells were suspended in serum‐free medium (3.0 × 105/mL) and seeded into the upper chamber. A culture medium containing 10% FBS was added to the basolateral chamber and left to incubate at 37°C for 24 h. Then, transwell cultures were washed twice with sterile PBS, fixed with methanol for 30 min, and then dyed with 0.1% crystal violet for 30 min at room temperature. Cell invasion was observed under an Olympus X51 inverted microscope (Olympus, Tokyo, Japan).
RNA extraction and reverse-transcription quantitative PCR (RT-qPCR)
TRIzol (Invitrogen, Carslbad, CA) reagent was used to extract total RNA from HT29 and SW480 cells according to the manufacturer’s protocol. RT-qPCR was performed by using the SYBR Premix Ex TaqTMII kit (Takara, Tokyo, Japan). The primers for E-cadherin, Vimentin, Snail, Twist, and β-actin amplification were as follows: E-cadherin forward, 5“-GGA TGT GAA TGA AGC CCC CA −3” and reverse, 5“-CCT GGG CAG TGT AGG ATG TG-3”, Vimentin forward, 5“-AGC TGC TAA CTA CCA GGA CAC TAT TG-3” and reverse, 5“-CGA AGG TGA CGA GCC ATC TC -3”, Snail forward, 5“- CTC AGC AGG GTG GTT ACT GG-3” and reverse, 5“-GCG CCC ATT ATA GGA ACC CA-3”, Twist forward, 5“- GGCACCATCCTCACACCTCT-3” and reverse, 5“-TGT CAC CAG GAC AAA TGG GG-3”, β-actin forward, 5“-CTC CAT CGT CCA CCG CAA ATG CTT CT-3” and reverse, 5“- GCT CCA ACC GAC TGC TGT CAC CTT C-3”. RT-qPCR was performed with a program of 5 min at 95°C and then 45 cycles at 95°C (30 s), 95°C (5 s), 55°C (30 s), and 72°C (30 s). The cycle threshold (CT) values of the samples were analyzed using Thermo Scientific PikoReal software 2.1 (Thermo Fisher Scientific, Waltham, MA, USA). The 2‐ΔΔCT method (ΔΔCT =ΔCTtreatment- ΔCTcontrol and ΔCT = Ct target - Ct reference.) was used to calculate the relative expression. Each candidate gene was internally normalized against β-actin.
Western blot analysis
HT29 and SW480 cells were lysed in RIPA buffer (Signaling Technology, Inc., Danvers, MA, USA) to collect total proteins. The protein content was quantitated using a BCA kit (Beyotime, Shanghai, China). Total protein (30 µg/sample) was separated via 10% SDS-PAGE and transferred to nitrocellulose membranes (Pall Life Sciences, New York, USA). The membranes were blocked with 5% nonfat dry milk for 1 h to prevent nonspecific binding of antibodies. After probed with primary antibodies overnight at 4°C, the membranes were washed and incubated with appropriate peroxidase-conjugated secondary antibodies. The corresponding protein primary antibodies were as follows: Oct4 (No. ab200834, Abcam, Cambridge, MA, USA; 1: 10,000), E-cadherin (No. ab40772, Abcam, Cambridge, MA, USA; 1: 10,000), Vimentin (No.A19607, Abclonal, Wuhan, China; 1:500), c-Kit (No. ab178527, Abcam, Cambridge, MA, USA; 1:1000), SCF (No. ab52603, Abcam, Cambridge, MA, USA; 1: 10,000), and β-actin (No. ab8227, Abcam, Cambridge, MA, USA; 1:1000). Finally, the bands were visualized using the ECL system (Thermo Scientific, Rockford, IL, USA).
Immunofluorescence (IF) stain
C-kit and SCF membrane expression was evaluated by IF stain. Cell climbing slices of HT29 and SW480 cells were washed with PBS 3 times, and fixed with 3.7% formaldehyde. After several rinses with PBS, the cells were permeabilized with 0.2% Triton X-100 for 5 min. Cells were then blocked for 20 min with 10% goat serum sealing fluid. Then, the permeabilized cells were incubated with SCF antibody (No. ab52603, Abcam, Cambridge, MA, USA; 1:100) or c-Kit antibody (No. ab178527, Abcam, Cambridge, MA, USA; 1:100) at 4°C overnight and washed 3 times with PBS. Then, the cell nuclei were stained with DAPI (ZLI-9557; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 5 min. Finally, the slides were analyzed with a fluorescence microscope Olyvia (Olympus, Tokyo, Japan) at 200 × and 400 × magnifications. Image processing was conducted with Image J (National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
All cell experiments were repeated at least in triplicate. The data were represented as means ± standard deviation (SD). Statistical analysis was performed with the SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) with Tukey’s post hoc test and Student’s t-test were used for statistical analysis. Differences with a P < 0.05 were considered to indicate statistically significant.
Results
Oct4 was up-regulated in human colon cancer tissues
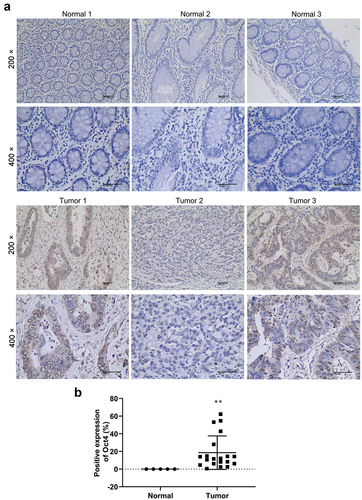
To investigate Oct4 expression in human colon cancer tissues, samples (colon cancer, n = 20; paracancerous, n = 5) from Shanxi Bethune Hospital were collected. IHC was applied to determine the expression of Oct4 in both colon cancer and paracancerous tissues. The protein expression level of Oct4 was up-regulated in colon cancer tissues than that in paracancerous tissues in patients with colon cancer ().
Figure 1. Oct4 was up-regulated in human colon cancer tissues. (a) Oct4 expression in 20 colon cancer tissues and 5paracancerous tissues was tested by immunohistochemistry (IHC) stain. Magnification is 200 × and 400 × .(b) Histogram of immunohistochemistry. Values are means ± sd, Student’s t-test was used for statistical analysis. **P <0.01 vs normal.
Overexpression of Oct4 promoted proliferation, migration, invasion, and EMT of colon cancer cells
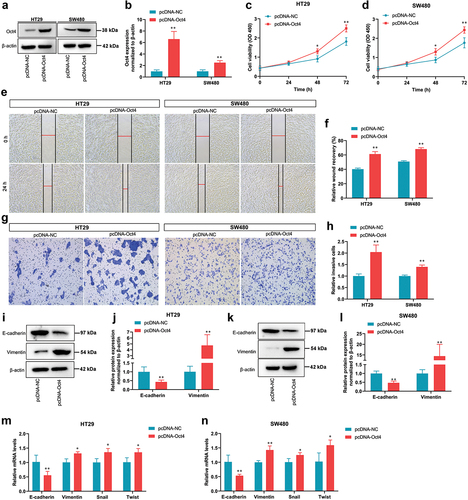
To explore the potential function of Oct4 in colon cancer, the overexpression vector pcDNA-Oct4 was constructed and overexpressed Oct4 in HT29 and SW480 cells by liposome transfection (). The effect of Oct4 overexpression on cell proliferation was evaluated by a CCK-8 assay. Our results indicated that Oct4 overexpression caused an increase in cell proliferation of Oct4 in HT29 and SW480 cells compared with the pcDNA-NC group (). In wound healing assay, Oct4 overexpression in HT29 and SW480 cells enhanced cell migration (). Meanwhile, transwell assay results showed that invasion cells were noticeably increased in HT29 and SW480 cells transfected with pcDNA-Oct4 (). To confirm whether Oct4 overexpression regulates the expression of EMT transition markers, which are closely related to metastatic properties, the mRNA or/and protein expression of EMT transition factors was determined. Compared with empty vector-transfected cells, the expression of one epithelial marker E-cadherin was decreased, whereas the expression of mesenchymal markers vimentin was increased in pcDNA-Oct4 transfected HT29 and SW480 cells (). In addition, Oct4 overexpression significantly promoted the mRNA expression of the mesenchymal markers vimentin, Twist, and Snail in HT29 and SW480 cells (). Collectively, these results demonstrate that the overexpression of Oct4 promoted proliferation, migration, invasion, and EMT of colon cancer cells.
Figure 2. Overexpression of Oct4 promoted proliferation, migration, invasion, and EMT of colon cancer cells. (a and b) Oct4 expression in HT29 and SW480 cells infected with pcDNA-NC or pcDNA-Oct4 was evaluated by Western Blot analysis. β-actin was a loading control. (c and d) the proliferation of HT29 and SW480 cells was evaluated by CCK-8 assay at 0 h, 24 h, 48 h, and 72 h after transfection. (e and f) Representative images of HT29 and SW480 cell migration following transfection with pcDNA-NC or pcDNA-Oct4. (g and h) Transwell invasion assay was performed in HT29 and SW480 cells. (i and k) Representative western blot analysis of E-cadherin and Vimentin expression in HT29 and SW480 cells. β-actin was a loading control. (j and l) Quantification of densitometries of western blot band. (m and n) E-cadherin, Vimentin, Snail, and Twist mRNA expression was assayed by RT-qPCR. Values are means ± sd, n = 3. Student’s t-test was used for statistical analysis. **P <0.01 vs pcDNA-NC.
Overexpression of Oct4 activated SCF/c-Kit signaling pathway in colon cancer cells
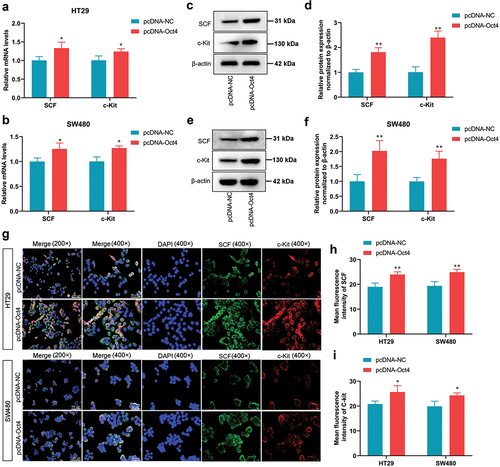
SCF/c-Kit is a critical signaling pathway that regulated cancer progression [Citation24]. Next, we wondered whether OCT4 overexpression could activate the SCF/c-Kit signaling pathway in HT29 and SW480 cells. The increases in SCF and c-Kit mRNA and protein levels were observed in pcDNA-Oct4-transfected HT29 and SW480 cells compared with control HT29 and SW480 cells (). Additionally, IF stain showed that Oct4 overexpression enhanced SCF and c-Kit expression in the cell membrane of HT29 and SW480 cells (). Taken together, these results strongly suggest that overexpression of Oct4 could activate SCF and c-Kit expression in the cell cytoplasm and cell membrane of colon cancer cells.
Figure 3. Overexpression of Oct4 activated SCF/c-Kit signaling pathway in CC cells. HT29 and SW480 cells were transfected with pcDNA-NC or pcDNA-Oct4. (A and B) the mRNA expression levels of SCF and c-Kit were tested by RT-qPCR. (C-F) the protein expression levels of SCF and c-Kit were assayed by western blot. β-actin was a loading control. (G-I) Fluorescence images show the expression of SCF and c-Kit on the membrane of colon cancer cells by immunofluorescence assay. Magnification is 200 × and 400 × .Values are means ± sd, n = 3. Student’s t-test was used for statistical analysis. *P <0.05 vs pcDNA-NC, **P <0.01 vs pcDNA-NC.
The inhibition of the SCF/c-Kit signaling pathway reversed the pro-oncogenic effects of Oct4 overexpression in colon cancer cells
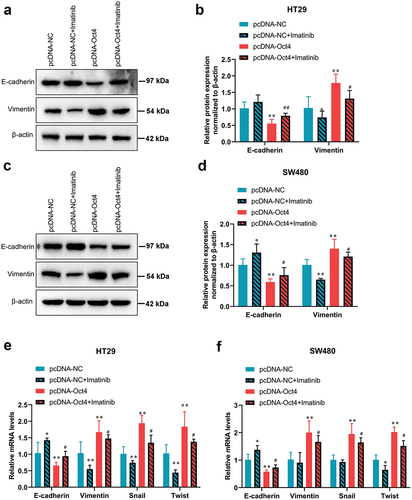
To investigate whether SCF/c-Kit mediates the pro-oncogenic effects of Oct4 overexpression, the HT29 and SW480 cells were treated with c-Kit inhibitor imatinib when pcDNA-Oct4 transfection. As shown in , the CCK-8 assay demonstrated that imatinib dose-dependently inhibited the proliferation of HT29 and SW480 cells compared with the pcDNA-Oct4 transfected groups. Therefore, cells were treated with 100 nM imatinib for 24 h for subsequent experiments. Moreover, the results of the wound healing assay manifested that the healing of the scratch was significantly reduced following imatinib co-treatment compared with the pcDNA-Oct4-transfected groups (). Meanwhile, the wound healing assay indicated that imatinib blocked the increased colon cancer cell migration capacity which was induced by Oct4 overexpression (). pcDNA-Oct4-transfected HT29 and SW480 cells displayed characteristics of EMT by displaying lower expression levels of E-cadherin and increased levels of vimentin, which were both reversed by imatinib co-treatment (). In addition, the mRNA expression of EMT-related transcription factors Snail and Twist were eliminated by imatinib co-treatment compared with the pcDNA-Oct4-transfected groups (). Taken together, our results indicated that inhibition of SCF/c-Kit signaling reversed the effects caused by Oct4 overexpression in colon cancer cells.
Figure 4. The inhibition of the SCF/c-Kit signaling pathway reversed proliferation, migration, and invasion of Oct4 overexpression in CC cells. HT29 and SW480 cells were treated with c-Kit inhibitor imatinib (0, 25, 50, and 100 nM) when pcDNA-NC or pcDNA-Oct4 transfection. (A and B) the proliferation of HT29 and SW480 cells was evaluated by a CCK-8 assay. Student’s t-test was used for statistical analysis. (C and D) Wound healing assay to determine cell migration. (E and F) Transwell analysis of cell invasion activities. Values are means ± sd, n = 3. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for statistical analysis. **P <0.01 vs pcDNA-NC, ##P <0.01 vs pcDNA-Oct4.
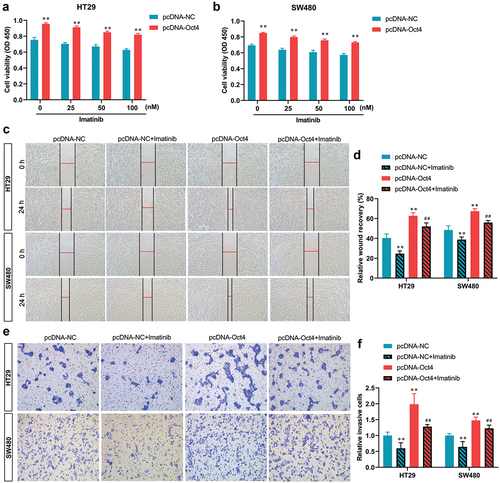
Figure 5. The inhibition of the SCF/c-Kit signaling pathway blocked the promotion of EMT due to Oct4 overexpression in colon cancer cells. HT29 and SW480 cells were treated with c-Kit inhibitor imatinib (100 nM) when pcDNA-NC or pcDNA-Oct4 transfection. (A-D)Representative western blot analysis of E-cadherin and Vimentin expression in HT29 and SW480 cells. β-actin was a loading control. (E and F) RT-qPCR analysis of E-cadherin, Vimentin, Snail, and Twist mRNA expression. Values are means ± sd, n = 3. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for statistical analysis. **P <0.05 vs pcDNA-NC, *P <0.01 vs pcDNA-NC, #P <0.05 vs pcDNA-Oct4, ##P <0.01 vs pcDNA-Oct4.
Discussion
In the present study, Oct4 was proved to up-regulate in CC samples of CC patients. Biological functional studies revealed that Oct4 overexpression promoted proliferation, migration, invasion, and EMT of CC cells by activating the SCF/c-Kit signaling pathway.
In lung cancer, Oct4 is correlated with metastasis and can be a prognostic marker for lung cancer patients. Indeed, high Oct4 expression predicted recurrence and poor outcomes in right-sided CC (RCC) patients [Citation25,Citation26]. High Oct4 expression was connected with tumor differentiation, later Dukes stage, and invasion of lymphatic in right-sided colon cancer (RCC) [Citation26]. Similarly, our data demonstrated that Oct4 was up-regulated in CC tissues of CC patients, implying that Oct4 may be a useful marker for CC identification. Moreover, Oct4 contributes to the growth and invasion of lung cancer cells through transcriptionally regulating NEAT1 and MALAT1 expression [Citation27]. Meanwhile, knockdown of OCT4 may sensitize non-small-cell lung cancer cells to cisplatin [Citation28]. Oct4 expression is correlated with Stat1 expression in lung adenocarcinoma patients and contributed to shorter overall survival [Citation29]. A recent review showed that Oct4 up-regulation was correlated with advanced clinical stage, tumor grade, lymph node metastasis, lymphatic invasion, and distal metastasis in colorectal cancer [Citation30]. Oct4 knockdown in head and neck squamous carcinoma reduced cell self-renewal capacity and led to partial tumor cell radiosensitization by regulating the homologous recombination factors PSMC3IP and RAD54 L [Citation31]. These studies indicate that Oct4 is involved in the tumorigenicity of different cancer types. In the present study, we demonstrated that Oct4 overexpression facilitated proliferation, migration, and invasion of colon cancer cells. We also found that Oct4 overexpression reduced the E-cadherin level and induced the Vimentin, Snail, and Twist levels. These results indicated that Oct4 might also facilitate EMT phenotype in CC cells, thus promoting migration and invasion. Virtually, Oct4 induced EMT-related transcription factor ZEB1 in rectal cancer cells, indicating that Oct4 is also involved in the EMT of rectal cancer [Citation32].
The SCF/c-Kit signaling pathway is proven to be oncogenic in human cancers, but its expression, regulation, and role in CC are unknown. In our study, we found that Oct4 overexpression induced SCF and c-Kit expression in CC cells. A previous study found that SCF/c-Kit interaction increased the recruitment of mast cells within the primary tumor and enhanced lung and bone metastases in breast tumor-bearing arthritic mice, implying that the SCF/c-Kit signaling pathway plays a critical role in tumor microenvironment remodeling and metastasis [Citation33]. In hepatocellular carcinoma, SCF/c-KIT and TGF-β/SMAD networks neutralized TGF-β-mediated cell cycle inhibition and induced tumor cell proliferation, EMT, migration, and invasion [Citation34]. SCF/c-kit signaling significantly promoted colorectal carcinoma progression by stimulating activator protein-1 (AP-1) binding with CLDN-3 and enhancing its transcription activity [Citation35]. In addition, SCF/c-Kit signaling enhanced the invasion of pancreatic cancer cells via the PI3K/Akt and Ras/MEK/ERK pathways and increased the expression of HIF-1α under normoxic conditions [Citation36]. We revealed that the inhibition of the SCF/c-Kit signaling pathway reversed pro-oncogenic effects of Oct4 overexpression in CC cells, including inhibition of CC cell proliferation, migration, invasion, and EMT. Further research is needed to elucidate the downstream signaling cascade of the SCF/c-Kit pathway in offsetting the CC cancer-promoting effects of Oct4. In actuality, a previous report confirmed that inhibiting SCF/c-KIT signaling by imatinib weakened mucus secretion by reducing PKCδ activity and phosphorylation level of p-MARCKS in mucinous colorectal adenocarcinoma [Citation37]. Our results offer novel insights into the role of SCF/c-KIT signaling in regulating CC progression.
Potential limitations of our study include that the function of Oct4 in CC was only evaluated in cell lines. In future studies, the effect of Oct4 and SCF/c-KIT signaling in the CC nude mouse model will be examined.
In conclusion, we demonstrated that Oct4 overexpression promoted CC cell proliferation, migration, invasion, and EMT via SCF/c-KIT signaling activation. These findings provide insight for understanding the mechanism of CC development. Oct4 might be a potential therapeutic target and prognostic marker for CC patients.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. 2018;68:7–30. DOI:10.3322/caac.21442
- Labianca R, Beretta GD, Kildani B, et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–133.
- Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732.
- Nfonsam VN, Jecius H, Chen D, et al. Increasing incidence of colon cancer in the young: assessing the tumor biology. J Am Coll Surg. 2019;229:79–90.
- Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021;22:1071–1080.
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–943.
- Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369.
- Mody K, Bekaii-Saab T. Clinical trials and progress in metastatic colon cancer. Surg Oncol Clin N Am. 2018;27:349–365.
- Freeman HJ. Early stage colon cancer. World J Gastroenterol. 2013;19:8468–8473. DOI:10.3748/wjg.v19.i46.8468
- Sohn EJ, Moon HJ, Lim JK, et al. Regulation of the protein stability and transcriptional activity of OCT4 in stem cells. Adv Biol Regul. 2021;79:100777.
- Mohiuddin IS, Wei SJ, Kang MH. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochimica et Biophysica Acta Mol Basis Dis. 2020;1866:165432.
- Lengner CJ, Welstead GG, Jaenisch R. The pluripotency regulator Oct4: a role in somatic stem cells? Cell Cycle (Georgetown, Tex). 2008;7:725–728. DOI:10.4161/cc.7.6.5573.
- Liang C, Xu Y, Ge H, et al. Clinicopathological and prognostic significance of OCT4 in patients with hepatocellular carcinoma: a meta-analysis. Onco Targets Ther. 2018;11:47–57.
- Lu CS, Shiau AL, Su BH, et al. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. 2020;13:62. DOI:10.1186/s13045-020-00887-1
- Lu H, Xie Y, Tran L, et al. Chemotherapy-Induced S100A10 recruits KDM6A to facilitate OCT4-mediated breast cancer stemness. J Clin Invest. 2020;130:4607–4623.
- Jiang WL, Zhang PF, Li GF, et al. Oct-4 is associated with gastric cancer progression and prognosis. Onco Targets Ther. 2016;9:517–522.
- Zhao X, Lu H, Sun Y, et al. Prognostic value of octamer binding transcription factor 4 for patients with solid tumors: a meta-analysis. 2020;99:e22804. DOI:10.1097/md.0000000000022804
- Chen Z, Zhang L, Zhu Q, et al. Clinical value of octamer-binding transcription factor 4 as a prognostic marker in patients with digestive system cancers: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:567–576.
- Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17:58.
- Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619–1649.
- Su B, Huang T, Jin Y, et al. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer. 2021;24:352–367.
- Annese T, Tamma R, Bozza M, et al. Autocrine/paracrine loop between SCF(+)/c-Kit(+) mast cells promotes cutaneous melanoma progression. Front Immunol. 2022;13:794974.
- Tonary AM, Macdonald EA, Faught W, et al. Lack of expression of c-KIT in ovarian cancers is associated with poor prognosis. Int J Cancer. 2000;89:242–250.
- Liang J, Wu YL, Chen BJ, et al. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435–443.
- Hu J, Li J, Yue X, et al. Expression of the cancer stem cell markers ABCG2 and OCT-4 in right-sided colon cancer predicts recurrence and poor outcomes. Oncotarget. 2017;8:28463–28470.
- Wang QH, Zhang M, Shi CT, et al. High Oct4 predicted worse prognosis of right-sided colon cancer patients. Future Oncol. 2018;14:2279–2291.
- Jen J, Tang YA, Lu YH, et al. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. 2017;16:104. DOI:10.1186/s12943-017-0674-z
- Liu X, Ma M, Duan X, et al. Knockdown of OCT4 may sensitize NSCLC cells to cisplatin. Clin Transl Oncol. 2017;19:587–592.
- Su YC, Chen YC, Tseng YL, et al. The pro-survival Oct4/Stat1/Mcl-1 axis is associated with poor prognosis in lung adenocarcinoma patients. 2021;10. DOI:10.3390/cells10102642
- Fang W, Ni M, Zhang M, et al. Prognostic value of OCT4 in colorectal cancer: analysis using immunohistochemistry and bioinformatics validation. Biomark Med. 2020;14:1473–1484.
- Nathansen J, Lukiyanchuk V, Hein L, et al. Oct4 confers stemness and radioresistance to head and neck squamous cell carcinoma by regulating the homologous recombination factors PSMC3IP and RAD54L. Oncogene. 2021;40:4214–4228.
- Shao M, Bi T, Ding W, et al. OCT4 potentiates radio-resistance and migration activity of rectal cancer cells by improving epithelial-mesenchymal transition in a ZEB1 dependent manner. 2018;3424956. DOI:10.1155/2018/3424956
- Das Roy L, Curry JM, Sahraei M, et al. Arthritis augments breast cancer metastasis: role of mast cells and SCF/c-Kit signaling. Bcr. 2013;15:R32.
- Rojas A, Zhang P, Wang Y, et al. A positive TGF-β/c-KIT feedback loop drives tumor progression in advanced primary liver cancer. Neoplasia. 2016;18:371–386.
- Wang Y, Sun T, Sun H, et al. SCF/C-Kit/JNK/AP-1 signaling pathway promotes Claudin-3 expression in colonic epithelium and colorectal carcinoma. Int J Mol Sci. 2017;18:10.3390/ijms18040765.
- Zhang M, Ma Q, Hu H, et al. Stem cell factor/c-kit signaling enhances invasion of pancreatic cancer cells via HIF-1α under normoxic condition. Cancer Lett. 2011;303:108–117.
- Li G, Yang S, Shen P, et al. Scf/c-KIT signaling promotes mucus secretion of colonic goblet cells and development of mucinous colorectal adenocarcinoma. Am J Cancer Res. 2018;8:1064–1073.
