ABSTRACT
The PI3K/Akt/GSK3β pathway is crucial in regulating cardiomyocyte growth and survival. It has been shown that activation of this pathway alleviates the negative impact of ischemia-reperfusion. Glycogen synthase kinase-3 (GSK3β) induces apoptosis through stimulation of transcription factors, and its phosphorylation has been suggested as a new therapeutic target for myocardial ischemia-reperfusion injury (MIRI). GSK3β regulatory role is mediated by the reperfusion injury salvage kinase (RISK) pathway, and its inhibition by Akt activation blocks mitochondrial permeability transition pore (mPTP) opening and enhances myocardial survival. The present article discusses the involvement of the PI3K/Akt/GSK3β pathway in cardioprotective effects of natural products against MIRI.
Abbreviations: Akt: protein kinase B; AMPK: AMP-activated protein kinase; ATP: adenosine triphosphate; Bad: bcl2-associated agonist of cell death; Bax: bcl2-associated x protein; Bcl-2: B-cell lymphoma 2; CK-MB: Creatine kinase-MB; CRP: C-reactive-protein; cTnI: cardiac troponin I; EGCG: Epigallocatechin-3-gallate; Enos: endothelial nitric oxide synthase; ER: endoplasmic reticulum; ERK ½: extracellular signal‑regulated protein kinase ½; GSK3β: glycogen synthase kinase-3; GSRd: Ginsenoside Rd; GSH: glutathione; GSSG: glutathione disulfide; HO-1: heme oxygenase-1; HR: hypoxia/reoxygenation; HSYA: Hydroxysafflor Yellow A; ICAM-1: Intercellular Adhesion Molecule 1; IKK-b: IκB kinase; IL: interleukin; IPoC: Ischemic postconditioning; IRI: ischemia-reperfusion injury; JNK: c-Jun N-terminal kinase; Keap1: kelch-like ECH-associated protein- 1; LDH: lactate dehydrogenase; LVEDP: left ventricular end diastolic pressure; LVP: left ventricle pressure; LVSP: left ventricular systolic pressure; MAPK: mitogen-activated protein kinase; MDA: malondialdehyde; MIRI: myocardial ischemia-reperfusion injury; MnSOD: manganese superoxide dismutase; mPTP: mitochondrial permeability transition pore; mtHKII: mitochondria-bound hexokinase II; Nrf-1: nuclear respiratory factor 1; Nrf2: nuclear factor erythroid 2-related factor; NO: nitric oxide; PGC-1α: peroxisome proliferator‑activated receptor γ coactivator‑1α; PI3K: phosphoinositide 3-kinases; RISK: reperfusion injury salvage kinase; ROS: reactive oxygen species; RSV: Resveratrol; SOD: superoxide dismutase; TFAM: transcription factor A mitochondrial; TNF-α: tumor necrosis factor-alpha; VEGF-B: vascular endothelial growth factor B
1. Introduction
Myocardial ischemia-reperfusion injury (MIRI) is a critical condition that leads to an inflammatory response, which causes further cellular damage and organ function impairment through induction of apoptosis by inducing oxidative stress, calcium overload, mitochondrial permeability transition pore (mPTP) opening, and hypercontracture [Citation1,Citation2].
The term ischemia denotes insufficient oxygen supply due to arterial inflow obstruction. During ischemia, anaerobic metabolism dominates and reduces cell pH and ATP levels [Citation3]. Thus, membrane ATP-dependent ionic pump function is altered. Anaerobic metabolism deactivates sodium-potassium and calcium pumps that preserve sodium and calcium inside and potassium outside the cell, on the cell membrane. Also, this condition disrupts calcium pumps on the endoplasmic reticulum, limiting calcium reuptake. The cytosolic Ca2+ rise increases mitochondrial Ca2+ and inner membrane permeability [Citation4]. In addition, a higher level of sodium in cells reduces sodium-hydrogen pump activity. Sodium and calcium ions retention causes hyperosmolarity and cell swelling, while hydrogen retention decreases cellular pH, leading to enzyme dysfunction and nuclear chromatin accumulation [Citation5].
The process of blood returning to the ischemic myocardium is called reperfusion. The potentially detrimental aspect of myocardial reperfusion injury involves reactive oxygen species (ROS) production, microvascular and endothelial dysfunction, myocardial metabolism changes, and activation of neutrophils, platelets, and complement [Citation6]. The ROS formation in reperfusion injury occurs through molecular oxygen reduction or other sources including the function of the enzymes, such as xanthine oxidase, cytochrome oxidase, and cyclooxygenase, and the oxidation of catecholamines [Citation7]. In ischemia, ATP is catabolized to hypoxanthine, and xanthine dehydrogenase (XDH) is converted via limited proteolysis and sulfhydryl oxidation to xanthine oxidase (XO). Upon reperfusion, an influx of O2 reacts with hypoxanthine (or xanthine) and XO to generate superoxide (O2−) and hydrogen peroxide (H2O2), which increase ROS generation [Citation8]. Superoxide anion induces lipid peroxidation, cell permeability disruption, and oxidation of proteins and DNA, leading to cell death [Citation9,Citation10]. Free radicals stimulate the endothelium to release platelet-activating factor (PAF), attracting more neutrophils and amplifying the production of oxidant radicals [Citation11]. Notably, lack of cell antioxidants further augments ROS levels. At the same time, responses to endothelium-dependent vasoconstrictors, such as endothelin-1 and oxygen-free radicals increase and result in endothelial injury and microvascular dysfunction [Citation12,Citation13].
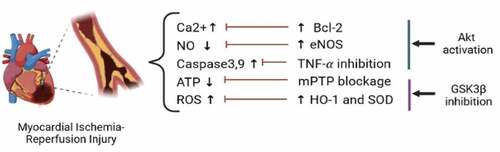
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/glycogen synthase kinase-3 β (GSK3β) pathway is one of the vital intracellular signaling pathways, which controls cell metabolism, growth, and proliferation, and stress response [Citation14,Citation15]. This pathway activates PI3K, which then phosphorylates Phosphatidylinositol-4,5-bisphosphate (PIP2), forming phosphatidylinositol-3,4,5-trisphosphate (PIP3) as a membranous second messenger. Upon binding PIP3, Akt undergoes a conformational change, facilitating its phosphoinositide-dependent kinase-1 (PDK1)-dependent phosphorylation [Citation16]. Once phosphorylated, Akt becomes active, moves from the membrane, and phosphorylates/inactivates GSK3β [Citation17]. GSK3β participates in some apoptotic signaling transduction by phosphorylating transcription factors that regulate apoptosis; GSK3β induces activation of pro-apoptotic factors, such as p53 [Citation18] and suppression of survival-promoting factors, so its phosphorylation/inactivation enhances cell survival [Citation19]. Within this intracellular signaling, PI3K/Akt phosphorylates several downstream targets, including NF-KB, to upregulate cell survival signaling pathways. When NF-kB forms a complex with IkB, it remains in the cytoplasm. Akt phosphorylates IkB and degrades it [Citation20]. For instance, it was shown that in human osteosarcoma cells, the liberated NF-kB enters the nucleus to increase cell survival [Citation21]. PI3K/Akt also regulates the Fas/Fas-ligand system, an essential pathway for cellular apoptosis. Fas and Fas-ligand are members of the Tumor necrosis factor (TNF) family. Activation of this system leads to recruitment of Fas-associated death domain (FADD) and triggers cell apoptosis [Citation22]. PI3K/Akt blocks forkhead protein, which promotes Fas-ligand [Citation23]. Furthermore, PI3K/Akt blocks the pro-apoptotic protein Bad to increase the anti-apoptotic protein bcl-2 level [Citation24] and reduces cytosolic calcium [Citation25]. PI3K/Akt activation induces endothelial nitric oxide synthase (eNOS) phosphorylation, which leads to NO secretion [Citation26] and suppression of c-Jun N-terminal kinase (JNK) thus enhancing cell survival [Citation27,Citation28]. GSK3β phosphorylation inhibits mPTP opening and stabilizes peroxisome proliferator‑activated receptor γ coactivator‑1α (PGC-1α) which increases mitochondrial respiration by activating PI3K/Akt and ERK1/2 involved in the reperfusion injury salvage kinase (RISK) pathway [Citation29–31]. Moreover, GSK3β phosphorylation results in cell protection against oxidation and inflammation through Nrf2 stimulation by superoxide dismutase (SOD), heme oxygenase-1 (HO-1), manganese superoxide dismutase (MnSOD), and catalase activation [Citation32]. The present article discusses the involvement of PI3K/Akt/GSK3β pathway in cardioprotective effects of natural products against MIRI ().
Figure 1. The protective mechanisms mediated through regulation of PI3K/Akt/GSK3β against myocardial ischemia-reperfusion injury. ATP, adenosine triphosphate; Bcl‐2, B-cell lymphoma 2; eNOS, endothelial nitric oxide synthase; HO-1, heme oxygenase-1; mPTP, mitochondrial permeability transition pore; NO, Nitric oxide; ROS, reactive oxygen species; SOD, superoxide dismutase; and TNF-α, tumor necrosis factor-alpha.
2.1. Syringic acid
Syringic acid is a phenolic acid found in olives, grapes, and dates, and it exerts antioxidant, anti-inflammatory, and anti-endotoxic properties [Citation33]. Recent studies have revealed that syringic acid protects hippocampal neuronal and H9C2 cells in hypoxia/reoxygenation (HR), and in other organs the spinal cord and kidney under ischemia-reperfusion (IR) [Citation34–37]. In a rat model of MIRI, syringic acid administration (50 mg/kg for three consecutive days before reperfusion) diminished infarct size, decreased apoptosis, and enhanced cardiac function through PI3K/Akt/GSK3β phosphorylation. This pathway increases the ratio of Bcl-2/Bax and reduces the contents of cleaved-9 and 3. In this pathway, GSK3β is phosphorylated by activated Akt on its serine 9 residue. The phosphorylated GSK3β suppresses mPTP opening and inhibits cytochrome c release from the mitochondria ( and ) [Citation38].
2.2. Berberine
Berberine, an isoquinoline alkaloid, is the main active ingredient of Rhizoma coptidis, a famous traditional Chinese herb with hypoglycemic and anti-inflammatory properties [Citation39–41]. This compound could attenuate endothelial dysfunction and cardiovascular disorders in diabetic rats and reduce mortality in patients with severe congestive heart failure [Citation42,Citation43]. In pressure overload-induced cardiac hypertrophy models, berberine treatment reduced infarct size in nondiabetic rats with ischemia-reperfusion injury (IRI) and reduced left ventricular myocardium size [Citation44,Citation45]. During cardiac ischemia, AMPK is activated to increase myocardial glucose uptake, glycolysis, and fatty acid oxidation for energy production and heart protection [Citation46]. It is noticeable that although crosstalk between Akt and AMPK signaling is still not apparent in cardiac myocytes and vascular endothelial cells, Akt activation appears to be regulated by the AMPK pathway [Citation47]. Berberine pretreatment was shown to induce AMP/ATP and ADP/ATP ratio, AMPK activation, phosphorylation of AKT, and inhibition of GSK3β to save mitochondria. Also, berberine alleviated malondialdehyde (MDA) levels and cardiac arrhythmia in diabetic rats with IRI ( and ) [Citation48].
2.3. Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG), the primary catechin of green tea, is known to have cardioprotective effects [Citation49] mediated through its anti-inflammatory property [Citation50]. EGCG administration before [Citation51] or after [Citation52] ischemic insult limited infarct size in the heart. It mitigated regional MIRI by activating RISK, a group of pro-survival protein kinases, and a combination of two parallel cascades, PI3K-Akt and MEK1-ERK1/2 [Citation53], and preventing cell death through JNK suppression. The PI3K/Akt pathway seems to be involved in EGCG-induced GSK3β inactivation. It was indicated that EGCG phosphorylates GSK3β through Akt activation and inhibits mPTP opening [Citation54]. In addition, while MAPKs cause cell death following IRI [Citation55], EGCG suppressed p38/JNK phosphorylation while increasing Akt/GSK3β phosphorylation ( and ) [Citation54].
2.4. Hydroxysafflor yellow A
Hydroxysafflor Yellow A (HSYA) is one of the active components of Honghua (Carthamus tinctorius L.) which is used for coronary heart disease treatment [Citation56–58]. It is evident that apoptosis plays a crucial role during MIRI and involves mitochondrial permeabilization leading to cytochrome c release and caspase-3 activation [Citation59,Citation60]. Mitochondrial function is associated with the RISK pathway [Citation61,Citation62]. It was demonstrated that mitochondrially bound hexokinase II (mtHKII) is a crucial element of ischemic preconditioning. Hexokinase II could be activated by Akt, thus restoring mitochondrial energy, reducing ROS generation, and inhibiting apoptosis [Citation63]. HSYA was shown to significantly reduce lactate dehydrogenase (LDH), oxidative stress, caspase 3 levels, and cell apoptosis, while increasing cell viability, mitochondrial energy metabolism, Akt activation, and GSK3β suppression in H9c2 cells ( and ) [Citation63].
2.5. Resveratrol
Resveratrol is a polyphenolic compound extracted from berries, peanuts, and particularly grape skins with various health-beneficial effects [Citation64–68]. Its biological activities include lifespan extension [Citation69], antidiabetic [Citation70,Citation71], and anticancer properties [Citation72,Citation73]. Its anti-apoptotic effect is mediated through the vascular endothelial growth factor B (VEGF-B) up-regulation in pancreatic cancer cells [Citation74]. One of the most well-known effects of resveratrol is cardioprotection [Citation75,Citation76]. Resveratrol improves heart functions, upregulates MnSOD and catalase, reduces infarct size and CK-MB release, and inhibits cell death during MIRI. In addition, resveratrol showed a lowering effect on ROS generation in H9c2 cells. It was reported that resveratrol upregulated VEGF-B, which induces Akt and GSK3β phosphorylation, and showed cardioprotection against IRI. Furthermore, resveratrol reduced Bax protein expression in heart tissue ( and ) [Citation77].
2.6. Kaempferol
Kaempferol is a flavonoid commonly found in kale, beans, tea, and spinach. Numerous preclinical studies have shown kaempferol pharmacological functions including antioxidant, anti-inflammatory, and anticancer activities [Citation78–80]. In an IRI model of isolated rat heart, kaempferol improved the recovery phase and reduced intracellular oxidation, myocardial ischemia, and apoptosis through GSK3β phosphorylation. Also, kaempferol enhanced SOD activity and glutathione/oxidized glutathione (GSH/GSSG) ratio while decreasing MDA level and preventing myocardial membrane peroxidation. During MIRI, a large amount of TNF-α is produced, and kaempferol could reduce TNF-α levels by PI3K stimulation [Citation81]. This decrease has been speculated to partially contribute to reduced myocardial infarction size and prevention of caspase-3 and −9 cleavage [Citation82]. Kaempferol induced all these effects by PI3K/Akt/GSK3β pathway phosphorylation and, consequently, mPTP opening suppression ( and ) [Citation81].
2.7. Safranal
Safranal, a monoterpene aldehyde isolated from saffron (Crocus sativus), has shown radical-scavenging and anti-apoptotic properties [Citation83], and conferred protection against indomethacin-induced gastric ulcers [Citation84], pentylenetetrazol-induced status epilepticus [Citation85], and cancer [Citation86]. Safranal also showed an anti-ischemic effect during renal IRI [Citation87]. It was shown that this compound prevents MIRI progression and improves cardiac performance in rats by phosphorylation of Akt/GSK3β/eNOS and inhibition of IKK-b/NF-kB pathways. Also, it provides anti-inflammatory and anti-apoptotic effects by TNF-α suppression. Safranal upregulated the endogenous antioxidant defense system and reduced nitrotyrosine levels. Thus, safranal limited the infarct size and improved inotropic reserves and contractile function. Enhanced Akt/GSK3β phosphorylation negatively regulates MIRI by activating the sarcoplasmic reticulum Ca2 +-ATPase2a which reduces cytosolic Ca2+ overload and improves contractile function [Citation88]. Inhibition of IKK-b/NF-kB/caspase-3, Bax, and TNF-α improved heart function and decreased infarct size following MIRI [Citation89,Citation90]. Safranal indirectly upregulates Bcl-2, which leads to Ca2+ overload reduction [Citation91], and normalized LDH and CK-MB activities in MIRI [Citation92]. Also, Akt phosphorylates and activates eNOS [Citation93], and oxidative stress augments IRI by promoting functional NO deficiency. Increased superoxide/ROS and NO/tyrosine produce nitrotyrosine, a marker for ROS-arbitrated NO oxidation [Citation94]. Notably, safranal-suppressed nitrotyrosine levels were correlated with increased eNOS phosphorylation in MIRI. Therefore, one of the mechanisms by which safranal ameliorated MIRI, is Akt phosphorylation and decreased nitrotyrosine level ( and ) [Citation95].
2.8. Astragaloside IV
Astragaloside IV is one of the effective constituents of Astragalus membranaceus, with various pharmacological effects, including immunity enhancement [Citation96], anti-inflammation [Citation97], antioxidation [Citation98], and anti-virus [Citation99] properties. It has been reported that astragaloside IV attenuates viral myocarditis [Citation100], myocardial fibrosis [Citation101], and heart failure [Citation102]. In addition, previous studies have shown that it protects the myocardium against IRI with different mechanisms [Citation103–106]. Besides, astragaloside IV improved left ventricular systolic pressure (LVSP), fractional shortening, and ejection fraction, and reduced LDH, CK-MB levels, heart-to-body weight ratio, and myocardial infarct size in MIRI through PI3K/Akt/GSK3β phosphorylation ( and )[Citation107].
2.9. Kaempferide
Kaempferide is a flavonoid obtained from Alpinia officinarum roots with excellent antioxidant [Citation108], anticancer [Citation109], and antihypertension properties [Citation110]. Its pretreatment in MIRI improved LVSP, fractional shortening, and ejection fraction, while left ventricular end-diastolic pressure (LVEDP), CK-MB and LDH levels, and myocardial infarct size were decreased. Kaempferide treatment also decreased C-reactive-protein (CRP), interleukin (IL)-6, TNF-α, MDA, ROS, and apoptosis and enhanced SOD activity. So, according to these results, this compound’s cardioprotective effects depend on its anti-inflammatory and anti-oxidative features. The GSK3β/caspase-3-dependent pathway phosphorylation has been proven to protect cardiomyocytes from IRI and inhibit apoptosis [Citation111]. So, GSK3β phosphorylation and caspase-3 inhibition in rats could be responsible for the kaempferide cardioprotection against IRI. Together, these results suggest that the potential benefits of kaempferide in MIRI are likely mediated via activation of PI3K/Akt/GSK3β/caspase-3 pathways ( and ) [Citation112].
2.10. Ginsenoside Rd
Ginsenoside Rd is one of the active components of Panax ginseng that scavenges free radicals [Citation113], inhibits Ca2+-influx via a receptor and store-operated Ca2+ channels [Citation114], and protects against neuronal apoptosis [Citation115]. It is a lipophilic compound that readily diffuses across biological membranes, so that it may be advantageous for the heart [Citation116]. A study reported that ginsenoside Rd could attenuate infarct size and enhance myocardial structure in rats [Citation117]. In a cell culture model, Panax notoginseng saponins prevented cardiomyocyte apoptosis induced by glucose and oxygen deprivation injury via PI3K/Akt signaling [Citation118]. Ginsenoside Rd augmented cardiac function, increasing ±LVdP/dt max and decreasing LVEDP, and reduced cardiomyocytes intracellular ROS generation. Ginsenoside Rd also attenuated cellular damage in cultured neonatal rat cardiomyocytes (NRCs) subjected to IR through ROS, mitochondrial membrane potential, cytochrome c release mitigation, and Bcl-2/Bax ratio enhancement. Akt overexpression raises Bcl-2 levels, and phosphorylated GSK3β suppresses mPTP opening by binding to adenine nucleotide translocase; these findings support the involvement of the Akt/GSK3β signaling pathway in ginsenoside Rd cardioprotection ( and ) [Citation119].
2.11. Curcumin
Curcumin is a yellow pigment from Curcuma longa (turmeric) commonly used as a spice/food coloring agent. It is relatively safe and nontoxic and exerts diverse biological effects such as anti‐inflammatory [Citation120], antidiabetic, antioxidant [Citation121,Citation122], immunomodulatory [Citation123], and anti-carcinogenic functions [Citation124]. Also, evidence suggests that curcumin has cardioprotective effects against MIRI [Citation125]. Curcumin reduces the phosphorylation of JNK and p38-MAPKs and infarct size by the PI3K/Akt and ERK1/2 pathway phosphorylation. It was indicated that curcumin administration before left anterior descending coronary artery occlusion protects against focal MIRI through induction of PI3K/Akt and ERK1/2 [Citation75]. While in IR, the pro-survival kinases PI3K/Akt and ERK1/2 may be overwhelmed by p38-MAPK and JNK [Citation126], resulting in myocardial apoptosis and necrosis, curcumin suppresses p38 and JNK phosphorylation. Curcumin administration resulted in myocardial protection by inhibiting GSK3β and mPTP opening ( and ) [Citation75].
2.12. Fisetin
Fisetin is a flavonoid found in many plants, such as strawberries, apples, persimmons, onions, and cucumbers. This compound has antioxidant, anti-inflammatory, and anti-apoptotic effects, exhibited neuroprotective properties, and inhibited cancer cells in several preclinical studies [Citation127,Citation128]. Also, cardioprotective potentials of fisetin were shown in vitro and in vivo [Citation129]. The compound caused a marked improvement in cardiac function, decreased myocyte injury markers, such as LDH and CK, and attenuated mitochondrial swelling. Fisetin also maintained mitochondria hyperpolarized and boosted their antioxidant capacity; these observations suggest that fisetin inhibits mPTP opening and improves mitochondrial function, thereby preventing IR-induced myocardial tissue injury. In addition, fisetin blunted excessive oxidative stress in lysosomes and microsomes obtained from MIRI-affected hearts [Citation130]. Fisetin could activate Nrf-2 and augment the expression of HO-1 to protect against oxidative insults [Citation131]. It was indicated that though MIRI reduced the level of PGC-1α, nuclear respiratory factor 1 (Nrf-1), and transcription factor A mitochondrial (TFAM), fisetin marginally enhanced their expression [Citation130]. GSK3β suppresses PGC-1α activation through its phosphorylation and thus prepares PGC-1α for degradation via the ubiquitin-proteasome pathway [Citation132]. In a mouse model of MIRI, GSK3β activity in myocardial tissues was raised, and this increase was suppressed by fisetin treatment. In summary, fisetin confers cardioprotection against MIRI by bolstering mitochondrial physiology, suppressing oxidative stress, and augmenting mitochondrial biogenesis, and these effects are mediated via inhibition of GSK3β activity ( and ) [Citation130,Citation133].
2.13. Leonurine
Leonurine is an alkaloid obtained from Herba leonuri and it has an extensive range of biological activities, including anti-inflammatory [Citation134], antioxidant [Citation135], anti-tumor [Citation136], and cardiovascular protective effects [Citation137]. It also induced Akt phosphorylation and hypoxia-inducible factor 1 expression, survival, and VEGF expression in rats with myocardial ischemia. Besides, leonurine inhibited apoptosis in H9c2 cells under hypoxia by increasing the Bcl‑2/Bax ratio [Citation138]. This compound also diminished the infarct size, alleviated collagen deposition, inhibited cardiomyocyte apoptosis, prevented left ventricular dilation, and improved cardiac function by activating the PI3K/Akt/GSK3β signaling pathway. Together, leonurine improved cardiac function in MIRI through up‑regulation of anti‑apoptotic protein Bcl‑2 and down‑regulation of pro‑apoptotic protein Bax and cleaved caspase-3 ( and ).
2.14. Lycopene
Lycopene is a carotenoid present in tomatoes and other red fruits and vegetables, and it exerts antioxidant properties and reduces the risk of coronary heart disease [Citation139]. Some studies demonstrated that lycopene protects against IRI by inhibiting the development of endoplasmic reticulum (ER) stress [Citation140] and blocking mPTP opening [Citation141]. It was found that lycopene and Ischemic postconditioning (IPoC) activated Akt and ERK1/2, subsequently inhibiting GSK3β and mPTP opening in hypercholesterolemic rats heart. This finding suggests that restoration of IPoC by lycopene may be partly attributed to the reactivation of the RISK pathway. ER stress has been demonstrated to impair RISK pathway activation, suppress Akt and ERK1/2 phosphorylation, and further phosphorylated GSK3β-mediated suppression of mPTP opening [Citation142]. Notably, the effect of lycopene administration in IPoC on the RISK pathway was similar to that of an ER stress inhibitor. Thus, it may be inferred that lycopene reactivates Akt and ERK1/2 in hypercholesterolemic rats heart partly through inhibition of ER stress ( and ) [Citation143].
2.15. Rhein
Rhein, an anthraquinone isolated from rhubarb, has been used for many years in China to treat constipation, gastrointestinal hemorrhage, and ulcers [Citation144]. Also, rhein protects the pancreatic β cells from hyperglycemia-induced cell apoptosis [Citation145]. Rhein was shown to protect H9c2 cells from HR injury by reducing ROS production via PI3K/Akt signaling pathway. Rhein was found to increase phosphorylated Akt and GSK3β, and GSK3β silencing abolished rhein’s anti-apoptotic effects, suggesting that rhein protects the myocardium against IRI through Akt/GSK3β pathway. In addition, rhein inhibited apoptosis by suppressing P38 phosphorylation ( and ) [Citation27].
2.16. Salvianolic acid A
Salvianolic acid A, the main ingredient of Salvia miltiorrhiza, has various pharmacological activities, such as preventing myocardial ischemia and regulating the immune system [Citation146,Citation147]. It was indicated that salvianolic acid A preserved cardiac function against IRI by reducing the myocardial infarct area and enzyme leakage [Citation148]. The compound could attenuate cardiomyocyte apoptosis and decrease the expression of cleaved caspase-3 and Bax/Bcl-2 ratio, suggesting that it inhibits MIRI by suppressing apoptosis [Citation149]. Also, salvianolic acid A enhanced ATP levels, reduced ROS production, and protected cardiomyocytes against H2O2-induced injury [Citation150]. It preserved the mitochondrial membrane potential and blocked mPTP opening through Akt/GSK3β phosphorylation, which boosts cardiomyocyte tolerance to HR. The salvianolic acid A protective effects were attributed to GSK3β phosphorylation and, consequently, mitochondria survival ( and ) [Citation149].
2.17. Shikonin
Shikonin is a natural naphthoquinone pigment purified from the root of Lithospermum erythrorhizon [Citation151]. It alleviates brain and liver IR outcomes [Citation152,Citation153] and protects against isoproterenol-induced heart damage [Citation154]. Shikonin protection against HR injury in H9c2 cells was suggested to be mediated through attenuating excessive ROS generation and apoptosis. It prevented mitochondrial membrane potential collapse and cytochrome c release to the cytosol to inhibit apoptosis during HR associated with mitochondria dysfunction through Akt/GSK3β phosphorylation ( and ) [Citation155].
2.18. Spinosin and 6‴-feruloylspinosin
Spinosin and 6‴-feruloylspinosin, two C-glycoside flavonoids obtained from Semen Ziziphi spinosae (Suanzaoren), have shown anxiolytic and hypnotic effects [Citation156]. In addition, spinosin ameliorated neurogenesis, memory deficit, cognitive performance, and Alzheimer’s disease in mice [Citation157–159]. These compounds reduced myocardial damage and cellular apoptosis, promoted autophagy flux, and upregulated PGC-1α, Nrf2, and HO-1 which were associated with GSK3β inhibition [Citation160]. Overproduction of ROS and free radicals in the mitochondria triggers myocardial damage in the reperfusion phase. Increased ROS levels open mPTP which boosts ROS production and causes myocardial damage [Citation161]. Approaches such as GSK3β inhibition, which limit mPTP induction by increasing the mPTP-ROS threshold, protect myocardial cells [Citation162]. Spinosine and 6‴-feruloylspinosin restrain GSK3β activity and protect myocardial cells. GSK3β inhibition induces multiple cell survival pathways, including pro-survival autophagy [Citation163] and PGC-1α/Nrf2/HO-1 [Citation164]. It was further identified that both spinosin and 6‴-feruloylspinosin could enhance the expression of LC3B-II, while 6’’‘‑feruloylspinosin could reduce the level of p62, indicating activation of autophagy. PGC-1α is an inductive transcription activator in repairing ROS-induced mitochondrial damage and biogenesis and regulating energy metabolism. GSK3β can phosphorylate PGC-1α and promote its ubiquitin-dependent degradation; however, its inhibition induces PGC-1α accumulation to protect cells [Citation165]. The cytoprotective effect of PGC-1α is related to Nrf2 upregulation and subsequently, induction of the expression of HO-1 gene. This enzyme catalyzes the degradation of pro-oxidant heme into three important antioxidant products, bilirubin, carbon monoxide, and ferrous ion [Citation166]. Besides, Nrf2 induces the expression of superoxide dismutase 2 (SOD-2). Overall, the cardioprotective effects of spinosin and 6‴-feruloylspinosin were shown to be mediated by inhibition of GSK3β and upregulation of PGC-1α, Nrf2, and HO-1 proteins level ( and ) [Citation160].
Table 1. Natural compounds that protect against myocardial ischemia-reperfusion injury via phosphorylation of PI3K/Akt/GSK3β.
2.19. Urolithin A, B
The link between gut microbiota and its metabolites and MIRI has been indicated, and it was reported that different diets might influence MIRI consequences [Citation167–169]. Ellagitannins (ETs) are a class of polyphenols that exist in pomegranates, walnuts, and berries [Citation170]. Gut microbiota hydrolyzes ETs to ellagic acid (EA) which is further hydrolyzed into Urolithin A (UA) and Urolithin B (UB) with antioxidant and anti-inflammatory properties [Citation171,Citation172]. UB was found to protect against colonic fibroblast inflammation [Citation173], lipopolysaccharide-induced microglia and macrophage activation [Citation174], and high glucose-induced cardiomyocyte injury. Interestingly, UB could decrease myocardial infarction (MI) size, elevate cardiac function in rats after IR, and protect against HR injury in H9C2 cardiomyocytes. UB inhibited autophagy by activating Akt/mTOR/ULK1 pathway and protected against oxidative stress and caspase 3-dependent cell apoptosis [Citation175]. UA health benefits include neuroprotection effects against Alzheimer’s disease and attenuating oxidized-LDL-induced endothelial dysfunction by up-regulation of NO expression and eNOS mRNA expression [Citation176]. UA also protected the heart against IRI by significantly improving cardiac function and reducing myocardial apoptosis. It prevented ROS generation, decreased MDA levels and apoptosis, while increased SOD activation in an HR model, and inhibited myocardial apoptosis following IR through PI3K/Akt pathway activation in mice ( and ) [Citation177].
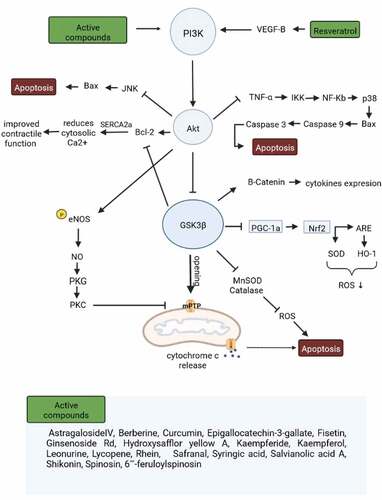
Figure 2. The regulatory effects of natural compounds on the PI3K/Akt/GSK3β signaling pathway. Ischemia-reperfusion in cardiomyocytes induces apoptosis, and some natural products enhance cell survival through PI3K/Akt/GSK3β regulation (phosphorylation). Akt, Ak strain transforming; ARE, antioxidant response element; BAX, Bcl2‐associated X protein; Bcl‐2, B-cell lymphoma 2; eNOS, endothelial nitric oxide synthase; GSK3β, glycogen synthase kinase 3 beta; HO-1, heme oxygenase-1; IKK, IκB kinase; JNK, c-Jun N-terminal kinase; mPTP, mitochondrial permeability transition pore; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor erythroid 2-related factor; NO, Nitric oxide; PGC‑1α, peroxisome proliferator‑activated receptor γ coactivator‑1α; PI3K, Phosphoinositide 3-kinases; PKC, Protein kinase C; PKG, protein kinase G; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha; VEGF-B, vascular endothelial growth factor B.
3. Conclusion
Myocardial ischemia-reperfusion injury (MIRI) is a tissue damage that occurs when the blood returns to tissue after a period of ischemia. It can increase the risk of developing congestive heart failure and arrhythmias, and mortality. Based on the literature, cardiotoxic compounds induce heart damage via various mechanisms, such as inducing ROS generation, oxidative stress, and apoptosis following dysregulation of PI3K/Akt/GSK3β signaling [Citation179,Citation180], so, phosphorylation of this pathway may be a promising therapeutic approach for cardiac IRI. It has been reported that MIRI is accompanied by induction of apoptosis via increasing the level of Bcl-2/Bax, ROS generation, and mPTP opening while reducing NO production. The phytochemicals discussed in the present article have been shown to alleviate MIRI through phosphorylation of the PI3K/Akt/GSK3β signaling pathway, thus preventing untoward consequences and cell death.
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Frank A, Bonney M, Bonney S, et al. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. In: Eckle T, editor. Seminars in cardiothoracic and vascular anesthesia. Vol.16. No. 3. Los Angeles, CA: SAGE Publications Sage CA; 2012. p. 123–132.
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100.
- Mahdiani S, Omidkhoda N, Rezaee R, et al. Induction of JAK2/STAT3 pathway contributes to protective effects of different therapeutics against myocardial ischemia/reperfusion. Biomed Pharmacother. 2022;155:113751.
- Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12(5):815–833.
- Wu M-Y, Yiang G-T, Liao W-T, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46(4):1650–1667.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135.
- Verma S, Fedak PW, Weisel RD, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105(20):2332–2336.
- Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551.
- Fitridge R, Thompson M. Mechanisms of vascular disease: a reference book for vascular specialists. Adelaide (AU): University of Adelaide Press; 2011.
- Yeh C-C, Hou M-F, Tsai S-M, et al. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clinica Chimica Acta. 2005;361(1–2):104–111.
- Baxter GF. The neutrophil as a mediator of myocardial ischemia-reperfusion injury: time to move on. Basic Res Cardiol. 2002;97(4):268–275.
- Carden DL, Granger DN. Pathophysiology of ischaemia–reperfusion injury. J Pathol. 2000;190(3):255–266.
- Granger DN. Ischemia‐reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6(3):167–178.
- Marqués M, Kumar A, Cortés I, et al. Phosphoinositide 3-kinases p110α and p110β regulate cell cycle entry, exhibiting distinct activation kinetics in G1 phase. Mol Cell Biol. 2008;28(8):2803–2814.
- Zeng B, Liu L, Liao X, et al. Thyroid hormone protects cardiomyocytes from H2O2-induced oxidative stress via the PI3K-AKT signaling pathway. Exp Cell Res. 2019;380(2):205–215.
- Walkowski B, Kleibert M, Majka M, et al. Insight into the role of the PI3K/Akt pathway in ischemic injury and post-infarct left ventricular remodeling in normal and diabetic heart. Cells. 2022;11(9):1553.
- Fyffe C, Falasca M. 3-Phosphoinositide-dependent protein kinase-1 as an emerging target in the management of breast cancer. Cancer Manage Res. 2013;5:271.
- Watcharasit P, Bijur GN, Zmijewski JW, et al. Direct, activating interaction between glycogen synthase kinase-3β and p53 after DNA damage. Proc Nat Acad Sci. 2002;99(12):7951–7955.
- Zhai P, Sciarretta S, Galeotti J, et al. Differential roles of GSK-3β during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109(5):502–511.
- Nidai Ozes O, Mayo LD, Gustin JA, et al. NF-κB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature. 1999;401(6748):82–85.
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obesity Rev. 2009;10(6):610–616.
- Whiteside TL. The role of death receptor ligands in shaping tumor microenvironment. Immunol Invest. 2007;36(1):25–46.
- Zhu Q, Liu J-Y, H-W X, et al. Mechanism of counterattack of colorectal cancer cell by Fas/Fas ligand system. World J Gastroenterol. 2005;11(39):6125.
- Zhang C, Li C, Chen S, et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017;11:1–11.
- Bonneau B, Prudent J, Popgeorgiev N, et al. Non-apoptotic roles of Bcl-2 family: the calcium connection. Biochim Biophys Acta -Mol Cell Res. 2013;1833(7):1755–1765.
- Namkoong S, Kim C-K, Cho Y-L, et al. Forskolin increases angiogenesis through the coordinated crosstalk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal. 2009;21(6):906–915.
- Liu J, Li Y, Tang Y, et al. Rhein protects the myocardiac cells against hypoxia/reoxygenation-induced injury by suppressing GSK3β activity. Phytomedicine. 2018;51:1–6.
- Zhao H-F, Wang J, Tony to S-S. The phosphatidylinositol 3-kinase/akt and c-Jun N-terminal kinase signaling in cancer: alliance or contradiction? Int J Oncol. 2015;47(2):429–436.
- Yang S, Li H, Tang L, et al. Apelin-13 protects the heart against ischemia-reperfusion injury through the RISK-GSK-3β-mPTP pathway. Arch Med Sci. 2015;11(5):1065.
- Ong SB, Dongworth R, Cabrera‐fuentes H, et al. Role of the MPTP in conditioning the heart–translatability and mechanism. Br J Pharmacol. 2015;172(8):2074–2084.
- Souder DC, Anderson RM. An expanding GSK3 network: implications for aging research. Geroscience. 2019;41(4):369–382.
- Lv H, Liu Q, Wen Z, et al. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324.
- Srinivasulu C, Ramgopal M, Ramanjaneyulu G, et al. Syringic acid (Sa)‒a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother. 2018;108:547–557.
- Cao Y, Zhang L, Sun S, et al. Neuroprotective effects of syringic acid against OGD/R-induced injury in cultured hippocampal neuronal cells. Int J Mol Med. 2016;38(2):567–573.
- Ding SK, Wang LX, Guo LS, et al. Syringic acid inhibits apoptosis pathways via downregulation of p38 MAPK and JNK signaling pathways in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Mol Med Rep. 2017;16(2):2290–2294.
- Tokmak M, Yuksel Y, Sehitoglu MH, et al. The neuroprotective effect of syringic acid on spinal cord ischemia/reperfusion injury in rats. Inflammation. 2015;38(5):1969–1978.
- Sancak EB, Akbas A, Silan C, et al. Protective effect of syringic acid on kidney ischemia-reperfusion injury. Ren Fail. 2016;38(4):629–635.
- Liu G, Zhang B-F, Hu Q, et al. Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3β signaling pathway. Biochem Biophys Res Commun. 2020;531(2):242–249.
- Mahmoudi M, Zamani Taghizadeh Rabe S, Balali-Mood M, et al. Immunotoxicity induced in mice by subacute exposure to berberine. J Immunotoxicol. 2016;13(2):255–262.
- Hashemzaei M, Rezaee R. A review on pain‐relieving activity of berberine. Phytother Res. 2021;35(6):2846–2853.
- Rezaee R, Monemi A, SadeghiBonjar MA, et al. Berberine alleviates paclitaxel-induced neuropathy. J Pharmacopuncture. 2019;22(2):90.
- Chen K, Li G, Geng F, et al. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K–Akt signaling in diabetic rats. Apoptosis. 2014;19(6):946–957.
- Wang C, Li J, Lv X, et al. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol. 2009;620(1–3):131–137.
- Li M-H, Zhang Y-J, Yu Y-H, et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol. 2014;728:67–76.
- Hong Y, Hui S-C, Chan T-Y, et al. Effect of berberine on regression of pressure-overload induced cardiac hypertrophy in rats. Am J Chin Med. 2002;30(04):589–599.
- Sambandam N, Lopaschuk GD. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Progress Lipid Res. 2003;42(3):238–256.
- Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells: evidence for an AMPK→ Rac1→ Akt→ endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282(28):20351–20364.
- Chang W, Li K, Guan F, et al. Berberine pretreatment confers cardioprotection against ischemia–reperfusion injury in a rat model of type 2 diabetes. J Cardiovasc Pharmacol Ther. 2016;21(5):486–494.
- Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea–a review. Am Coll Nutr. 2006;25(2):79–99.
- Jeong W-S, Kim I-W, Hu R, et al. Modulatory properties of various natural chemopreventive agents on the activation of NF-κB signaling pathway. Pharm Res. 2004;21(4):661–670.
- Song D-K, Jang Y, Kim JH, et al. Polyphenol (-)-epigallocatechin gallate during ischemia limits infarct size via mitochondrial KATP channel activation in isolated rat hearts. J Korean Med Sci. 2010;25(3):380–386.
- Aneja R, Hake PW, Burroughs TJ, et al. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10(1):55–62.
- Rossello X, Yellon DM. The RISK pathway and beyond. Basic Res Cardiol. 2018;113(1):1–5.
- Kim SJ, Li M, Jeong CW, et al. Epigallocatechin-3-gallate, a green tea catechin, protects the heart against regional ischemia-reperfusion injuries through activation of RISK survival pathways in rats. Arch Pharm Res. 2014;37(8):1079–1085.
- Kim SJ, Jeong CW, Bae HB, et al. Protective effect of sauchinone against regional myocardial ischemia/reperfusion injury: inhibition of p38 MAPK and JNK death signaling pathways. J Korean Med Sci. 2012;27(5):572–575.
- Liu S-X, Zhang Y, Wang Y-F, et al. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow a conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int J Cardiol. 2012;160(2):95–101.
- Wang J, Zhang Q, Mei X, et al. Hydroxysafflor yellow a attenuates left ventricular remodeling after pressure overload-induced cardiac hypertrophy in rats. Pharm Biol. 2014;52(1):31–35.
- Chen M, Wang M, Yang Q, et al. Antioxidant effects of hydroxysafflor yellow a and acetyl-11-keto-β-boswellic acid in combination on isoproterenol-induced myocardial injury in rats. Int J Mol Med. 2016;37(6):1501–1510.
- Li F, Fan X, Zhang Y, et al. Cardioprotection by combination of three compounds from ShengMai preparations in mice with myocardial ischemia/reperfusion injury through AMPK activation-mediated mitochondrial fission. Sci Rep. 2016;6(1):1–14.
- Schubert C, Raparelli V, Westphal C, et al. Reduction of apoptosis and preservation of mitochondrial integrity under ischemia/reperfusion injury is mediated by estrogen receptor β. Biol Sex Differ. 2016;7(1):1–9.
- Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12(3):217–234.
- Davidson SM, Hausenloy D, Duchen MR, et al. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38(3):414–419.
- Min J, Wei C. Hydroxysafflor yellow a cardioprotection in ischemia–reperfusion (I/R) injury mainly via Akt/hexokinase II independent of ERK/GSK-3β pathway. Biomed Pharmacother. 2017;87:419–426.
- Alamolhodaei NS, Tsatsakis AM, Ramezani M, et al. Resveratrol as MDR reversion molecule in breast cancer: an overview. Food Chem Toxicol. 2017;103:223–232.
- Hashemzaei M, Tabrizian K, Alizadeh Z, et al. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Toxicol Rep. 2020;7:1571–1577.
- Tabrizian K, Musavi S, Rigi M, et al. Behavioral and molecular effects of intrahippocampal infusion of auraptene, resveratrol, and curcumin on H-89-induced deficits on spatial memory acquisition and retention in Morris water maze. Human Exp Toxicol. 2019;38(7):775–784.
- Tabrizian K, Shahraki J, Bazzi M, et al. Neuro‐protective effects of resveratrol on carbon monoxide‐induced toxicity in male rats. Phytother Res. 2017;31(9):1310–1315.
- Hashemzaei M, Barani AK, Iranshahi M, et al. Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol. 2016;46:110–115.
- Valenzano DR, Terzibasi E, Genade T, et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300.
- Do GM, Jung UJ, Park HJ, et al. Resveratrol ameliorates diabetes‐related metabolic changes via activation of AMP‐activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56(8):1282–1291.
- González‐rodríguez Á, Santamaría B, Mas‐gutierrez JA, et al. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1‐independent manner. Mol Nutr Food Res. 2015;59(8):1431–1442.
- Aires V, Limagne E, Cotte AK, et al. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol Nutr Food Res. 2013;57(7):1170–1181.
- Hashemzaei M, Karami SP, Delaramifar A, et al. Anticancer effects of co-administration of daunorubicin and resveratrol in MOLT-4, U266 B1 and RAJI cell lines. Farmacia. 2016;64(1):36–42.
- Yang L, Yang L, Tian W, et al. Resveratrol plays dual roles in pancreatic cancer cells. J Cancer Res Clin Oncol. 2014;140(5):749–755.
- Liao Z, Liu D, Tang L, et al. Long‐term oral resveratrol intake provides nutritional preconditioning against myocardial ischemia/reperfusion injury: involvement of VDAC1 downregulation. Mol Nutr Food Res. 2015;59(3):454–464.
- Li J, Xie C, Zhuang J, et al. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: role of the TLR4/NF-κB signaling pathway. Mol Med Rep. 2015;11(2):1120–1126.
- Yang L, Zhang Y, Zhu M, et al. Resveratrol attenuates myocardial ischemia/reperfusion injury through up-regulation of vascular endothelial growth factor B. Free Radical Biol Med. 2016;101:1–9.
- Park MJ, Lee EK, Heo H-S, et al. The anti-inflammatory effect of kaempferol in aged kidney tissues: the involvement of nuclear factor-κ B via nuclear factor-inducing kinase/I κ B kinase and mitogen-activated protein kinase pathways. J Med Food. 2009;12(2):351–358.
- Verma AR, Vijayakumar M, Mathela CS, et al. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47(9):2196–2201.
- Gates MA, Tworoger SS, Hecht JL, et al. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121(10):2225–2232.
- Zhou M, Ren H, Han J, et al. Protective effects of Kaempferol against myocardial ischemia/reperfusion injury in isolated rat heart via antioxidant activity and inhibition of Glycogen Synthase Kinase-3 β. Oxid Med Cell Longevity. 2015;2015:1–8.
- Cain BS, Meldrum DR, Dinarello CA, et al. Tumor necrosis factor-alpha and interleukin-1 beta synergistically depress human myocardial function. Crit Care Med. 1999;27(7):1309–1318.
- Pan P, Qiao L, Wen X. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson’s disease through regulating Keap1/Nrf2 signaling pathway. Cell Mol Biol. 2016;62(14):11–17.
- Kianbakht S, Mozaffari K. Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. J Med Plants. 2009;8(29):30–38.
- Pathan S, Zaidi S, Jain G, et al. Anticonvulsant evaluation of safranal in pentylenetetrazole-induced status epilepticus in mice. Int J Essent Oil Ther. 2009;3(2/3):106–108.
- Rezaee R, Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran J Basic Med Sci. 2013;16(1):12.
- Sadeghnia H, Boroushaki M, Mofidpour H. Effect of safranal, a constituent of saffron (Crocus sativus l.), on lipid peroxidation level during renal ischemia-reperfusion injury in rats. Iran J Basic Med Sci. 2005;8(3): 179–185.
- Catalucci D, Zhang D-H, DeSantiago J, et al. Akt regulates L-type Ca2+ channel activity by modulating Cavα1 protein stability. J cell Biol. 2009;184(6):923–933.
- Hochhauser E, Kivity S, Offen D, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284(6):H2351–9.
- Maekawa N, Wada H, Kanda T, et al. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-α. J Am Coll Cardiol. 2002;39(7):1229–1235.
- Basset O, Boittin F-X, Cognard C, et al. Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem J. 2006;395(2):267–276.
- Wang H, Zheng B, Che K, et al. Protective effects of safranal on hypoxia/reoxygenation‑induced injury in H9c2 cardiac myoblasts via the PI3K/AKT/GSK3β signaling pathway. Exp Ther Med. 2021;22(6):1–15.
- Sugden PH. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circ Res. 2003;93(12):1179–1192.
- Roberts CK, Vaziri ND, Wang XQ, et al. Enhanced NO inactivation and hypertension induced by a high-fat, refined-carbohydrate diet. Hypertension. 2000;36(3):423–429.
- Bharti S, Golechha M, Kumari S, et al. Akt/gsk-3β/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia–reperfusion injury in rats. Eur J Nutr. 2012;51(6):719–727.
- Zhang A, Zheng Y, Que Z, et al. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140(11):1883–1890.
- Wang B, Chen M-Z. Astragaloside IV possesses antiarthritic effect by preventing interleukin 1β-induced joint inflammation and cartilage damage. Arch Pharm Res. 2014;37(6):793–802.
- Qiu L-H, Xie X-J, Zhang B-Q. Astragaloside IV improves homocysteine-induced acute phase endothelial dysfunction via antioxidation. Biol Pharm Bull. 2010;33(4):641–646.
- Zhang Y, Zhu H, Huang C, et al. Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-γ. J Cardiovasc Pharmacol. 2006;47(2):190–195.
- Gui J, Chen R, Xu W, et al. Remission of CVB 3‐induced myocarditis with astragaloside IV treatment requires A20 (TNFAIP 3) up‐regulation. J Cell Mol Med. 2015;19(4):850–864.
- Chen P, Xie Y, Shen E, et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol. 2011;658(2–3):168–174.
- Dong Z, Zhao P, Xu M, et al. Astragaloside IV alleviates heart failure via activating PPARα to switch glycolysis to fatty acid β-oxidation. Sci Rep. 2017;7(1):1–15.
- Zhang W-D, Chen H, Zhang C, et al. Astragaloside IV from Astragalus membranaceus shows cardioprotection during myocardial ischemia in vivo and in vitro. Planta Med. 2006;72(01):4–8.
- Tu L, Pan CS, Wei XH, et al. Astragaloside IV protects heart from ischemia and reperfusion injury via energy regulation mechanisms. Microcirculation. 2013;20(8):736–747.
- Si J, Wang N, Wang H, et al. HIF-1α signaling activation by post-ischemia treatment with astragaloside IV attenuates myocardial ischemia-reperfusion injury. PLoS ONE. 2014;9(9):e107832.
- Zheng Q, Zhu J-Z, Bao X-Y, et al. A preclinical systematic review and meta-analysis of astragaloside IV for myocardial ischemia/reperfusion injury. Front Physiol. 2018;9:795.
- Wei D, Xu H, Gai X, et al. Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3β signaling pathways. Acta Cir Bras. 2019;34(7): e201900708.
- Bian Q-Y, Wang S-Y, L-J X, et al. Two new antioxidant diarylheptanoids from the fruits of Alpinia oxyphylla. J Asian Nat Prod Res. 2013;15(10):1094–1099.
- Martineti V, Tognarini I, Azzari C, et al. Inhibition of in vitro growth and arrest in the G0/G1 phase of HCT8 line human colon cancer cells by kaempferide triglycoside from Dianthus caryophyllus. Phytother Res. 2010;24(9):1302–1308.
- Maruyama H, Sumitou Y, Sakamoto T, et al. Antihypertensive effects of flavonoids isolated from Brazilian green propolis in spontaneously hypertensive rats. Biol Pharm Bull. 2009;32(7):1244–1250.
- Pei Y-H, Chen J, Xie L, et al. Hydroxytyrosol protects against myocardial ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Mediators Inflamm. 2016;2016:1232103.
- Wang D, Zhang X, Li D, et al. Kaempferide protects against myocardial ischemia/reperfusion injury through activation of the PI3K/Akt/GSK-3β pathway. Mediators Inflamm. 2017;2017:5278218.
- Ye R, Han J, Kong X, et al. Protective effects of ginsenoside Rd on PC12 cells against hydrogen peroxide. Biol Pharm Bull. 2008;31(10):1923–1927.
- Guan Y-Y, Zhou J-G, Zhang Z, et al. Ginsenoside-Rd from panax notoginseng blocks Ca2+ influx through receptor-and store-operated Ca2+ channels in vascular smooth muscle cells. Eur J Pharmacol. 2006;548(1–3):129–136.
- Li XY, Liang J, Tang YB, et al. Ginsenoside Rd prevents glutamate‐induced apoptosis in rat cortical neurons. Clin Exp Pharmacol Physiol. 2010;37(2):199–204.
- Shi Y, Han B, Yu X, et al. Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury in rats. Pharm Biol. 2011;49(9):900–906.
- Wang Z, Li M, Wu W-K, et al. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther. 2008;22(6):443–452.
- Chen S, Liu J, Liu X, et al. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J Ethnopharmacol. 2011;137(1):263–270.
- Wang Y, Li X, Wang X, et al. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS ONE. 2013;8(8):e70956.
- Panahi Y, Hosseini MS, Khalili N, et al. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015;34(6):1101–1108.
- Panahi Y, Alishiri GH, Parvin S, et al. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: results of a randomized controlled trial. J Diet Suppl. 2016;13(2):209–220.
- Hassani S, Sepand M, Jafari A, et al. Protective effects of curcumin and vitamin E against chlorpyrifos-induced lung oxidative damage. Human Exp Toxicol. 2015;34(6):668–676.
- Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili S-A, et al. Curcumin: a natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev. 2018;17(2):125–135.
- Mirzaei H, Naseri G, Rezaee R, et al. Curcumin: a new candidate for melanoma therapy? Int J Cancer. 2016;139(8):1683–1695.
- Mokhtari‐zaer A, Marefati N, Atkin SL, et al. The protective role of curcumin in myocardial ischemia–reperfusion injury. J Cell Physiol. 2019;234(1):214–222.
- Kim SJ, Yoo KY, Jeong CW, et al. Urinary trypsin inhibitors afford cardioprotective effects through activation of PI3K-Akt and ERK signal transduction and inhibition of p38 MAPK and JNK. Cardiol. 2009;114(4):264–270.
- Naeimi AF, Alizadeh M. Antioxidant properties of the flavonoid fisetin: an updated review of in vivo and in vitro studies. Trends Food Sci Technol. 2017;70:34–44.
- Khan N, Afaq F, Khusro FH, et al. Dual inhibition of phosphatidylinositol 3‐kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer. 2012;130(7):1695–1705.
- Pal HC, Pearlman RL, Afaq F. Fisetin and its role in chronic diseases. Adv Exp Med Biol. 2016;928:213–244.
- Shanmugam K, Ravindran S, Kurian GA, et al. Fisetin confers cardioprotection against myocardial ischemia reperfusion injury by suppressing mitochondrial oxidative stress and mitochondrial dysfunction and inhibiting glycogen synthase kinase 3β activity. Oxid Med Cell Longevity. 2018;2018:9173436.
- Lee SE, Jeong SI, Yang H, et al. Fisetin induces Nrf2‐mediated HO‐1 expression through PKC‐δ and p38 in human umbilical vein endothelial cells. J Cell Biochem. 2011;112(9):2352–2360.
- Xu R, Hu Q, Ma Q, et al. The protease Omi regulates mitochondrial biogenesis through the GSK3β/PGC-1α pathway. Cell Death Amp Dis. 2014;5(8):e1373.
- Shanmugam K, Boovarahan SR, Prem P, et al. Fisetin attenuates myocardial ischemia-reperfusion injury by activating the reperfusion injury salvage kinase (RISK) signaling pathway. Front pharmacol. 2021;12:566470.
- Song X, Wang T, Zhang Z, et al. Leonurine exerts anti-inflammatory effect by regulating inflammatory signaling pathways and cytokines in LPS-induced mouse mastitis. Inflammation. 2015;38(1):79–88.
- Sun J, Huang SH, Zhu YC, et al. Anti-oxidative stress effects of Herba leonuri on ischemic rat hearts. Life Sci. 2005;76(26):3043–3056.
- Mao F, Zhang L, Cai M-H, et al. Leonurine hydrochloride induces apoptosis of H292 lung cancer cell by a mitochondria-dependent pathway. Pharm Biol. 2015;53(11):1684–1690.
- Zhu YZ, Wu W, Zhu Q, et al. Discovery of Leonuri and therapeutical applications: from bench to bedside. Pharmacol Ther. 2018;188:26–35.
- Liu X, Pan L, Gong Q, et al. Leonurine (SCM-198) improves cardiac recovery in rat during chronic infarction. Eur J Pharmacol. 2010;649(1–3):236–241.
- Rao AV, Ray M, Rao L. Lycopene. Adv Food Nutr Res. 2006;51:99–164.
- Xu J, Hu H, Chen B, et al. Lycopene protects against hypoxia/reoxygenation injury by alleviating ER stress induced apoptosis in neonatal mouse cardiomyocytes. PLoS ONE. 2015;10(8):e0136443.
- Yue R, Hu H, Yiu KH, et al. Lycopene protects against hypoxia/reoxygenation-induced apoptosis by preventing mitochondrial dysfunction in primary neonatal mouse cardiomyocytes. PLoS ONE. 2012;7(11):e50778.
- Miki T, Miura T, Hotta H, et al. Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho–glycogen synthase kinase-3β–mediated suppression of mitochondrial permeability transition. Diabetes. 2009;58(12):2863–2872.
- Duan L, Liang C, Li X, et al. Lycopene restores the effect of ischemic postconditioning on myocardial ischemia‑reperfusion injury in hypercholesterolemic rats. Int J Mol Med. 2019;43(6):2451–2461.
- Huang Q, Lu G, Shen HM, et al. Anti‐cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27(5):609–630.
- Liu J, Chen Z, Zhang Y, et al. Rhein protects pancreatic β-cells from dynamin-related protein-1–mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 2013;62(11):3927–3935.
- Xue L, Wu Z, Ji X-P, et al. Effect and mechanism of salvianolic acid B on the myocardial ischemia-reperfusion injury in rats. Asian Pac J Trop Med. 2014;7(4):280–284.
- Wang B, Sun J, Shi Y, et al. Salvianolic Acid B inhibits high‐fat diet‐induced inflammation by activating the Nrf2 pathway. J Food Sci. 2017;82(8):1953–1960.
- Fan H, Yang L, Fu F, et al. Cardioprotective effects of salvianolic Acid a on myocardial ischemia-reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med. 2012;2012:508938.
- Li X-L, Fan J-P, Liu J-X, et al. Salvianolic acid a protects neonatal cardiomyocytes against hypoxia/reoxygenation-induced injury by preserving mitochondrial function and activating Akt/GSK-3β signals. Chin J Integr Med. 2019;25(1):23–30.
- Peng L, Junguo R, Changling D, et al. The effects of three components of Salviae miltiorrhizae radix on anoxia and peroxidation injuries in neonatal cardiomyocytes. Pharmacol Clin Chin Mater Med. 2009; 25(5): 29–31.
- Chen X, Yang L, Oppenheim JJ, et al. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16(3):199–209.
- Wang Z, Liu T, Gan L, et al. Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur J Pharmacol. 2010;643(2–3):211–217.
- Liu T, Zhang Q, Mo W, et al. The protective effects of shikonin on hepatic ischemia/reperfusion injury are mediated by the activation of the PI3K/Akt pathway. Sci Rep. 2017;7(1):1–13.
- Yang J, Wang Z, Chen D-L. Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed Pharmacother. 2017;93:1343–1357.
- Wang S, Zhu Y, Qiu R. Shikonin protects H9C2 cardiomyocytes against hypoxia/reoxygenation injury through activation of PI3K/Akt signaling pathway. Biomed Pharmacother. 2018;104:712–717.
- Liu J, Zhai W-M, Yang Y-X, et al. GABA and 5-HT systems are implicated in the anxiolytic-like effect of spinosin in mice. Pharmacol Biochem Behav. 2015;128:41–49.
- Lee Y, Jeon SJ, Lee HE, et al. Spinosin, a C-glycoside flavonoid, enhances cognitive performance and adult hippocampal neurogenesis in mice. Pharmacol Biochem Behav. 2016;145:9–16.
- Ko SY, Lee HE, Park SJ, et al. Spinosin, a C-Glucosylflavone, from Zizyphus jujuba var. spinosa Ameliorates Aβ1–42 Oligomer-Induced Memory Impairment in Mice. Biomol Ther. 2015;23(2):156.
- Xu F, He B, Xiao F, et al. Neuroprotective effects of spinosin on recovery of learning and memory in a mouse model of Alzheimer’s disease. Biomol Ther. 2019;27(1):71.
- Gu M, He P, Lyu C, et al. Spinosin and 6’’‘‑feruloylspinosin protect the heart against acute myocardial ischemia and reperfusion in rats. J Mol Med Rep. 2019;20(5):4253–4261.
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta –Bioenergetics. 2006;1757(5–6):509–517.
- Juhaszova M, Zorov DB, Yaniv Y, et al. Role of glycogen synthase kinase-3β in cardioprotection. Circ Res. 2009;104(11):1240–1252.
- Marchand B, Arsenault D, Raymond-Fleury A, et al. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem. 2015;290(9):5592–5605.
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995.
- Rogov V, Dötsch V, Johansen T, et al. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Molecular Cell. 2014;53(2):167–178.
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354.
- Djekic D, Shi L, Brolin H, et al. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Heart Assoc. 2020;9(18):e016518.
- Gencer B, Li XS, Gurmu Y, et al. Gut microbiota‐dependent trimethylamine N‐oxide and cardiovascular outcomes in patients with prior myocardial infarction: a nested case control study from the PEGASUS‐TIMI 54 trial. J Am Heart Assoc. 2020;9(10):e015331.
- Nemet I, Saha PP, Gupta N, et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180(5):862–77. e22.
- Landete J. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int. 2011;44(5):1150–1160.
- Dey P. Gut microbiota in phytopharmacology: a comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol Res. 2019;147:104367.
- Espín JC, Larrosa M, García-Conesa MT, et al. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med. 2013;2013: 270418.
- González-Sarrías A, Larrosa M, Tomás-Barberán FA, et al. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br j nutr. 2010;104(4):503–512.
- Piwowarski JP, Kiss AK, Granica S, et al. Urolithins, gut microbiota‐derived metabolites of ellagitannins, inhibit LPS‐induced inflammation in RAW 264.7 murine macrophages. Mol Nutr Food Res. 2015;59(11):2168–2177.
- Zheng D, Liu Z, Zhou Y, et al. Urolithin B, a gut microbiota metabolite, protects against myocardial ischemia/reperfusion injury via p62/Keap1/Nrf2 signaling pathway. Pharmacol Res. 2020;153:104655.
- Ryu D, Mouchiroud L, Andreux PA, et al. Urolithin a induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nature Med. 2016;22(8):879–888.
- Tang L, Mo Y, Li Y, et al. Urolithin a alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochem Biophys Res Commun. 2017;486(3):774–780.
- Xu L, Jiang X, Wei F, et al. Leonurine protects cardiac function following acute myocardial infarction through anti‑apoptosis by the PI3K/AKT/GSK3β signaling pathway. J Mol Med Rep. 2018;18(2):1582–1590.
- Ajzashokouhi A, Bostan H, Jomezadeh V, et al. A review on the cardioprotective mechanisms of metformin against doxorubicin. Human Exp Toxicol. 2020;39(3):237–248.
- Wang J, Liu J, Xie L, et al. Bisoprolol, a β1 antagonist, protects myocardial cells from ischemia–reperfusion injury via PI3K/AKT/GSK3β pathway. Fundam Clin Pharmacol. 2020;34(6):708–720.
