ABSTRACT
Atherosclerosis (AS) is a chronic inflammatory disease, which leads to atherosclerotic rupture, lumen stenosis and thrombosis, and often endangers life. Circular RNAs (circRNAs) are a special class of non-coding RNA molecules, whose abnormal expression has been proved to be closely related to human diseases, including AS. Both the abnormal regulation of circRNAs and the sponging effect on miRNAs would lead to changes in gene expression in the form of epigenetic modification, ultimately leading to the formation of AS. CircRNAs can be used as peripheral blood markers of AS, and play an important regulatory role in the proliferation, migration, inflammation and apoptosis of vascular smooth muscle cells, endothelial cells and macrophage, which are key cells for the development of AS. The in-depth understanding of circRNAs in AS not only provides a new method for the diagnosis of AS, but also provides a new idea for the treatment of AS.
1 Introduction
Atherosclerosis (AS) is a chronic vasculitis associated with oxidative stress and endothelial dysfunction, which is an important cause of cardiovascular death in human. AS is characterized by abnormal endothelial cell function, formation of foam cells, abnormal proliferation, migration and apoptosis of vascular smooth muscle cells (VSMCs) due to lipid accumulation in arterial blood vessel wall, and ultimately lead to the formation of atherosclerotic plaques and atherosclerosis [Citation1–3].
Endothelial cells (ECs) are essential for maintaining vascular homeostasis. In the initial stage of AS, ECs are stimulated and damaged by external factors, leading to abnormal proliferation, migration and apoptosis of endothelial cells, resulting in endothelial dysfunction [Citation4]. VSMCs are mainly distributed in the middle layer of the artery, and their role is to maintain the integrity of the artery wall. Abnormal proliferation, migration and secretion of extracellular matrix (ECM) of VSMCs in early AS promote the damage of AS, which is a key step in the occurrence and development of AS [Citation5,Citation6]. The proliferation of VSMCs in the late development of AS can prevent the rupture of plaque fibrous cap, and increase plaque stability is beneficial to AS. Aging and apoptosis of VSMCs promote the thinning of plaque fibrous cap, promote the occurrence of inflammation and increase the vulnerability of atherosclerotic plaque [Citation7,Citation8].
Circular RNAs (circRNAs) are generated by reverse splicing of precursor mRNA of thousands of gene exons in eukaryotes, and are characterized by cell type and tissue specificity and low relative expression level [Citation9]. CircRNAs are mainly located in the cytoplasm or stored in exosomes, which are not affected by exonuclease. They have high expression stability, wide distribution and are not easy to degrade. CircRNAs have been proved to exist in a variety of eukaryotes. Moreover, it accounts for a large proportion in the expression of transcripts, and the expression level of some transcripts is significantly higher than that of others [Citation10,Citation11]. CircRNA decision function, in a special rings circRNA as competitive endogenous RNA (ceRNA) combined with microRNAs(miRNAs), by acting as miRNA sponges, regulating gene expression. CircRNAs play an important biological function in diseases of nervous system, cardiovascular disease and cancer, and has become a hot spot in RNA research [Citation12–14]. CircRNAs are involved in basic processes of life, such as apoptosis, cell cycle and proliferation. They play up-regulation and down-regulation roles in the pathogenesis of many diseases, and serve as biomarkers and therapeutic targets for these diseases, but the understanding of their operating mechanism is still limited [Citation15,Citation16].
Wang et al [Citation17], found that 624 circRNAs were significantly up-regulated and 171 circRNAs were significantly down-regulated in patients with coronary heart disease. Hsa_circ_0001879 and hsa_circ_0004104 may play a key role in coronary heart disease (CAD). Circ_0004104 in AS promotes vascular endothelial cell (VEC) damage by regulating the expression of downstream gene TNFAIP8, as a miR-100 molecular sponge. Although circ_0004104 can alleviate oxidized low-density lipoprotein (ox-LDL) induced VEC injury, miR-100 inhibitors reverse the inhibitory effect of circ_0004104 gene knockout on VEC injury [Citation18]. Compared with the control group, 182 up-regulated and 176 down-regulated circrnas were identified in patients with large atherosclerosis (LAA) stroke. These differentially expressed circrnas are mainly related to chromatin modification, autophagy, platelet activation and proliferation of neural precursor cells. Evidence shows that circRNA plays a role in the molecular mechanism of ECs and VSMCs in AS, revealing the close relationship between circrna and as [Citation19,Citation20].
In view of the key role of circRNAs in AS, this work systematically summarizes the role of circRNAs in the pathogenesis of AS, including the role of circRNA in the diagnosis of AS, the regulatory role of circRNA in AS pathogenesis, and the intervention role of circRNA in the treatment of AS.
2 CircRnas as diagnostic markers of atherosclerosis
Compared with healthy control, serum circPTPRA in AS is significantly up-regulated, and circPTPRA regulates cell proliferation and apoptosis through miR-636/SP1 axis. CircPTPRA can be used AS a serum biomarker in AS patients to distinguish between AS patients and healthy persons [Citation21]. The expression level of hsa_circ_0001445 in plasma of AS is negatively correlated with the degree and severity of coronary atherosclerosis, and reducing the expression level of hsa_circ_0001445 is conducive to promoting the development of AS [Citation22].
Microarray analysis of circRNAs in VSMCs induced by high glucose reveals a total of 983 differentially expressed circRNAs, of which 458 are up-regulated and 525 are down-regulated. Among them, 31 circRNAs are up-regulated and 22 circRNAs are down-regulated, with a 2-fold change (P < 0.05), and the role of circWDR77-miR-124-FGF2 regulatory pathway in proliferation and migration of VSMCs is confirmed, which may provide a new theoretical basis for vascular diseases [Citation23].
CircUSP36 is up-regulated in ox-LDL-treated ECs, and regulates the expression level of WNT4 by competitively binding miR-637, thus inhibiting the survival and migration ability of endothelial cells and causing cell damage. CircUSP36 has high value in the diagnosis of AS [Citation24].
Circ0010283 is highly expressed in serum samples from atherosclerotic patients, while circ0010283 knockdown inhibits ox-LDL-induced proliferation, migration, and invasion of HVSMC, as well as migration-related protein matrix metalloproteinase 2 (MMP2) and MMP9 expression. Circ_0010283 can regulate the expression of PAPPA by mediating miR-133a-3p and participate in ox-LDL induced HVSMC dysfunction [Citation25,Citation26].
In human aortic endothelial cells (HAECs) induced by ox-LDL, circ_0093887 is down-regulated and miR-876-3p is up-regulated. Circ_0093887 plays a role by regulating the expression levels of CCND2 and SUCNR1 through the sponge miR-876-3p. Not only can overexpressed circ_0093887 eliminate HAECs damage, but also miR-876-3p inhibitors mitigate the effect of circ_0093887 knockout on HAECs [Citation27]. CircZNF609 not only has anti-inflammatory effect, after overexpression in mouse macrophage RAW264.7, the expression levels of inflammatory factors IL-6 and TNF-α are significantly decreased, IL-10 is significantly increased, and the expression level of peripheral blood leukocytes (PBLs) of CAD patients is significantly decreased, which has moderate diagnostic value [Citation28].
Changes in circRNA levels can be used as a predictor of plaque rupture. By comparing the levels of circRNA_284 and miRNA-221 in serum of patients with cerebral ischemic events, it is found that the ratio of circRNA_284 to miR-221 increases significantly after carotid plaque rupture, which is helpful for rapid identification and diagnosis of patients with urgent plaque rupture And asymptomatic patients, suggesting its potential as a biomarker for plaque rupture [Citation29]. ().
Figure 1. The biogenesis of circRnas.
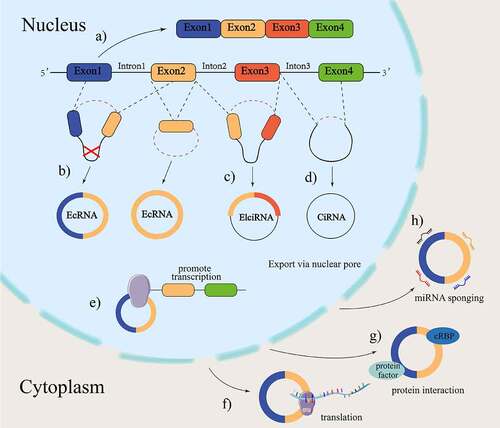
3 CircRnas are involved in the pathogenesis of atherosclerosis
Under the induction of various risk factors (environmental pollution, smoking, diet and), first of all, ECs are damaged, endothelial dysfunction occurs, leading to monocyte adhesion/migration, lipid uptake, and the formation of “foam cells” (fat stripes). Subsequently, VSMCs in the middle layer migrate to the subendothelial space, leading to the formation of atherosclerotic plaques and atherosclerosis. When the large necrotic core is covered by a thin fibrous cap, the plaques are easy to rupture [Citation30,Citation31].
CircGNAQ is significantly down-regulated in senescent ECs, and overexpression of circGNAQ inhibits EC senescence and AS progression. This suggests that circGNAQ plays a key role in EC senescence and the pathogenesis of atherosclerosis, and the management of circGNAQ provides a potential treatment for limiting the progression of atherosclerosis [Citation32]. CircDIP2C and TET2 are significantly down-regulated in AS, and miR-556-5p is up-regulated. Mechanistically, circDIP2C promotes HUVEC injury through TET2 mediated by sponge miR-556-5P [Citation33].
The level of hsa_circRNA_0001599 is positively correlated with stroke scale score and infarct volume. ROC analysis of hsa_circRNA_0001599 in LAA stroke patients shows that the area under the curve is 0.805 (95% confidence interval: 0.748 to 0.862, P < 0.001), and the sensitivity and specificity of the diagnosis of LAA stroke are 64.41% and 89.93%, respectively, which further indicates that circRNA is related to the pathogenesis of AS [Citation19].
3.1 CircRNA and ECs
Endothelial cells play an important role in vascular homeostasis, and loss of endothelial anatomical integrity is the initial stage of AS. When endothelium is exposed to various non-genetic risk factors (such as hypoxia, pro-inflammatory cytokines and oxidative stress), endothelial cells will be damaged and endothelial dysfunction will occur [Citation4]. The impaired vascular dynamic balance leads to the proliferation, migration, apoptosis and senescence of ECs, and promotes angiogenesis and inflammation, leading to the adhesion/migration of monocytes, lipid uptake and formation of “foam cells”, and then a series of events lead to the formation of early lesions of AS [Citation34]. ().
Figure 2. CircRnas in ECs of as.
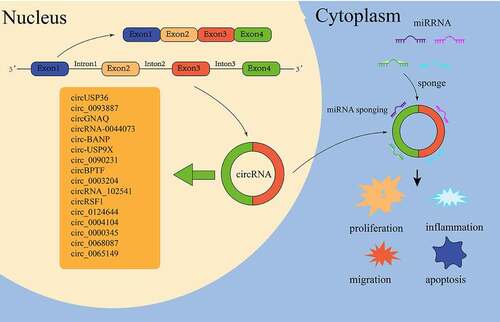
3.1.1 CircRNA regulates the proliferation and migration of ECs
CircRNA -0,044,073 can promote the proliferation and migration of human umbilical vein smooth muscle cells (HUVSMCs) and human umbilical vein endothelial cells (HUVECs). Meanwhile, miR-107 is a key target of circRNA -0,044,073, and its level will be inhibited by circRNA -0,044,073. CircRNA -0,044,073 accelerates the JAK/STAT signaling pathway, which is activated in patients with atherosclerosis. Therefore, circRNA -0,044,073 may provide a new treatment for AS by targeting miR-107 to increase cell proliferation and migration [Citation35].
MiR-148a-3p is significantly up-regulated in atherosclerosis compared with healthy people. miR-148a-3p inhibits FOXO4 and FOXO3 expression by disrupting circ_0003575 function, stimulates proliferation and migration of endothelial cells, and exacerbates atherosclerosis [Citation36].
Hsa_circ_0001445 is down-regulated in AS, and the serine-rich splicing-factor 1 (SRSF1)/β-catenin axis is activated by up-regulation of hsa_circ_0001445, which reverses ox-LDL-induced HUVEC proliferation, tube formation, and mitochondrial membrane potential inhibition [Citation37].
Hsa_circ_0003575 is significantly up-regulated in ox-LDL-induced HUVEC. Meanwhile, functional loss experiments showed that silenced hsa_circ_0003575 is beneficial to HUVEC angiogenesis and promoted cell proliferation and inhibited apoptosis, which reveals the expression profile of HUVECs. It provides therapeutic strategies for VEC injury in atherosclerosis [Citation38].
Knockdown of circ-BANP down-regulates the expression of TXNIP, the direct target of circ-BANP, and promotes the activity, migration and invasion of VSMCs, alleviates the injury of HUVEC [Citation39]. Hsa_circ_0004543 was significantly up-regulated in ox-LDL-treated HUVECs and positively correlated with ox-LDL concentration within a certain range. Silencing of hsa_circ_0004543 promoted cell proliferation, migration and reduced apoptosis [Citation40].
Serum circ-USP9X is up-regulated in patients with AS. Silenced circ-USP9X regulates the expression of chloride channel 4 (CLIC4) through sponge miR-599 and reduces the endothelial cell injury. The effect of circ-USP9X silenced HUVEC injury is reversed by miR-599 inhibitors, while CLIC4 overexpression reverses the mediating effect of elevated miR-599 on ox-LDL -induced HUVEC injury [Citation41].
Compared with the control group, circ_USP36 expression is significantly up-regulated. Circ_USP36 gene knockout can reduce ROBO1 expression through miR-197-3p and thus alleviate ox-LDL-induced HUVEC dysfunction. Overexpression of ROBO1 reverses the accumulation mediated effect of miR-197-3p in ox-LDL-induced HUVEC [Citation42]. Knockout of circ_USP36 increases the miR-182-5p expression and down-regulates KLF5, alleviating ox-LDL-mediated HUVSMCs damage, and may provide therapeutic targets for atherosclerosis [Citation43].
3.1.2 CircRnas regulate ECs inflammation
Pyotopia is a novel inflammatory programmed cell death. HAECs treated with ox-LDL can lead to pyotopia, which is associated with significantly increased lactate dehydrogenase (LDH) release and levels of inflammatory cytokines IL-1β and IL-18. In ox-LDL-treated HAEC, circ_0090231 acts by competitively binding miR-635, and silencing circ_0090231 reduces HAECs damage and inflammation. This suggests that circ_0090231/miR-635/NLRP3 axis can affect the development of AS by regulating cell pyrophosis [Citation44].
In mercury-induced HUVEC, circBPTF knockout eliminated inflammatory damage in HUVEC by increasing miR-384 expression. LIN28B is a target of miR-384, and circBPTF positively regulates the expression of LIN28B through miR-384. This suggests that circBPTF knockout protects humans from mercury-induced inflammatory damage and oxidative stress by mediating the miR-384/LIN28B axis in HUVECs [Citation45].
CircUSP36 is highly expressed in AS, and VCAM1 is the target of miR-98-5p and is reversely regulated by miR-98-5p. Highly expressed circ-USP36 up-regulates the expression of VCAM1 through competitive binding with miR-98-5, promoting ox-LDL-induced apoptosis, inflammation and activity inhibition of HUVEC [Citation46]. CircUSP36 sponges miR‑20a‑5p, indirectly regulates the expression of ROCK2. Knockdown of circUSP36 reduces the ROCK2 level and reverses the HUVEC damage and inflammation through miR-20a-5p [Citation47].
Silenced circ_0003645 inhibits lactate dehydrogenase leakage (LDH leakage), apoptosis and the expression of IL-6, TNF-α, ICAM-1, VCAM-1, NF-κB mRNA and NF-κB protein through the NF-κB pathway, and promotes cell viability [Citation48]. Circ_0003204 knockdown inhibits the release of inflammatory cytokines IL-6, IL-1β and TNF-α of HUVEC by up-regulating the level of miR-330-5p, and reduced cell apoptosis and oxidative stress. TLR4 is the target of miR-330-5p, and enhancing the expression of TLR4 alleviates the injury of HUVECs. NF-κB signaling pathway also plays a key role in the regulation of HUVECs by circ_0003204/miR-330-5p/TLR4 axis [Citation49].
CircANRIL is a key circRNA in CAD development. In coronary atherosclerosis model of SD rats, circANRIL not only reduces serum calcium ion and blood lipid levels, but also decreased the contents of LDH, SOD, MDA, TNF-α and IL-6. CircANRIL silencing reduces the apoptosis of VECs and the level of inflammatory factors, which plays an important role in the progression of AS [Citation50,Citation51].
3.1.3 CircRNA regulates apoptosis of ECs
Overexpression of miR-296-5p significantly eliminates the effect of circRNA_102541 on the proliferation and apoptosis of HUVEC cells. PLK1 is a target of miR-296-5P, and PLK1 knockout can accelerate the inhibition of proliferation and promotion of apoptosis of HUVEC cells by miR-296-5p [Citation52].
In AS, CircRSF1 plays a role by regulating miR-135B-5p/HDAC1 axis. By up-regulating circRSF1 expression, cell apoptosis and Il-1 β, IL-6, TNF-α and IL-8 levels are significantly reduced, and transfection of miR-135B-5P can save cell apoptosis. Knockdown of HDAC1 reverses the effect of anti-miR-135B-5p on ox-LDL-treated HUVECs cell damage [Citation53]. Circ_0124644 positively regulates the PAPP-A, the target of miR-149-5p, and promotes apoptosis of HUVEC. The mechanism is that circ_0124644 acts as a sponge for miR-149-5p and enhances ox-LDL-induced HUVECs injury by adsorption of miR-149-5p [Citation54].
The silencing of miR-758 and up-regulation of miR-758 by CCND2 deletion offset the promoting effect of circRSF1 on cell growth, migration and tube formation. CircRSF1 and CCND2 can competitively bind to miR-758, and circrF1 positively regulates the expression of CCND2 through miR-758. CircRSF1 can protect ox-LDL-induced EC injury in vitro through miR-758/CCND2 axis, suggesting circRSF1 is a potential target for atherosclerosis therapy [Citation55].
Knockout of hsa_circ_0003204 inhibites ox-LDL-induced apoptosis of HUVEC, and significantly decreases the expression of E-cadherin, but increased the expression of N-cadherin and vimentin [Citation56]. Circ_0003204 aggravates ox-LDL-induced HUVECs injury by regulating miR-942-5p/HDAC9 pathway [Citation57].
Ox-LDL can up-regulate circ_0004104 level of HUVECs, and promote apoptosis, inflammation and oxidative stress of HUVECs. Circ_0004104 gene knockout mitigates oxidative low-density lipoprotein-induced HUVECs dysfunction through regulation of miR-328-3p mediated TRIM14 [Citation58].
Hsa_circ_0000345 promotes HUVEC cell viability and cell cycle progression, and prevents cell apoptosis. Circ_0000345 functions by sponging miR-129-5p in HUVEC to enhance TET2 mRNA and protein expression and alleviates ox-LDL-induced HUVEC damage [Citation59]. Up-regulated circ_0068087 in AS inhibits the HUVEC activity and induces apoptosis, inflammation and oxidative stress by down-regulating miR-186-5p [Citation60].
Hsa_circ_0000345 and circ_0065149 can activate cell viability, inhibit cell apoptosis, promote cell proliferation and inhibit cell invasion. In addition, hsa_circ_0000345 increases the level of HIF-1α, and miR-330-5p regulates NF-κB and pro-inflammatory cytokine levels as a target of circ_0065149 [Citation61,Citation62]. ().
Table 1. Regulation and function of circRnas in ECs.
3.2 CircRNA and VSMCs
VSMCs are an important part of blood vessels, which affect the occurrence and development of cardiovascular and cerebrovascular diseases. First, VSMC proliferation, migration and synthesis of extracellular matrix promote the formation of as damage. However, VSMC proliferation in late plaque can prevent the rupture of plaque fiber cap and increase plaque stability, which is beneficial to as. On the contrary, VSMC aging and apoptosis promote the thinning of plaque fibrous cap, and macrophages derived from its phenotypic transformation may promote inflammation, lead to the formation of necrotic core, increase plaque vulnerability, and aggravate as lesions [Citation63]. ().
Figure 3. CircRNA in VSMCs of as.
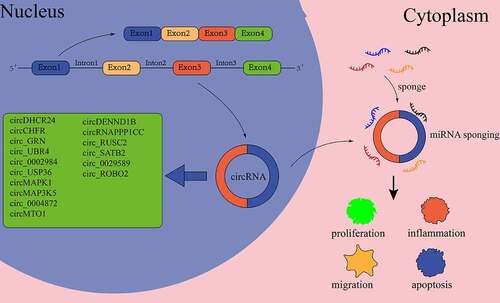
3.2.1 CircRnas regulate the proliferation and migration of VSMCs
Has_circ_0113656 (circDHCR24) is significantly up-regulated in PDGF-BB induced HA VSMCs. Upregulation of circDHCR24 promotes proliferation, migration and phenotypic transformation of VSMCs in PATIENTS with AS, thereby aggravating vascular restenosis. Mechanistically, circDHCR24 inhibites MMP9 by sponging miR-149-5p [Citation64]. CircCHFR regulates FOXO1 expression by targeting miR-370 and the transcription factor FOXO1 promotes cyclin D1 expression. Overexpressed circCHFR promotes proliferation and migration of VSM through the miR-370/FOXO1/CyclinD1 axis pathway [Citation65].
Circ_GRN promotes proliferation, migration and inflammation of VSMCs in AS via miR-214-3p/FOXO1 axis. Overexpression of miR-214-3p eliminates abnormal proliferation, migration, and inflammation of HVSMC, but this effect is reversed by up-regulation of FOXO1 [Citation66]. Circ_CHFR positively regulates Wnt3 expression and promotes VSMC growth, migration and inflammation by sponging miR-214. Both down-regulation of Wnt3 and silencing of circ-CHFR attenuated ox-LDL damage to VSMC [Citation67].
Silenced circUBR4 down-regulates FRS2 by targeting miR-185-5p, thereby inhibiting ox-LDL-induced proliferation and migration of VSMC. Silencing miR-185-5p reverses the effect of circUBR4 knockout on ox-LDL-induced VSMCs [Citation68]. Knockdown of circ_UBR4 can positively regulate FOXO4 by targeting miR-637, inhibit ox-LDL-induced overproliferation and migration of HVSMC, and overexpression of FOXO4 can eliminate this inhibition [Citation69]. Circ_UBR4 is up-regulated in human AS serum and ox-LDL-induced human VSMCs. Circ_UBR4 silencing inhibits VSMCs proliferation and migration, but is reversed by ROCK1 upregulation. The expression of miR-107 is negatively correlated with circ_UBR4, and the effect of circ_UBR4 knockdown on VSMCs is eliminated by silencing miR-107 through up-regulation of ROCK1 [Citation70].
The expression of circ_0002984 in AS is significantly up-regulated. Li et al [Citation71], confirmed that the up-regulation of miR-326-3p reversed the damage of VMSCs caused by circ_0002984 overexpression. In addition, miR-326-3p inhibitor-mediated VSMC function was reversed by knockout of vesicle-associated membrane protein 3 (VAMP3). Knockdown of circ_USP36 increased miR-182-5p expression and down-regulated KLF5, alleviating oxidative low-density lipoprotein-mediated DAMAGE to HUVSMCs, and may provide therapeutic targets for atherosclerosis [Citation43].
In atherosclerotic model, circMAPK1 up-regulates miR-22-3p level by silencing circMAPK1 and inhibits the MECP2 level, thereby inhibiting proliferation and migration of VSMCs. It was proved that circMPAK1 promotes the proliferation and migration of VSMCs through miR-22-3p/MECP2 axis, providing a clue for the treatment of atherosclerosis [Citation72]. The down-regulation of circMPAK 5 is related to the injury and proliferation of VSMCs and coronary artery lesions. Overexpression of circMPAK5 will inhibit the proliferation of VSMCs through the circMAP3K5/miR-22-3p/TET2 axis, thereby reducing the formation of neointima. CircMAP3k5 plays an important role in the pathogenesis of intimal hyperplasia related diseases [Citation73].
Circ_0004872 increases TXNIP level by secreting miR-513A-5p, and accelerates proliferation and migration of PDGF-BB-induced HA-VSMC. Circ_0004872 can effectively inhibit the proliferation, migration and dedifferentiation of PDGF-BB-induced HA-VSMCs [Citation74]. Knockout circ_0029589 can regulate the level of STIM1 through miR-214-3p and inhibit the proliferation and migration of VSMCs, which is weakened by miR-214-3p gene knockout [Citation75].
In human AS serum and ox-LDL-stimulated VSMC, circMTO1 expression decreases and miR-182-5p expression increases. The overexpression of circMTO1 inhibites the proliferation and promotes the apoptosis of ox-LDL-stimulated VSMCs. On the contrary, the overexpression of miR-182-5p and the knockout of miR-182-5P target RASA1 gene reverse the effect of circMTO1 overexpression on ox-LDL-stimulated VSMCs. This indicates that circMTO1 has potential in the treatment of AS [Citation76].
3.2.2 CircRnas regulate the inflammation of VSMCs
Inflammation is a key mediator of AS, and inflammatory cytokines play an important role in the development and treatment of AS. The expression of circDENND1B is negatively correlated with the progression of atherosclerosis and foam cell formation, and the up-regulation of circDENND1B significantly reduces ox-LDL-induced foam cell formation by promoting cholesterol efflux. CircDENND1B is also involved in the anti-atherosclerosis effect of IL-1β monoclonal antibody in vivo and in vitro [Citation77].
Aging VSMCs are present in atherosclerotic plaques and are essential for the progression of disease and are associated with inflammatory response. Aging VSMC may induce proinflammatory status of adjacent cells in an IL-1α dependent manner, which directly leads to chronic inflammation associated with AS [Citation78].
Lipopolysaccharide (PG-LPS) from Porphyromonas gingivalis (Pg) can cause pyroptosis of VSMCs and instability of atherosclerotic plaques. Knockout of circRNAPPP1CC reduces PG-LP-induced coke death of VSMCs and inhibites the expression of HMGB1, TLR9, AIM2 and cleaved caspase-1. In addition, PPP1CC directly targets and competitively adsorbs miR-103A-3p and miR-107, thereby weakening the inhibitory effect of these miRNAs on HMGB1 expression [Citation79].
Alagliptin inhibits the damage of TUMOR necrosis factor-α (TNF-α) and IL-6 on VSMCs in patients with AS. Silencing of SIRT1 weakens the protective effect of alagliptin on VSMC senescence [Citation80]. Therefore, in the treatment of AS, targeted reduction of inflammation can delay VSMC injury, and finally achieve the purpose of treating AS.
3.2.3 CircRnas regulate apoptosis of VSMCs
Transcription on chromosome 9 p21 near INK4 loci in the annular antisense noncoding circANRIL, through combined with nucleoli protein PES1, prevents VSMCs and macrophages in the maturing of the ribosomal RNA, damages the ribosomes biosynthesis and p53 activation [Citation81]. Circ_RUSC2 is up-regulated in proliferative VSMCs, and there is multiple miR-661 binding site on circ_RUSC2, and their expression are not affected by each other. In addition, circ_RUSC2 can promote the expression of miR-661 target gene SYK at the binding site, and regulate the proliferation, apoptosis, phenotypic regulation and migration of VSMC. These findings will provide a theoretical basis for studying the circRNA function of VSMCs and provide new ideas for the diagnosis and treatment of cardiovascular diseases [Citation82].
The expressions of circ-SATB2 and STIM1 are up-regulated, the miR-939 is down-regulated, and the expressions of circ-SATB2 and miR-939 did not interfere with each other. Overexpression of circ-SATB2 inhibits the expression of SM22α, a marker of contractile vascular SMC, and regulates the VSMC injury by promoting the expression of STIM1. MiR-939 not only promotes cell apoptosis, but also positively regulates SM22α [Citation83].
Silencing of circ_0029589 inhibits proliferation and migration of ox-LDL-treated VSMCs but induces apoptosis by regulating miR-424-5P/IGF2 axis. MiR-424-5p is a target of circ_0029589, whose knockout reverses the effect of circ_0029589 interference on ox-LDL-stimulated VSMC proliferation, migration, invasion and apoptosis [Citation84].
In AS, the expression of circ_ROBO2 is significantly up-regulated and the expression of miR-149 is down-regulated, and circ_ROBO2 activates the TRAF6/NF-κB signal by sponging miR-149. Knockdown of circ_ROBO2 significantly inhibits the proliferation and migration of human arterial smooth muscle cells (HASMCs), increases the apoptosis rate, and inhibites the NF-κB signal transduction. The biological effect of circ_ROBO2 knockout is not reversed by silencing miR-149 or activating NF-κB signal [Citation85]. ().
Table 2. Regulation and function of circRnas in VSMCs.
3.3 CircRNA and macrophages
Macrophages release inflammatory factors in pathological state, stimulate cell apoptosis and necrosis, and subsequently form AS plaques, which play an important role in the pathological process of AS [Citation86]. In macrophages, ox-LDL activates autophagy by inducing endoplasmic reticulum (ER) stress, and autophagy promotes the clearance of damaged proteins and organelles to promote the survival of macrophages. Although autophagy is generally considered as a protective process against AS stress, overactivated autophagy can induce autophagy death of VSMCs, resulting in reduced collagen synthesis and plaque instability [Citation87].
CircTM7SF3 level is significantly up-regulated in serum samples of AS patients and ox-LDL-induced THP-1-derived macrophages. The mechanism is that circTM7SF3 sponges miR-206, promotes THP-1 apoptosis, inflammation and oxidative stress [Citation88]. CircSCAP and phosphodiesterase 3B (PDE3B) levels increase while miR-221-5p levels decreased. CircSCAP knockout inhibits ox-LDL-induced lipid deposition, inflammation, and oxidative stress in THP-1 cells via miR-221-5p/PDE3B axis. Overexpression of PDE3B, a target of miR-221-5p, largely counteracts the effect mediated by miR-221-5p accumulation in ox-LDL-induced THP-1 cells [Citation89].
Ox-LDL can inhibit metabolism by up-regulation of Beclin1 and accumulation of excessive autophagic spots, thus stimulates abnormal autophagy, which may lead to apoptosis of ECs, while hsa_circ_0030042 can reduce ox-LDL-induced autophagic accumulation and. Hsa_circ_0030042 is significantly down-regulated in coronary heart disease (CHD), and after overexpression, it acts as an endogenous eukaryotic promoter 4A-III (eIF4A3) sponge to inhibit ox-LDL-induced abnormal autophagy of HUVECs and maintain plaque stability in vivo [Citation90].
CircHIPK3 is down-regulated in HFD mice and ox-LDL-HUVECs. Overexpression of circHIPK3 can reverse the decrease of autophagy level in AS and reduce lipid accumulation. Therefore, circHIPK3 can inhibit the lipid content of HUVEC by activating autophagy and reduce the damage of HUVEC, opening up a new approach for the treatment of AS [Citation91].
A total of 7276 circRNAs are differentially expressed in the model of cholesterol efflux promoted by Astaxanthin (AST) in THP-1 cells. Among them, circUGGT2, circPCMTD1 and circBRWD1 with the largest differences may sponge miRNA. Therefore, the regulatory genes ABCA1, ABCG1 and SR-BI promote macrophage foaming and cholesterol efflux, which may be used AS markers for the diagnosis of AS [Citation92].
The hsa_circ_0029589 in macrophages of patients with acute coronary syndrome (ACS) decreases, while the level of N6-methyladenosine (m6A) and M6A-methyltransferase (METTL3) of hsa_circ_0029589 significantly increases. IFN regulatory factor-1 (IRF-1) promotes pyroptosis and inflammation of macrophages in ACS and AS by promoting its m6A modification and inhibiting circ_0029589 [Citation93].
4 Conclusions and research prospects
CircRNAs can be involved in several as-related processes, including the regulation of proliferation, migration and apoptosis of ECs and SMCs, inflammatory responses, and lipid metabolism. CircRNA expression is very stable, so multiple circRNAs can be used as biomarkers for the occurrence of a variety of diseases [Citation94]. For example, it has been proved that hsa_circ_0001445 and circZNF609 are expected to be diagnostic markers of AS, However, the current research is still in the early stage and the molecular mechanism needs to be further clarified. CircRNA plays an important biological function in gene regulation, almost participates in regulating various physiological and pathological processes, and is closely related to the pathological mechanism of different diseases. In-depth study of circRNAs in the pathophysiological process of AS opens the way to decipher the function and mechanism of circRNA in other diseases [Citation95].
Current studies show that the biological function of circRNAs related to AS are mainly involved in the regulation of AS in the form of miRNA molecular sponge, but not all circRNAs have perfect miRNA capture sites, and whether circRNAs can directly affect the progress of AS by regulating mRNAs is an issue that needs to be verified urgently. Although many circRNAs related to AS have been discovered, the specific molecular mechanism of only a few circRNAs has been elucidated, and most of them remain at the level of in vitro experiments. The ultimate goal of the scientific development of animal experiments is to explore the mysteries of human life by studying the life phenomena of animals themselves and then applying them to human beings. Therefore, in order to comprehensively understand the mechanism of circRNAs in the progress of AS, it is urgent to first solve the problem of how circRNAs performs biological functions in animal.
Astragaloside IV (ASV) plays an important role in inhibiting apoptosis, inflammation, and oxidative stress, and ASV protects HUVEC from oxidative LDL-induced damage by targeting the circ_0000231/miR-135A-5p/CLIC4 axis. This may provide a new insights into the development of effective strategies for the treatment of AS [Citation96,Citation97]. Due to the multi-component and multi-target action mechanism of Chinese herbal medicine and Traditional Chinese medicine compound, the effective ingredients are not easy to determine. These substances may occur complex chemical reactions during processing, which further increases the difficulties for the study of Traditional Chinese medicine compound. The connection between the Chinese herbal medicine with the AS research is still very limited. Based on the rapid development of bioinformatics and network databases, it is possible to conduct network analysis of biological systems, and connect herbal medicine with AS through data mining, network pharmacology, network analysis and other technologies, which may open up new ideas for the diagnosis and treatment of AS.
Acknowledgements
We would like to thank the National Natural Science Foundation of China (No.82274310), Anhui Science and Technology Department (No.2022e07020028), Anhui Education Department (KJ2021A0588, KJ2021A0606, gxgwfx2022019) and Anhui University of traditional Chinese medicine (No.2020rcZD001) for supporting the research of this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, the data are not publicly available due to restrictions.
Additional information
Funding
References
- Engelen SE, Robinson AJB, YX Z, et al. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol. 2022;19(8):522–542.
- Tabas I, García-Cardeña G, Owens GK Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):13–22.
- Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241.
- JR Z, HJ S. LncRnas and circular RNAs as endothelial cell messengers in hypertension: mechanism insights and therapeutic potential. Mol Biol Rep. 2020;47(7):5535–5547.
- DF R-B, Maira A, Sibinga NES. The atypical cadherin FAT1 limits mitochondrial respiration and proliferation of vascular smooth muscle cells. Front Cardiovasc Med. 2022;9:905717.
- Worssam MD, Jørgensen HF. Mechanisms of vascular smooth muscle cell investment and phenotypic diversification in vascular diseases. Biochem Soc Trans. 2021;49(5):2101–2111.
- Basatemur GL, Jørgensen HF, Clarke MCH, et al. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–744.
- Li T, Wang B, Ding H, et al. Effect of extracellular vesicles from multiple cells on vascular smooth muscle cells in atherosclerosis. Front Pharmacol. 2022;13:857331.
- Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428–442.
- Gu J, Su C, Huang F, et al. Past, present and future: the relationship between circular RNA and immunity. Front Immunol. 2022;13:894707.
- Jiao S, Wu S, Huang S, et al. Advances in the identification of circular RNAs and research into circrnas in human diseases. Front Genet. 2021;12:665233.
- Li H, Liu X, Sun N, et al. Differentially expressed circular non-coding RNAs in atherosclerotic aortic vessels and their potential functions in endothelial injury. Front Cardiovasc Med. 2021;8:657544.
- Kang L, Jia H, Huang B, et al. Identification of differently expressed mRNAs in atherosclerosis reveals CDK6 is Regulated by circHipk3/mir-637 axis and promotes cell growth in human vascular smooth muscle cells. Front Genet. 2021;12:596169.
- Kristensen LS, Andersen MS, Stagsted LVW, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691.
- Wen Y, Chun Y, Lian ZQ, et al. circRna‑0006896‑mir1264‑dnmt1 axis plays an important role in carotid plaque destabilization by regulating the behavior of endothelial cells in atherosclerosis. Mol Med Rep. 2021;23(5):311.
- Caba L, Florea L, Gug C, et al. Circular RNA-is the circle perfect? Biomolecules. 2021;11(12):1755.
- Wang L, Shen C, Wang Y, et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis. 2019;286:88–96.
- Ji P, Song X, Lv Z. Knockdown of circ_0004104 alleviates oxidized low-density lipoprotein-induced vascular endothelial cell injury by regulating miR-100/TNFAIP8 Axis. J Cardiovasc Pharmacol. 2021;78(2):269–279.
- Li S, Hu W, Deng F, et al. Identification of Circular RNA hsa_circ_0001599 as a novel biomarker for large-artery atherosclerotic stroke. DNA Cell Biol. 2021;40(3):457–468.
- Wen C, Li B, Nie L, et al. Emerging Roles of extracellular vesicle-delivered circular RNAs in atherosclerosis. Front Cell Dev Biol. 2022;10:804247.
- LL Z. CircRNA-PTPRA promoted the progression of atherosclerosis through sponging with miR-636 and upregulating the transcription factor SP1. Eur Rev Med Pharmacol Sci. 2020;24(23):12437–12449.
- Vilades D, Martínez-Camblor P, Ferrero-Gregori A, et al. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: performance as a biomarker. Faseb J. 2020;34(3):4403–4414.
- Chen J, Cui L, Yuan J, et al. Circular RNA WDR77 target FGF-2 to regulate vascular smooth muscle cells proliferation and migration by sponging miR-124. Biochem Biophys Res Commun. 2017;494(1–2):126–132.
- Huang JG, Tang X, Wang JJ, et al. A circular RNA, circUSP36, accelerates endothelial cell dysfunction in atherosclerosis by adsorbing miR-637 to enhance WNT4 expression. Bioengineered. 2021;12(1):6759–6770.
- Ding P, Ding Y, Tian Y, et al. Circular RNA circ_0010283 regulates the viability and migration of oxidized low‑density lipoprotein‑induced vascular smooth muscle cells via an miR‑370‑3p/HMGB1 axis in atherosclerosis. Int J Mol Med. 2020;46(4):1399–1408.
- Feng Z, Zhu Y, Zhang J, et al. Hsa-circ_0010283 regulates oxidized low-density lipoprotein-induced proliferation and migration of vascular smooth muscle cells by targeting the miR-133a-3p/pregnancy-associated plasma protein a axis. Circ J. 2020;84(12):2259–2269.
- Gao Y, Li G, Fan S, et al. Circ_0093887 upregulates CCND2 and SUCNR1 to inhibit the ox-LDL-induced endothelial dysfunction in atherosclerosis by functioning as a miR-876-3p sponge. Clin Exp Pharmacol Physiol. 2021;48(8):1137–1149.
- Liang B, Li M, Deng Q, et al. CircRNA ZNF609 in peripheral blood leukocytes acts as a protective factor and a potential biomarker for coronary artery disease. Ann Transl Med. 2020Jun;812:741
- Bazan HA, Hatfield SA, Brug A, et al. Carotid plaque rupture is accompanied by an increase in the ratio of serum circR-284 to miR-221 levels. Circ Cardiovasc Genet. 2017;10(4):e001720.
- Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866.
- Xu S, Pelisek J, Jin ZG. Atherosclerosis is an epigenetic disease. Trends Endocrinol Metab. 2018;29(11):739–742.
- Wu WP, Zhou MY, Liu DL, et al. circGNAQ, a circular RNA enriched in vascular endothelium, inhibits endothelial cell senescence and atherosclerosis progression. Mol Ther Nucleic Acids. 2021;26:374–387.
- Hu F, Chen X, Gao J, et al. CircDIP2C ameliorates oxidized low-density lipoprotein-induced cell dysfunction by binding to miR-556-5p to induce TET2 in human umbilical vein endothelial cells. Vascul Pharmacol. 2021;139:106887.
- Xu S, Kamato D, Little PJ, et al. Targeting epigenetics and non-coding RNAs in atherosclerosis: from mechanisms to therapeutics. Pharmacol Ther. 2019;196:15–43.
- Shen L, Hu Y, Lou J, et al. CircRNA‑0044073 is up-regulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR‑107. Mol Med Rep. 2019;19(5):3923–3932.
- Shang L, Quan A, Sun H, et al. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from apoe deficient mice through restricting circular RNA 0003575. Gene. 2019;711:143948.
- Liang G, Chen S, Xin S, et al. Overexpression of hsa_circ_0001445 reverses oxLdl‑induced inhibition of HUVEC proliferation via SRSF1. Mol Med Rep. 2021;24(1):507.
- Li CY, Ma L, Yu B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed Pharmacother. 2017;95:1514–1519.
- Chen G, Li Y, Zhang A, et al. Circular RNA circ-BANP regulates oxidized low-density lipoprotein-induced endothelial cell injury through targeting the miR-370/thioredoxin-interacting protein axis. J Cardiovasc Pharmacol. 2021;77(3):349–359.
- Han L, Li D, Hang Y, et al. Downregulation of hsa_circ_0004543 activates oxldl-induced vascular endothelial cell proliferation and angiogenesis. Front Genet. 2021;12:632164.
- Peng H, Sun J, Li Y, et al. Circ-USP9X inhibition reduces oxidized low-density lipoprotein-induced endothelial cell injury via the microRNA 599/chloride intracellular channel 4 axis. J Cardiovasc Pharmacol. 2021;78(4):560–571.
- Zhang Y, Li W, Li H, et al. Circ_usp36 silencing attenuates oxidized low-density lipoprotein-induced dysfunction in endothelial cells in atherosclerosis through mediating miR-197-3p/robo1 axis. J Cardiovasc Pharmacol. 2021;78(5):e761–772.
- Zhao Q, Lu YH, Wang X, et al. Circ_usp36/mir-182-5p/klf5 axis regulates the ox-LDL-induced injury in human umbilical vein smooth muscle cells. Am J Transl Res. 2020;12(12):7855–7869.
- Ge Y, Liu W, Yin W, et al. Circular RNA circ_0090231 promotes atherosclerosis in vitro by enhancing NLR family pyrin domain containing 3-mediated pyroptosis of endothelial cells. Bioengineered. 2021;12(2):10837–10848. DOI:10.1080/21655979.2021.1989260
- Zhang W, Sui Y. CircBPTF knockdown ameliorates high glucose-induced inflammatory injuries and oxidative stress by targeting the miR-384/LIN28B axis in human umbilical vein endothelial cells. Mol Cell Biochem. 2020;471(1–2):101–111.
- Peng K, Jiang P, Du Y, et al. Oxidized low-density lipoprotein accelerates the injury of endothelial cells via circ-USP36/miR-98-5p/vcam1 axis. IUBMB Life. 2021;73(1):177–187. DOI:10.1002/iub.2419
- Miao J, Wang B, Shao R, et al. CircUSP36 knockdown alleviates oxidized low‑density lipoprotein‑induced cell injury and inflammatory responses in human umbilical vein endothelial cells via the miR‑20a‑5p/ROCK2 axis. Int J Mol Med. 2021;47(4):40.
- Qin M, Wang W, Zhou H, et al. Circular RNA circ_0003645 silencing alleviates inflammation and apoptosis via the NF-κB pathway in endothelial cells induced by oxLDL. Gene. 2020;755:144900.
- Su Q, Dong X, Tang C, et al. Knockdown of circ_0003204 alleviates oxidative low-density lipoprotein-induced human umbilical vein endothelial cells injury: circulating RNAs could explain atherosclerosis disease progression. Open Med (Wars). 2021;16(1):558–569.
- Shi P, Ji H, Zhang H, et al. circANRIL reduces vascular endothelial injury, oxidative stress and inflammation in rats with coronary atherosclerosis. Exp Ther Med. 2020;20(3):2245–2251.
- Song CL, Wang JP, Xue X, et al. Effect of circular ANRIL on the inflammatory response of vascular endothelial cells in a rat model of coronary atherosclerosis. Cell Physiol Biochem. 2017;42(3):1202–1212.
- Du N, Li M, Yang D. Hsa_circrna_102541 regulates the development of atherosclerosis by targeting miR-296-5p/plk1 pathway. Ir J Med Sci. 2022;191(3):1153–1159.
- Zhang X, Lu J, Zhang Q, et al. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/hdac1 axis in atherosclerosis. Biol Res. 2021;54(1):11.
- Wang G, Li Y, Liu Z, et al. Circular RNA circ_0124644 exacerbates the ox-LDL-induced endothelial injury in human vascular endothelial cells through regulating PAPP-A by acting as a sponge of miR-149-5p. Mol Cell Biochem. 2020;471(1–2):51–61.
- Wei Z, Ran H, Yang C. CircRSF1 contributes to endothelial cell growth, migration and tube formation under ox-LDL stress through regulating miR-758/CCND2 axis. Life Sci. 2020;259:118241.
- Liu H, Ma X, Mao Z, et al. Circular RNA has_circ_0003204 inhibits oxLDL-induced vascular endothelial cell proliferation and angiogenesis. Cell Signal. 2020;70:109595.
- Wan H, You T, Luo W. Circ_0003204 regulates cell growth, oxidative stress, and inflammation in ox-LDL-induced vascular endothelial cells via regulating miR-942-5p/hdac9 axis. Front Cardiovasc Med. 2021;8:646832.
- Zhang C, Wang L, Shen Y. Circ_0004104 knockdown alleviates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells through targeting miR-328-3p/trim14 axis in atherosclerosis. BMC Cardiovasc Disord. 2021;21(1):207.
- Tiliwaldi H, Tursun A, Tohti A, et al. Circ_0000345 protects endothelial cells from oxidized low-density lipoprotein-induced injury by miR-129-5p/Ten-eleven translocation axis. J Cardiovasc Pharmacol. 2021;77(5):603–613.
- Li S, Huang T, Qin L, et al. Circ_0068087 silencing ameliorates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells depending on miR-186-5p-mediated regulation of roundabout guidance receptor 1. Front Cardiovasc Med. 2021;8:650374.
- Liu H, Ma X, Wang X, et al. Hsa_circ_0000345 regulates the cellular development of ASMCs in response to oxygenized low-density lipoprotein. J Cell Mol Med. 2020;24(20):11849–11857.
- Li D, Jin W, Sun L, et al. Circ_0065149 alleviates oxidized low-density lipoprotein-induced apoptosis and inflammation in atherosclerosis by targeting miR-330-5p. Front Genet. 2021;12:590633.
- Skuratovskaia D, Vulf M, Komar A, et al. Promising directions in atherosclerosis treatment based on epigenetic regulation using microRNAs and long noncoding RNAs. Biomolecules. 2019;9(6):226. DOI:10.3390/biom9060226
- Peng W, Li T, Pi S, et al. Suppression of circular RNA circDHCR24 alleviates aortic smooth muscle cell proliferation and migration by targeting miR-149-5p/mmp9 axis. Biochem Biophys Res Commun. 2020;529(3):753–759.
- Yang L, Yang F, Zhao H, et al. Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/foxo1/cyclin D1 pathway. Mol Ther Nucleic Acids. 2019;16:434–441.
- Li X, Li L, Dong X, et al. Circ_grn promotes the proliferation, migration, and inflammation of vascular smooth muscle cells in atherosclerosis through miR-214-3p/foxo1 axis. J Cardiovasc Pharmacol. 2021;77(4):470–479.
- Zhuang JB, Li T, Hu XM, et al. Circ_chfr expedites cell growth, migration and inflammation in ox-LDL-treated human vascular smooth muscle cells via the miR-214-3p/Wnt3/β-catenin pathway. Eur Rev Med Pharmacol Sci. 2020;24(6):3282–3292.
- Sun C, Li J, Li Y, et al. Circular RNA circUBR4 regulates ox-LDL-induced proliferation and migration of vascular smooth muscle cells through miR-185-5p/frs2 axis. Mol Cell Biochem. 2021;476(11):3899–3910.
- Ding Y, Tang T, Lu J, et al. Circ_ubr4 knockdown alleviates oxidized low-density lipoprotein-provoked growth and migration of human vascular smooth muscle cells by acting on the miR-637/FOXO4 pathway. J Cardiovasc Pharmacol. 2021;78(4):534–543.
- Zhang Y, Zhang C, Chen Z, et al. Blocking circ_ubr4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis. Open Life Sci. 2021;16(1):419–430.
- Li R, Jiang Q, Zheng Y. Circ_0002984 induces proliferation, migration and inflammation response of VSMCs induced by ox-LDL through miR-326-3p/vamp3 axis in atherosclerosis. J Cell Mol Med. 2021;25(16):8028–8038.
- Fu X, Niu T, Yang T, et al. CircMAPK1 promotes the proliferation and migration of vascular smooth muscle cells through miR-22-3p/methyl-CpG binding protein 2 axis. Nutr Metab Cardiovasc Dis. 2021;31(7):2189–2198.
- Zeng Z, Xia L, Fan S, et al. Circular RNA CircMAP3K5 acts as a MicroRNA-22-3p sponge to promote resolution of intimal hyperplasia via tet2-mediated smooth muscle cell differentiation. Circulation. 2021;143(4):354–371. DOI:10.1161/CIRCULATIONAHA.120.049715
- Fan K, Ruan X, Wang L, et al. Circ_0004872 promotes platelet-derived growth factor-BB-induced proliferation, migration and dedifferentiation in HA-VSMCs via miR-513a-5p/txnip axis. Vascul Pharmacol. 2021;140:106842.
- Ji N, Wang Y, Gong X, et al. CircMTO1 inhibits ox-LDL-stimulated vascular smooth muscle cell proliferation and migration via regulating the miR-182-5p/rasa1 axis. Mol Med. 2021;27(1):73.
- Huang Z, Li P, Wu L, et al. Hsa_circ_0029589 knockdown inhibits the proliferation, migration and invasion of vascular smooth muscle cells via regulating miR-214-3p and STIM1. Life Sci. 2020;259:118251.
- Xu F, Shen L, Chen H, et al. circDENND1B participates in the antiatherosclerotic effect of IL-1β monoclonal antibody in mouse by promoting cholesterol efflux via miR-17-5p/abca1 axis. Front Cell Dev Biol. 2021;9:652032.
- Gardner SE, Humphry M, Bennett MR, et al. Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α-dependent senescence-associated secretory phenotype. Arterioscler Thromb Vasc Biol. 2015;35(9):1963–1974.
- Liu J, Wang Y, Liao Y, et al. Circular RNA PPP1CC promotes Porphyromonas gingivalis-lipopolysaccharide-induced pyroptosis of vascular smooth muscle cells by activating the HMGB1/TLR9/AIM2 pathway. J Int Med Res. 2021;49(3):300060521996564.
- Zhao J, He X, Zuo M, et al. Anagliptin prevented interleukin 1β (IL-1β)-induced cellular senescence in vascular smooth muscle cells through increasing the expression of sirtuin1 (SIRT1). Bioengineered. 2021;12(1):3968–3977. DOI:10.1080/21655979.2021.1948289
- Holdt LM, Stahringer A, Sass K, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429.
- Sun J, Zhang Z, Yang S. Circ_rusc2 upregulates the expression of miR-661 target gene SYK and regulates the function of vascular smooth muscle cells. Biochem Cell Biol. 2019;97(6):709–714.
- YY M, JQ W, XX G, et al. Circ-SATB2 upregulates STIM1 expression and regulates vascular smooth muscle cell proliferation and differentiation through miR-939. Biochem Biophys Res Commun. 2018;505(1):119–125.
- Yu H, Zhao L, Zhao Y, et al. Circular RNA circ_0029589 regulates proliferation, migration, invasion, and apoptosis in ox-LDL-stimulated VSMCs by regulating miR-424-5p/igf2 axis. Vascul Pharmacol. 2020;135:106782.
- Lin DS, Zhang CY, Li L, et al. Circ_robo2/mir-149 axis promotes the proliferation and migration of human aortic smooth muscle cells by activating NF-κB signaling. Cytogenet Genome Res. 2021;161(8–9):414–424.
- Marchio P, Guerra-Ojeda S, Vila JM, et al. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845.
- Grootaert MOJ, Moulis M, Roth L, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114(4):622–634.
- Wang X, Bai M. CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc Disord. 2021;21(1):51.
- He Q, Shao D, Hao S, et al. CircSCAP Aggravates oxidized low-density lipoprotein-induced macrophage injury by upregulating PDE3B by miR-221-5p in Atherosclerosis. J Cardiovasc Pharmacol. 2021;78(5):e749–760.
- Yu F, Zhang Y, Wang Z, et al. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics. 2021;11(11):5404–5417. DOI:10.7150/thno.48389
- Wei MY, Lv RR, Teng Z. Circular RNA circHIPK3 as a novel circRNA regulator of autophagy and endothelial cell dysfunction in atherosclerosis. Eur Rev Med Pharmacol Sci. 2020;24(24):12849–12858.
- Liu J, Wei Y, Lin Y, et al. Expression of the circular RNAs in astaxanthin promotes cholesterol efflux from THP-1 cells based on RNA-seq. Genes Nutr. 2021;16(1):13.
- Guo M, Yan R, Ji Q, et al. IFN regulatory Factor-1 induced macrophage pyroptosis by modulating m6a modification of circ_0029589 in patients with acute coronary syndrome. Int Immunopharmacol. 2020;86:106800.
- Lin Z, Tang X, Wan J, et al. Functions and mechanisms of circular RNAs in regulating stem cell differentiation. RNA Biol. 2021;18(12):2136–2149.
- Beck-Joseph J, Lehoux S. Molecular interactions between vascular smooth muscle cells and macrophages in atherosclerosis. Front Cardiovasc Med. 2021;8:737934.
- Shao X, Liu Z, Liu S, et al. Astragaloside IV alleviates atherosclerosis through targeting circ_0000231/mir-135a-5p/clic4 axis in as cell model in vitro. Mol Cell Biochem. 2021;476(4):1783–1795.
- Qian W, Cai X, Qian Q, et al. Astragaloside IV protects endothelial progenitor cells from the damage of ox-LDL via the LOX-1/NLRP3 inflammasome pathway. Drug Des Devel Ther. 2019;13:2579–2589.
