ABSTRACT
Gastric cancer (GC) still poses a significant threat to human life. Hence, there is an urgent need to understand the mechanism of GC progression and develop novel therapeutics approach to treating GC. This study was conducted to evaluate the role of the lncRNA SNHG22 in the progression of GC. First, GC data from TCGA were analyzed using GEPIA. After the starbase database was used to predict SNHG22 target miRNA and miR-101-3p target mRNA. The predictions were validated using a dual-luciferase reporter assay, biotinylated RNA pull-down assay, and RIP-qRT-PCR. The relative expression of SNHG22, miR-101-3p, and E2F2 was measured by qRT-PCR and western blot (WB) analysis, while the mechanism of GC cell proliferation was elucidated through the colony formation and CCK-8 assay. Our result showed that SNHG22 was upregulated significantly in GC tissue samples from TCGA database, GC cell lines, and clinical tissue samples, and its expression was related to low survival rate of gastric cancer patients. Bioinformatics prediction predicted miR-101-3p as the potential target of SNHG22 and E2F2 genes as miR-101-3p target mRNA. We found that E2F2 expression was negatively associated with overall survival of GC patients. Functional study showed that silencing SNHG22 markedly inhibited the proliferation, migration, and invasion of GC cells as well as in vivo tumor growth. This was reversed after inhibiting miR-101-3p or overexpressing E2F2. The lncRNA SNHG22 promotes the proliferation, migration, and invasion of GC cells via the miR-101-3p/E2F2 axis. SNHG22 might be a potential prognostic indicator in gastric cancer.
KEYWORDS:
Introduction
Globally, gastric cancer is one of the most commons form of cancers [Citation1]. It is the third and fifth deadliest cancer from among males and females, respectively, and the most common gastrointestinal malignancy in Latin America and East Asia [Citation2]. Though GC incidence is decreasing, population growth and aging contributed to the increasing number of cases in 2015 [Citation3]. Conversely, in China, GC still represents a disease of exceptional mortality and morbidity, with several individuals being recognized for having an advanced-stage tumor with a poor prognosis and an average 5-year survival rate of not more than 20%. This is mostly because the early stages of GC remain clinically undetected, and most cases are performed at a late stage [Citation4,Citation5]. Moreover, only a few countries, like Japan, have an extensive program in place for early detection of GC. And a 90% 5-year survival rate is predicted if the tumor is detected and treated before invading the muscular layer of the stomach [Citation6]. Early GC detection also aids a decrease in mortality rate prior metastasis [Citation7]. Apart from the late detection of most GC cases and its significantly poor prognosis, most therapeutic plans are restricted to either chemotherapy or radiotherapy; with this, GC is still related to a high rate of mortality [Citation8]. Hence, to better understand cancer progression and develop novel therapeutics, high consideration has been given to the detection of putative genes as well as regulatory mechanisms that might be involved in the cell metastasis [Citation7]. As a result, detecting novel and specific GC biomarkers are gradually becoming essential.
About 2% of the human genome codes for proteins, while the remaining which comprises the non-coding RNAs (ncRNAs), play a central role in several cellular and physiological functions [Citation9]. Increasing evidence shows that ncRNAs deregulation is implicated in a diversity of human cancers, such as GC [Citation10–12]. Additionally, lncRNAs are newly emerging type of ncRNAs having a significant role in an array of biological functions [Citation13]. Several evidences have proved that lncRNAs control GC progressions such as proliferation, migration, invasion, metastasis, apoptosis, and other biological activities [Citation14] and may be related to specific signaling pathways; and their expression shows the occurrence of an active signaling event [Citation15,Citation16]. Typically, these molecules regulate tumor development by targeting specific genes in diverse signaling pathways [Citation17]. However, to date, there is still no report on the role of the lncRNA SNHG22 in GC progression.
In the present study, we evaluated the role of lncRNA SNHG22 in the migration, invasion, and proliferation of GC cells and its probable mechanism of regulation. The connection between abnormally expressed SNHG22 and clinicopathologic factors of GC individuals was also evaluated. Our result revealed that SNHG22 expression enhanced the migration, invasion, and proliferation of GC cells and SNHG22 might be a useful tool for effective and early diagnosis of GC and also a therapeutic target for its treatment.
Materials and methods
The Cancer Genome Atlas (TCGA) data set analysis and gastric cancer tissue sample collection
Gastric cancer datasets obtained from TCGA database (http://tcga-data.nci.nih.gov/tcga) were tested for differential expression using GEPIA (http://gepia.cancer-pku.cn/). Overall, 56 pairs GC clinical samples and normal tissues were collected after surgical resection. Immediately, samples collected were kept in freezing liquid nitrogen for further analysis and examination. The ethics committee of the Yantai Yuhuangding Hospital approved this study and all participants consented to this according to the declaration of Helsinki. The clinical features of the GC patients are presented in .
Table 1. The clinical characteristics of the studied gastric cancer patients.
RNA extraction
Trizol reagent (Invitrogen, Carlsbad, CA, USA) was utilized to extract the total RNA according to the manufacturer’s guidelines. RNA clean-up comprising a DNase I digestion step needed to be done using RNeasy spin columns (Qiagen). The integrity of the RNA was determined with a NanoDrop spectrophotometer (Thermo Fisher) together with the standard denatured agarose gel-electrophoresis. The RNAs were kept until further use at −80°C.
Cell culture and transient transfection
GC cell lines (AGS, HGC27, MKN-45, MKN74) and normal gastric epithelial cell-line GES-1 were acquired from the Digestive Disease Laboratory of Shanghai Sixth People’s Hospital. The cells were grown in DMEM medium enhanced with 100 μg/ml of streptomycin (HyClone), 100 U/ml of penicillin, 10% heat-inactivated FBS. Cells in this medium were maintained in a humidified atmosphere at 37°C with 5% CO2. To make SNHG22 stable overexpression vector, the SNHG22 sequence was cloned in a pcDNA 3.1 (pcDNA-SNHG22) vector (Invitrogen, Carlsbad, USA). MiR-101-3p mimics, miR-NC, short hairpin RNA targeting SNHG22 (sh-SNHG22), scramble shRNA (sh-NC) was produced by RiboBio (Shanghai, China). The cells were cultured in 6-well plates and then transfected with sh-SNHG22, miR-101-3p inhibitor, miR-101-3p mimics, pcDNA-SNHG22, or pcDNA-E2F2 through Lipofectamine 2000 (Invitrogen, USA). After 48 h of transfection, the cells were harvested and employed to further experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Reverse transcription was done with a Reverse Transcription kit (Promega) following the producer’s protocols. The qRT-PCR was done with SYBR Green Mix kit (Promega) in a Step One Real-time PCR System (ABI, USA). The PCR conditions and stages: stage 1 for 10 min at 95°C; stage 2, 40 cycles for 15 s at 95°C, and 57°C for 1 min; stage 3, for 15 s at 95°C, 60°C, and 95°C each. GAPDH and U6 were employed as an internal reference gene to standardize the relative expression of target genes using the 2−ΔΔCT method. Also, nuclear and cytosolic fractions of the AGS and HGC27 cells were isolated using the PARISTM Kit (Thermo Fisher) in accordance with the manufacturer’s guide. Then, the relative nuclear and cytoplasmic expression of SNHG22 in AGS and HGC27 cells was detected using qRT-PCR.
Cell proliferation assay
AGS and HGC27 cell lines with identified manipulation of gene-expression were seeded (4 × 106/well) into 96-well plates. After transfecting with oligonucleotides and culturing for 24 h, the viability of the cell was evaluated with the Cell Counting Kit (CCK)-8 (Dojindo Molecular Technologies, Japan) in accordance with the producer’s protocol. Concisely, cells were incubated with the CCK-8 reagent for 2 h at 37°C. The viability of the cell was quantified by assessing its absorbance at 450 nm.
Colony formation assay
AGS and HGC27 cells were trypsinized and then seeded (1 × 104 /well) into 6-well plates, transfected with sh-NC, sh-SNHG22, miR-101-3p inhibitor, or pcDNA-E2F2, and incubated for 7 days at 37°C. A dyeing solution comprising of 0.1% crystal violet and 20% methanol was applied to the colonies. After, the colonies were counted and analyzed.
Transwell assay
GC cell invasion and migration capabilities were assessed using Transwell (Corning, USA) coated with or without Matrigel (Clontech, CA). Briefly, AGS and HGC-27 cells were transfected with sh-Negative control, sh-SNHG22#1, SNGH22#2, miR-101-3p inhibitor, and pcDNA-E2F2. Then, the transfected cells were obtained and re-suspended in a serum-free RPMI-1640 medium. The cells (1 × 104) were subsequently seeded into the upper 24-well chambers and then RPMI1640 medium comprising of 20% FBS was added to the lower chambers as a chemoattractant. After treatment, the residual cells attached to the upper chamber were scraped with cotton swabs; while cells in the lower chamber were set for 15 min in 4% formaldehyde solution and stained with crystal violet (0.2%) for 5 min. Migrated and invasive cells were visualized and acquired with an inverted microscope (200X magnification).
Flow cytometric analysis
To investigate cell apoptosis, flow cytometric assay was carried out according to the previously reported procedures [Citation18]. Briefly, GC cells were harvested and washed three times with PBS and re-suspended cells in binding buffer to fix them. Subsequently, incubated the cells in darkness with 5 µL Annexin V-FITC and PI at room temperature for 15 min. Flow cytometry was performed using FACSCantoII flow cytometer (BD, USA), and FlowJo 10.0 software was used to determine the percent of apoptotic cells.
In vivo assays for tumor growth
To examine in vivo tumor growth, a xenograft model was built using 4 to 6-week-old athymic C57 nude male mice acquired from the Shanghai Laboratory Animal Center of China (Shanghai, China). A total of 4 × 106 suspended cells transfected with sh-SNHG22#1 or sh-NC were inoculated subcutaneously into the left-flank area of each mouse. Tumor growth was evaluated and volumes were calculated (0.5 × length × width) every 7 days. Being the inoculation, the mice were sacrificed, and individual tumors were excised, and the weight measured. The animal experiment was approved by Yantai Yuhuangding Hospital's ethical review board.
Western blot analysis
AGS and HGC-27 cells were obtained and lysed with lysis buffer (100 mM Tris-HCl, 25% glycerol, 1 Mm Mercaptoethanol, 2% SDS). Extracts of the cell were boiled in a loading buffer and separated on 15% SDS-PAGE gels. After, separated protein bands were transferred into PVDF membranes. The antibodies, E2F2 (ab138515, Abcam, USA), Bcl-2 (ab32124, Abcam, USA), cleaved-caspase-3 (ab32042, Abcam, USA) and anti-GAPDH (ab153802, Abcam, USA) were diluted at a proportion of 1:1000 and kept at 4°C overnight following the instructions. Secondary antibodies (Horseradish peroxidase, ab7090, Abcam, USA) were added and kept for 1 h at room temperature. The PVDF membranes were then washed using PBS three times and the immunoreactive bands were visualized with ECL-PLUS/Kit (GE Healthcare, USA) following the manufacturer’s instruction.
Target gene prediction
We predicted the lncRNA SNHG22 target miRNA with StarBase v2.0 (http://starbase.sysu.edu.cn) and also predicted the target mRNA (gene) of the miRNA using the same StarBase v2.0.
Dual-Luciferase reporter assay
To perform dual-luciferase report assay, we inserted the wild-type (WT) and mutant-type (MUT) sequences of SNHG22 and E2F2 into the downstream of the luciferase gene in pGL3-Luciferase reporter vector (Promega) and co-transfected with the miR-101-3p mimics and miR-NC into AGS and HGC27 cells followed by incubation for 48 h. Following the manufacturer’s instruction, the firefly luciferase activity was determined by the dual-luciferase assay system (Promega) with Renilla activity to standardize.
Biotinylated RNA pull-down assay
The RNA precipitation evaluation was done to determine the coupling capable of SNHG22 with the RISC complex, with synthesized SNHG22 as a probe, and the precipitation complex, AGO2 was detected using western blot and miR 101-3p with qRT-PCR. In vitro, the biotin-labeled lncRNA-SNHG22 RNA was transcribed using Biotin RNA Labeling Mix (Roche) and T7 RNA polymerase (Roche), and treated with RNase-free DNase I (Roche), with QIA quick Nucleotide Removal Kit (Qiagen) for recycling and with the RNeasy Mini Kit (Qiagen) for purification. SNHG22 was cloned at the NC and utilized for comparison in the precipitation experiments. Biotin-labeled lncRNA-SNHG22 RNAs were mixed with HGC27 and AGS proteins and incubated at 4°C for 1 h. With each binding reaction, streptomycin agarose beads (Invitrogen) were added and incubated for 1 h at room temperature. The western blot was done to detect AGO2, while the two groups of precipitates were employed for detecting miR-101-3p and miR-218-5p expression by qRT-PCR following the standard procedures.
RNA immunoprecipitation (RIP)
To further confirm if SNHG22 can successfully bind to miR-101-3p through Ago2, RNA immunoprecipitation was performed. RIP assay needed to be done using Magna RIP RNA Binding Protein Immunoprecipitation Kit (Millipore) in accordance with the manufacturer’s protocol. RIP assays antibodies against IgG and AGO2 were obtained from Abcam (ab5072, Rabbit polyclonal antibody, USA).
Statistical analysis
All experiments in this study were performed in triplicates. All data were analyzed statistically with SPSS 18.0 software and expressed as mean ± S.D. Also, all comparisons among three or more groups were analyzed using one-way ANOVA and then Tukey postdoc test. Whereas student’s t-test was utilized to calculate the differences between the two groups. The relationship between SNHG22 expression and clinicopathological data of GC was analyzed with Chi-square test and the p-value <0.05 was considered significant statistically for the study. The survival curve was drawn using Kaplan – Meier method and curve differences were measured by the log-rank test. The independent prognostic factors were detected through the univariate and multivariate Cox regression analyses.
Results
SNHG22 is overexpressed in gastric cancer tissue samples and associated with poor survival ability of patients
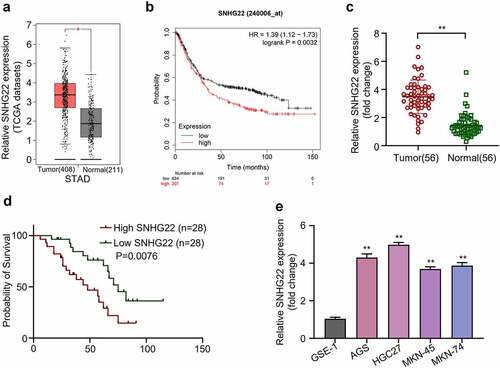
In the beginning of this study, we carried out differential expression analysis of SNHG22 in a gastric cancer dataset that is publicly available on the TCGA (The Cancer Genome Atlas) website, using GEPIA. As shown in , SNHG22 was significantly overexpressed in gastric cancer tissues compared to the normal ones (p < 0.05). Using KM-plotter (http://kmplot.com/analysis/index.php?p=service) we also concluded that the high expression level of SNHG22 in GC cases was significantly associated with the poor overall survival rate of GC patients ( p = 0.0032). We further performed qRT-PCR analysis on collected GC clinical samples which showed that SNHG22 expression level was significantly upregulated in GC tissues as compared to the adjacent normal ones, implying that the SNHG22 might be actively involved in the progression of GC in patients ( p < 0.01). To determine the correlation between the SNHG22 expression and the overall prognosis of GC patients, we used the median expression value of SNHG22 in GC clinical samples as the cutoff value. Fifty-six GC clinical samples were divided into the high- and low-expression groups (n = 28 each). Afterward, Kaplan–Meier (KM) method was utilized to plot the survival curves for the groups using the log-rank t-test. As shown in , GC patients with high SNHG22 expression level had a significantly worse overall survival rate as compared to those with low SNHG22 expression level (p = 0.0076). The expression level of SNHG22 in GC was subsequently measured in GC cell lines (AGS, HGC27, MKN-45, and MKN74) and normal gastric epithelial cell line (GSE-1) using qRT-PCR. The result showed that the SNHG22 was significantly upregulated in GC cell lines when compared to the gastric epithelial cells ( p < 0.01). Further, we found that the AGS and HGC27 cell lines had the highest SNHG22 expression level and were therefore used in subsequent experiments. displays the relationship between SNHG22 expression and clinicopathological data of GC. The result showed that the expression level of SNHG22 significantly correlated with the histological grade (p = 0.007), lymph node metastasis (p = 0.032), and distant metastasis (p = 0.029) but not with other clinical factors in GC patients. Together, these results suggested that the SNHG22 might be an oncogenic lncRNA involved in GC progression.
Figure 1. The expression level of SNHG22 is associated with poor survival ability of patients. (a) TCGA gastric cancer tissue sample data were analyzed using GEPIA. (b) KM-plotter analysis was utilized to evaluate the relationship between SNHG22 expression in gastric cancer cases and the patient’s overall survival rate. (c) Relative SNHG22 expression in collected clinical samples was detected by qRT-PCR analysis. (d) Kaplan – Meier survival curve analysis of the high- and low- SNHG22 -expression groups were evaluated. (e) Relative SNHG22 expression in gastric cell lines and normal gastric epithelial cell line (GSE-1) was detected by qRT-PCR analysis. All experiments were done in triplicates and experimental data are presented as the mean ± SD with a statistical significance level of P < 0.05 (**P < 0.01).
SNHG22 knockdown inhibits cell proliferation, induces apoptosis, and reduces in vivo tumor growth in gastric cancer
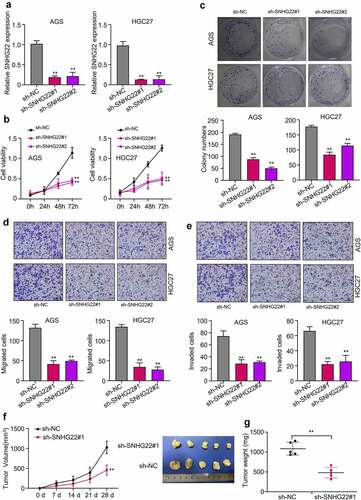
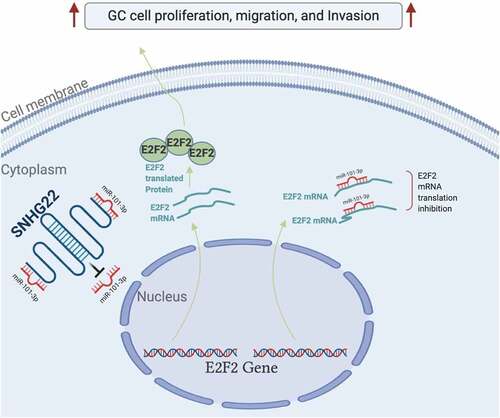
To determine the biological function of SNHG22 in GC, we knockdown SNHG22 in the AGS and HGC27 cell lines using two different shRNAs (sh-SNHG22#1, sh-SNHG22#2). After, the relative expression of SNHG22 needed to be measured using qRT-PCR. As shown in , the two sh-SNHG22 significantly inhibited the expression of SNHG22 in the AGS and HGC27 cell lines compared to the sh-NC negative control group (p < 0.01). Furthermore, CCK-8 result showed that silencing SNHG22 significantly reduced the viability of GC cell lines in a time-dependent manner (0 h, 24 h, 48 h, and 72 h after transfection) (, p < 0.01). We also found that the number of colonies formed in the GC cell lines was significantly reduced by sh-SNHG22#1 and sh-SNHG22#2 compared to the sh-NC (, p < 0.01). We further examined the biological consequence of silencing SNHG22 on the GC cell migration and invasion ability using transwell assay and found that silencing SNHG22 significantly decreased the migration and invasion ability of the AGS and HGC27 cell lines ( p < 0.01). Besides, we found that silencing SNHG22 significantly reduced tumor growth (tumor volume after 28 days of transfection) ( p < 0.01). Altogether, our data demonstrated that silencing SNHG22 could markedly inhibit GC cells proliferation, migration, and invasion, and also suppress in vivo tumor growth.
Figure 2. Silencing SNHG22 inhibits cell proliferation, induces apoptosis, and reduces in vivo tumor growth in gastric cancer (a) qRT-PCR analysis was used to detected the relative SNHG22 expression after the knockdown. (b) Measurement of cell viability of gastric cancer cell lines after SNHG22 knockdown using CCK8 assay. (c) Colony formation experiment was used to evaluate the proliferation of the GC cells after SNHG22 knockdown. (d) and (e) Detection of gastric cancer cell migration and invasion ability after SNHG22 knockdown using transwell assay. (f) and (g) In vivo tumor growth experiment was used to evaluate tumor growth ability after silencing SNHG22. Experimental data are presented as mean ± SD of three different experiments with a significance level defined as P < 0.05 (**P < 0.01).
SNHG22 targets miR-101-3p
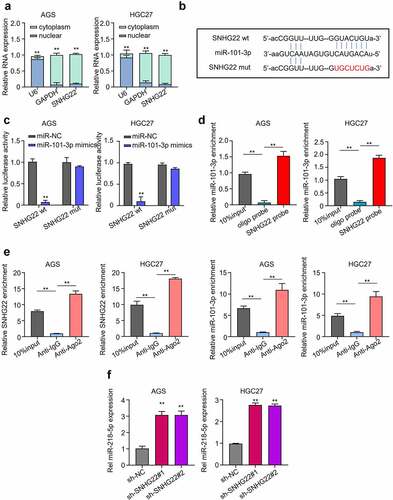
First, we determined the subcellular localization of SNHG22 in the AGS and HGC27 cell lines. Then, the relative expression of SNHG22 in each subcellular fraction collected needed to be measured using qRT-PCR. shows that the expression level of SNHG22 was significantly higher in the cytoplasm of GC cell lines compared to the nuclear, suggesting that SNHG22 is more likely to interact with cytoplasmic miRNAs in GC cells (p < 0.01). Utilizing the Starbase database, we predicted miR-101-3p as SNHG22 regulatory target miRNA and confirmed this through a Luciferase reporter assay (). Luciferase assay result showed that miR-101-3p mimics can significantly inhibit the luciferase activity of AGS and HGC27 cells co-transfected with wild type (WT) in SNHG22 plasmid, compared to the miR-NC. And could, however, not inhibit luciferase activity of the AGS and HGC27 cells that were co-transfected with the mutant type (Mut) SNHG22 plasmid (, p < 0.01). We further confirmed this lncRNA-miRNA binding ability through a biotinylated pull-down assay and RIP-qRT-PCR analysis. Our result showed that the SNHG22 probe could significantly pull down more miR-101-3p than the control Oligo probe group ( p < 0.01). Furthermore, we found that more SNHG22 and miR-101-3p were significantly enriched in the Anti-Ago2 group compared to the control Anti-IgG group, indicating that SNHG22 can directly interact with miR-101-3p in the AGS and HGC27 cell lines through the Ago2 protein of the RISC (RNA-induced silencing) complex ( p < 0.01). Besides, we found that the miR-101-3p was quite highly expressed after SNHG22 knockdown, suggesting that SNHG22 upregulation could inhibit the miR-101-3p expression ( p < 0.01).
Figure 3. SNHG22 sponges miR-101-3p. (a) qRT-PCR was used to detection of the subcellular location of SNHG22 in gastric cancer cell lines. (B) Starbase prediction of SNHG22 target miRNA. (C) Dual-luciferase reporter assay was utilized to confirm SNHG22-miR-101-3p binding ability. (D) Biotinylated RNA pull-down assay was performed to verify the relationship of SNHG22 and miR-101-3p. (E) RIP-qRT-PCR analysis Was used to verify the relationship of SNHG22 and miR-101-3p. (F) qRT-PCR analysis was performed to detected the expression of miR-101-3p in gastric cancer cell lines after SNHG22 knockdown. The experimental data are presented as the mean ± SD of at least three independent experiments. The significance level is defined as P <0.05 (**P < 0.01).
miR-101-3p directly targets E2F2
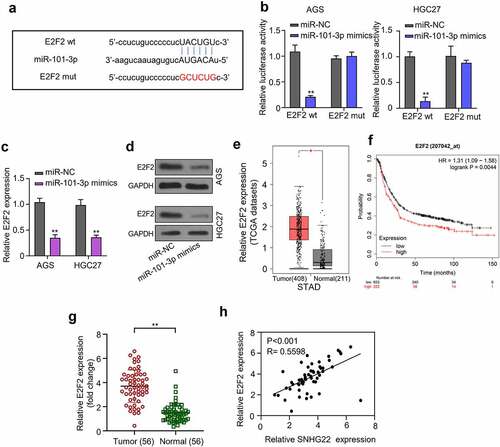
We also predicted miR-101-3p target mRNA using the starbase database. The prediction result revealed that the 3‘UTR of E2F2 harbors a binding site sequence for miR-101-3p seed region (). This result was also validated through a Luciferase reporter assay. Result showed that miR-101-3p mimics significantly inhibited the luciferase activity of AGS and HGC27 cells that were co-transfected with the wild type (WT) E2F2 plasmid but could not inhibit the luciferase activity of the cells after mutating the miR-101-3p putative binding site in the mutant type (Mut) E2F2 plasmid, which was co-transfected into the cell (, p < 0.01). Besides, qRT-PCR and Western blot analysis result revealed that E2F2 mRNA and protein expression was significantly downregulated in the AGS and HGC27 cells after transfecting with miR-101-3p mimics, compared to the miR-NC control group, suggesting that the overexpression of miR-101-3p can significantly repress E2F2 expression in GC cells ( p < 0.01). Additionally, using GEPIA, we analyzed E2F2 expression level in GC tumor samples from the TCGA database and found that the E2F2 was significantly upregulated in the GC tumor samples when compared to the normal ones ( p < 0.05). We also determined the relationship between the E2F2 expression level in GC samples and patient’s prognosis using the KM-plotter and found that high E2F2 expression predicted a poor overall prognosis of gastric cancer patients ( p = 0.0044). Furthermore, the E2F2 expression was significantly upregulated in GC tissues compared to the normal ones and there was a positive relationship between SNHG22 and E2F2, which expressed in GC samples (, H p < 0.01).
Figure 4. miR-101-3p directly targets E2F2. (a) Bioinformatics was used to predicted the miR-101-3p target gene. (b) Dual-luciferase reporter assay experiment was performed to confirmed the prediction. (c) qRT-PCR analysis was utilized to detect the expression of E2F2 mRNA in gastric cancer cell lines after transfection with miR-101-3p mimics. (d) Western blot analysis was utilized to detect the protein expression of E2F2 after transfection with miR-101-3p mimics. (e) GEPIA analysis was used to evaluate the E2F2 expression level in gastric cancer tumor samples from the TCGA database. (f) the relationship between E2F2 expression level in gastric cancer tissue samples and patient’s overall survival probability was evaluate by KM-plotter analysis. (g) Relative E2F2 expression in collected clinical samples was detected by qRT-PCR analysis. (h) the expression of SNHG22 was positively correlated with E2F2 in GC clinical samples. The experimental data are shown as mean ± SD of three independent experiments. The significance level is defined as P <0.05 (**P < 0.01).
SNHG22 regulates gastric cancer cell progression via the miR-101-3p/e2f2 axis
Finally, to ascertain whether SNHG22 regulates GC cell progression through the miR-101-3p/E2F2, we knockdown SNHG22 and co-transfected the GC cell lines with miR-101-3p inhibitor or pcDNA-E2F2. The functional effect of this on the proliferation, migration, and invasion of GC cells was evaluated through CCK-8 assay, and transwell assay. Western blotting analysis revealed that silencing SNHG22 inhibited E2F2 protein expression in the AGS and HGC27 cell lines. However, inhibiting miR-101-3p and overexpressing E2F2 in the SNHG22-knockdown cells significantly restored the E2F2 expression (). Furthermore, CCK-8 and colony formation assay result showed that SNHG22 knockdown significantly reduced GC cell proliferation while co-transfecting the cells with miR-101-3p inhibitor or pcDNA-E2F2 significantly restored the proliferative ability of the cells ( p < 0.01). Using a transwell assay, we found that silencing SNHG22 significantly inhibited the migration and invasion of the AGS and HGC27 cell lines which were restored after co-transfecting the cell with miR-101-3p inhibitor or pcDNA-E2F2 ( p < 0.01). Altogether, this data shows that SNHG22 substantially promote the proliferation, migration, and invasion of GC cell lines by regulating the miR-101-3p/E2F2 axis. A schematic representation of the lncRNA SNHG22 mechanism of regulation of GC cell progression is presented in .
Figure 5. SNHG22 regulates gastric cancer cell progression via the miR-101-3p/e2f2 axis. (a) Western blot analysis was performed the protein expression of E2F2 in gastric cancer cell lines after transfection. (b) CCK-8 assay was utilized to detect the cell viability after transfection. (c) Colony formation assay was performed the proliferation of gastric cancer cell lines transfected with miR-101-3p inhibitor or E2F2 overexpression. (d) and (e) Gastric cancer cell line migration and invasion ability were evaluated by Transwell assay. Data are presented as the mean ± SD of at least three independent experiments. The significance level is defined as P <0.05. (**P <0.01 vs sh-NC; ^^P <0.01 vs sh-SNHG22#1).
Figure 6. A schematic representation of the lncRNA SNHG22 mechanism of gastric cancer cell regulation was showed in the current study.
Furthermore, SNHG22 knockdown resulted in a significant increase in the rate of apoptosis in GC cell lines than the control. In contrast, miR-101-3p inhibition or E2F2 overexpression significantly reduced the apoptosis effect of SNHG22 knockdown in these cells (FigureS1A). In addition, western blotting analysis was utilized to detect the expression of apoptosis-related proteins (Bcl-2 and cleaved-caspase-3), the results revealed that SNHG22 knockdown markedly reduced Bcl-2 protein expression while induced cleaved-caspase-3 expression. In contrast, miR-101-3p inhibition or E2F2 overexpression significantly reverse this effect of SNHG22 knockdown in these cells (FigureS1B).
Discussion
The survival rate of gastric cancer remains very low across the globe and although the incidence rate is decreasing, continuous population growth and aging remain a major factor in the rising number of new cases in the year 2015 [Citation3]. Therefore, understanding the molecular mechanism of GC pathophysiology, and identifying effective biomarkers for early diagnosis, prognosis, and treatment of GC is of the greatest importance to medical researchers. Many studies have reported that SNHG22 plays important role and can be a potential treatment strategy in cancer progression [Citation19,Citation20]. For instance, SNHG22 sponges miR‑128‑3p to promote the progression of colorectal cancer by upregulating E2F3 [Citation21], SNHG22 induces cell migration, invasion, and angiogenesis of gastric cancer cells via miR-361-3p/HMGA1/Wnt/β-catenin axis [Citation22]. Similarly, Zhang et al reported that the overexpression of SNHG22 in epithelial ovarian carcinoma (EOC) induced chemotherapy resistance in EOC cells by regulating the miR-2467/Gal-1 signaling pathway and was associated with the overall poor prognosis of patients diagnosed with epithelial ovarian carcinoma [Citation23]. Based on these studies, in our study, we examined the differential expression of SNHG22 in the GC data from the TCGA database using GEPIA. Similarly, the expression of SNHG22 in GC clinical samples was comparable to that of adjacent normal tissues. Both results indicated that SNHG22 is significantly overexpressed in GC tissue samples and significantly associated with the poor prognosis of GC patients. This study for the first time determined the role of SNHG22 in the tumorigenesis and progression of GC.
Generally, ncRNA, including lncRNA, is deemed to serve as ceRNA (competing endogenous RNA), regulating the activities of miRNA to form a lncRNA-miRNA-mRNA signal axis [Citation24–26]. To understand this regulatory mechanism of the SNHG22 in our study, a bioinformatics prediction was conducted which predicted that SNHG22 directly targets miR-101-3p. Dual-luciferase reporter assay, biotinylated RNA pull-down assay, and RIP-qRT-PCR assay were used to verify the prediction. It has been reported that miR-101-3p played a negative role in tumorigenesis and progression, including lung adenocarcinoma [Citation27], thyroid cancer [Citation28], and other cancers [Citation29]. For example, miR-101-3p has been previously turned to promote autophagy in endometrial carcinoma cells by inhibiting the expression of the EZH2 gene [Citation30]. In addition, miR-101-3p can inhibit EMT to attenuate proliferation and metastasis in glioblastoma by targeting TRIM44 [Citation31]. Besides, circulating miR-101-3p, together with miR-106b-3p, and miR-1246, has been recognized as a diagnostic biomarker of hepatocellular carcinoma [Citation32]. In particular, serum miR-101-3p combined with pepsinogen contributes to the early diagnosis of GC [Citation33]. Many evidences confirm that miR-101-3p can regulate cancer through the mechanism of miRNA-mRNA signal axis [Citation34,Citation35]. In our study, we confirmed that the miR-101-3p directly targets the E2F2 gene in the GC cell. The E2F2 is a transcription factor gene whose aberrant expression has been implicated in the progression of many cancers and is reportedly associated with poor prognosis in various cancers [Citation36,Citation37]. For instance, miR-638 represses the stem cell characteristics of breast cancer cells by targeting E2F2 [Citation38]. H19 rises in GC and exerts a tumor-promoting function via miR-138/E2F2 axis [Citation39]. In the present study, the functional study indicated that silencing SNHG22 significantly upregulated miR-101-3p expression, repressed E2F2 mRNA and protein expression, and reduced the proliferation, migration, and invasion but induced apoptosis of GC cells, suggesting that SNHG22 could be a potential therapeutic target for treating GC in patients. Notably, we found that miR-101-3p inhibition significantly upregulated E2F2 expression, significantly increasing the proliferation, migration, and invasion but induced apoptosis of GC cells. The overexpression of E2F2 was also found to be associated with the poor prognosis of GC patients. Altogether, our data indicated that the SNHG22 is an oncogenic lncRNA that acts as ceRNA to sponge miR-101-3p and upregulate E2F2 expression. Thus, increasing the proliferation, migration, and invasion of GC cells. SNHG22 could be a promising prognostic tool or therapeutic target for treating GC.
Conclusion
In conclusion, the above results indicate that SNHG22 promotes the proliferation, migration, and invasion of gastric cancer cells by regulating the miR-101-3p/E2F2 axis. SNHG22 might be a potential prognostic indicator or therapeutic target for gastric cancer treatment.
Ethics approval and consent to participate
Overall, 56 pairs GC clinical samples and normal tissues were collected after surgical resection. Immediately, samples collected were kept in frozen liquid nitrogen for further analysis and examination. The ethics committee of the Yantai Yuhuangding Hospital approved this study and all participants consented to this according to the declaration of Helsinki.
Availability of data and materials
The data underlying this article are available in the article. If needed, please contact with the corresponding author. The e-mail address is [email protected].
Supplemental Material
Download TIFF Image (3.7 MB)Acknowledgement
We thank all the reviewers in this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2119515
Additional information
Funding
References
- Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29(6):895–905.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
- Kim YI, Kim YW, Choi IJ, et al. Long-Term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy. 2015;47(4):293–301.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19(1):198–205.
- Miyahara R, Niwa Y, Matsuura T, et al. Prevalence and prognosis of gastric cancer detected by screening in a large Japanese population: data from a single institute over 30 years. J Gastroenterol Hepatol. 2007;22(9):1435–1442.
- Song W, Liu YY, Peng JJ, et al. Identification of differentially expressed signatures of long non-coding RNAs associated with different metastatic potentials in gastric cancer. J Gastroenterol. 2016;51(2):119–129.
- Liu X, Meltzer SJ. Gastric cancer in the era of precision medicine. Cell Mol Gastroenterol Hepatol. 2017;3(3):348–358.
- Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981.
- Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(17):3850–3856.
- Tang W, Fu K, Sun H, et al. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer. 2018;17(1):137.
- Hu S, Zheng Q, Wu H, et al. miR-532 promoted gastric cancer migration and invasion by targeting NKD1. Life Sci. 2017;177:15–19.
- Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14(1):42–54.
- Yu H, Rong L. Emerging role of long non-coding RNA in the development of gastric cancer. World J Gastrointest Oncol. 2018;10(9):260–270.
- Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. DOI:10.1038/nature08975
- Majem B, Rigau M, Reventos J, et al. Non-Coding RNAs in saliva: emerging biomarkers for molecular diagnostics. Int J Mol Sci. 2015;16(4):8676–8698.
- Yang ZG, Gao L, Guo XB, et al. Roles of long non-coding RNAs in gastric cancer metastasis. World J Gastroenterol. 2015;21(17):5220–5230.
- Zhu X, Li Z, Li T, et al. Osthole inhibits the PI3K/AKT signaling pathway via activation of PTEN and induces cell cycle arrest and apoptosis in esophageal squamous cell carcinoma. Biomed Pharmacother. 2018;102:502–509.
- Li ZW, Zhang TY, Yue GJ, et al. Small nucleolar RNA host gene 22 (SNHG22) promotes the progression of esophageal squamous cell carcinoma by miR-429/SESN3 axis. Ann Transl Med. 2020;8(16):1007.
- Fang X, Zhang J, Li C, et al. Long non-coding RNA SNHG22 facilitates the malignant phenotypes in triple-negative breast cancer via sponging miR-324-3p and upregulating SUDS3. Cancer Cell Int. 2020;20(1):252.
- Yao J, Wang C, Dong X, et al. lncRNA SNHG22 sponges miR1283p to promote the progression of colorectal cancer by upregulating E2F3. Int J Oncol. 2021;59(3). DOI:10.3892/ijo.2021.5251
- Cui X, Zhang H, Chen T, et al. Long noncoding RNA SNHG22 induces cell migration, invasion, and angiogenesis of gastric cancer cells via microRNA-361-3p/hmga1/wnt/beta-catenin axis. Cancer Manag Res. 2020;12:12867–12883.
- Zhang PF, Wu J, Luo JH, et al. SNHG22 overexpression indicates poor prognosis and induces chemotherapy resistance via the miR-2467/gal-1 signaling pathway in epithelial ovarian carcinoma. Aging (Albany NY). 2019;11(19):8204–8216.
- Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358.
- Zhang J, Lou W. A key mRNA-miRNA-lncRNA competing endogenous RNA triple sub-network linked to diagnosis and prognosis of hepatocellular carcinoma. Front Oncol. 2020;10:340.
- Zhao Y, Yu Z, Ma R, et al. lncRNA-Xist/mir-101-3p/klf6/c/ebpalpha axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol Ther Nucleic Acids. 2021;23:536–551.
- Meng X, Sun Y, Liu S, et al. miR-101-3p sensitizes lung adenocarcinoma cells to irradiation via targeting BIRC5. Oncol Lett. 2021;21(4):282.
- Du YL, Liang Y, Cao Y, et al. LncRNA XIST promotes migration and invasion of papillary thyroid cancer cell by modulating MiR-101-3p/cldn1 axis. Biochem Genet. 2021;59(2):437–452.
- Gu Z, You Z, Yang Y, et al. Inhibition of MicroRNA miR-101-3p on prostate cancer progression by regulating cullin 4B (CUL4B) and PI3K/AKT/mTOR signaling pathways. Bioengineered. 2021;12(1):4719–4735.
- Wang C, Liu B. miR-101-3p induces autophagy in endometrial carcinoma cells by targeting EZH2. Arch Gynecol Obstet. 2018;297(6):1539–1548.
- Li L, Shao MY, Zou SC, et al. MiR-101-3p inhibits EMT to attenuate proliferation and metastasis in glioblastoma by targeting TRIM44. J Neurooncol. 2019;141(1):19–30.
- Moshiri F, Salvi A, Gramantieri L, et al. Circulating miR-106b-3p, miR-101-3p and miR-1246 as diagnostic biomarkers of hepatocellular carcinoma. Oncotarget. 2018;9(20):15350–15364. DOI:10.18632/oncotarget.24601
- Zeng W, Zhang S, Yang L, et al. Serum miR-101-3p combined with pepsinogen contributes to the early diagnosis of gastric cancer. BMC Med Genet. 2020;21(1):28.
- Cao S, Lin L, Xia X, et al. lncRNA SPRY4-IT1 regulates cell proliferation and migration by sponging miR-101-3p and regulating AMPK expression in gastric cancer. Mol Ther Nucleic Acids. 2019;17:455–464.
- Wang L, Shen J, Jiang Y. Circ_0027599/phdla1 suppresses gastric cancer progression by sponging miR-101-3p.1. Cell Biosci. 2018;8(1):58.
- Wang H, Zhang X, Liu Y, et al. Downregulated miR-31 level associates with poor prognosis of gastric cancer and its restoration suppresses tumor cell malignant phenotypes by inhibiting E2F2. Oncotarget. 2016;7(24):36577–36589.
- Zhou Q, Zhang F, He Z, et al. E2F2/5/8 serve as potential prognostic biomarkers and targets for human ovarian cancer. Front Oncol. 2019;9:161.
- Lin QY, Wang JQ, Wu LL, et al. miR-638 represses the stem cell characteristics of breast cancer cells by targeting E2F2. Breast Cancer. 2020;27(1):147–158.
- Yu J, Fang C, Zhang Z, et al. H19 rises in gastric cancer and exerts a tumor-promoting function via miR-138/E2F2 axis. Cancer Manag Res. 2020;12:13033–13042.
