ABSTRACT
The family protein of cyclins, as well as cyclin-dependent kinases (CDKs) cooperating with them, are broadly researched, as a matter of their dysfunction may lead to tumor transformation. Cyclins are defined as key regulators that have a controlling function of the mammalian nuclear cell divides. Cyclin Y (CCNY) is a recently characterized member of the cyclin family and was first identified from the human testis cDNA library. It is an actin-binding protein acting through decreased actin dynamics at a steady state and during glycine-induced long-term potentiation (LTP) and involves the inhibition of cofilin activation. What is more, CCNY is a positive regulatory subunit of the CDK14/PFTK1 complexes affected by the activation of the Wnt signaling pathway in the G2/M phase by recruiting CDK14/PFTK1 to the plasma membrane and promoting phosphorylation of LRP6. The expression of CCNY has been significantly mentioned within the cell migration and invasion activity both in vivo and in vitro. The aim of this review is evaluation of the expression of CCNY in the physiology processes and compare the expression of this protein in cancer cells, taking into account the impact of the level of expression on tumor progression.
Introduction
The cell cycle is regulated by the constitutive synthesis and destruction of cyclins. This family of proteins plays an important role in the regulation of the life cycle among eukaryotic organisms. The proteins constituting members of the cyclin family can be divided into those that are necessary for the control of the G1 to S phase transition (G1/S cyclins) and those that are essential for G2 to M phase transition maintenance (G2/M cyclins). Undisturbed progression through the cell cycle depends on the activity of the cyclins, appropriate cyclin-dependent kinases (CDKs) which are serine/threonine kinases, and their inhibitors. Close cooperation between this trio plays indispensable roles in the cell cycle regulation of physiological and pathological processes. The first mentioned is based on the regulation of DNA damage repair and transcriptional programs directing cells on differentiation, apoptosis, and metabolic flux. On the other hand, scientific research results strongly suggest that disturbances in the signaling pathways of mentioned processes are also associated with cyclins whose overexpression could lead to the initiation of carcinogenesis, promoting tumor mass growth, as well as metastasis. A particularly prominent protein that contributes significantly to tumor progression is cyclin Y (CCNY). Next to tumorigenesis, this protein is primarily equated with molecular noncanonical WNT signaling pathways that play central roles in many early and late developmental stages. Cyclin Y overexpression is observed among patients diagnosed among others with human hepatocellular carcinoma (HCC) or non-small lung cancer (NSLC). High expression of CCNY significantly contributes to intensifying cell proliferation and increased migration potential.Given the significant CCNY role in tumorigenesis and metastasis in varied cancer types, CCNY could be determined as a biomarker for tumor diagnosis and possibly also useful for the determination of more or less aggressive tumor biology.
Cyclins and CDKs
Mammalian cyclin-dependent kinases (CDKs), cyclins, and cyclin-dependent kinase inhibitors (CKIs) belong to families of proteins that play a key role in many cellular processes, eg.: cell proliferation, transcription, regulation of cell metabolism, epigenetic regulation, spermatogenesis, self-renewal of stem cells, and also in the process of cancer formation [Citation1]. Cyclin-dependent kinases are serine-threonine protein kinases, which are the basic and catalytic subunit of the protein complex, which regulate the cell cycle by transferring phosphorus groups to various proteins. Cdks contain a catalytic core consisting of an adenosine 5’-triphosphate (ATP) binding pocket, a T-loop activating motif, and a cyclin binding domain in the form of the VPSTAIRESLLKE often referred to as the PSTAIRE helix. Activation of Cdks, which involves attachment to the cyclin via the PSTAIRE helix to displace the T-loop and expose substrate-binding [Citation2]. Every Cdk has activating and inhibitory phosphorylation sites that make cell cycle control nuanced and precise. The phosphorylation sites are in the vicinity of the ATP binding site, and due to the inhibitory Wee1 and Myt1 kinases, the phosphorylation disrupts the correct fit of ATP. In the case of T-161, kinases are activators and phosphatases are inhibitors. Cdk activating kinase (CAK), which consists of cyclin H and Cdk7, acts on the T-loop phosphorylation site, leading to improved substrate binding, which enables full Cdks activation [Citation1]. The activity of Cdk kinases is dependent on subcellular localization. This contributes to the determination of the specific substrate to be phosphorylated to identify the function to be induced [Citation3].
Cyclins belong to the family of proteins identified by the “cyclin box”. It is a region that consists of about 100 amino acid residues and is responsible for the regulation of cyclins binding to the corresponding Cdk. Cyclins have a PEST sequence, a protein motif that is ubiquitinated, and rich in proline (P), glutamic acid (E), serine (S), and threonine (T). Correct expression of cyclins is crucial for the proper proliferation of cells, while disturbed expression of these proteins may lead to neoplastic transformation [Citation4].
While most cyclins promote the activity of Cdks, inhibitors of cyclin-dependent kinases inhibit their activity, eg by blocking the active sites of the Cdks, or by competing with cyclins for their binding site. Cdks inhibitors have been divided into two groups due to their structure and specificity. Members of the INK4 family (Cdk4 Inhibitor of CDK4) are the proteins p15Ink4b, p16Ink4, p18Ink4c, and p19Ink4d, the most important features of which are the possession of ankyrin repeats and inhibition mainly of Cdk4 and Cdk6. On the other hand, members of the CIP/KIP (Cdk interacting protein/kinase interacting protein) family, which include the p21, p27, and p57 proteins, are encoded by the CIP1, KIP1, and KIP2 genes, respectively, and inhibit the activity of Cdk responsible for the transition from G1 to S phase The above-mentioned proteins regulate the activity of complexes of cyclins D, E, A, and B with appropriate kinases [Citation5].
Cyclin Y
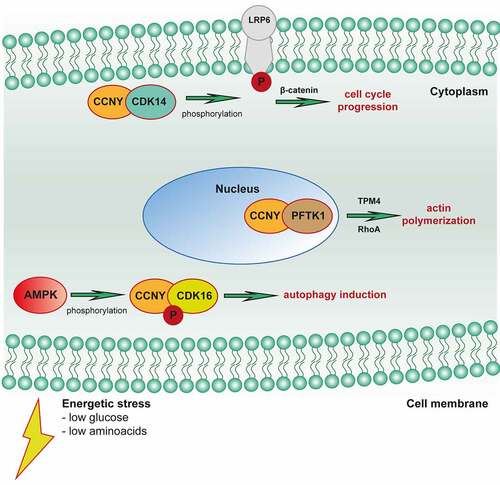
Cyclin Y is the highly conserved cyclin family protein, which is known mainly for its role in the regulation of the cell cycle and the transcription process. Comparing the amino acid sequence, the cyclins can be divided into two groups. First, cyclins act as a cell cycle regulator, whereas the second class modulates the activity of the RNA II polymerase [Citation6]. Cyclin Y is encoded by the CCNY gene located on the short arm of chromosome 10 at position p11.21 (www.genecards.org) [Citation7]. The structure of cyclin Y also differs from that of conventional cyclins (). Most cyclins have two cyclin folds, while cyclin Y has only one cyclin fold [Citation8].
Figure 1. The structure of cyclin Y. Cyclin Y has only one cyclin domain and is heavily phosphorylated.

It was originally identified as a protein that binds to CDK 14/PFTK1 in a yeast screening test (two-hybrid test), increasing its activity and changing its intracellular localization. In vitro and in vivo studies have shown that the PFTAIRE motif in PFTK1 and the Cyclin Y cyclin box is required for the interaction between these proteins [Citation9]. Later studies have shown that this protein combines with CDK 16/PCTK1 to form an active complex capable of controlling several biological processes. CDK 16 is a protein kinase that is highly expressed in the brain and testes and is activated by the membrane-bound cyclin Y [Citation10]. Cyclin Y/Cdk 14 complex can also phosphorylate and activate the Lrp6 co-receptor, which is the major regulator of the Wnt/B-cat signaling pathway [Citation11]. Cyclin Y plays an important role in many cellular processes: maintaining the properties of mammalian stem/progenitor cells, regulating the development of Drosophila, and also controlling the process of adipogenesis and lipid production. It has also been shown to participate in the regulation of cancer cell proliferation, including lung and kidney, and lowering the expression level of cyclin Y reduces the proliferation and growth of laryngeal cancer cells [Citation8,Citation10,Citation12].
This review focuses on the biological functions of cyclin Y and also describes the involvement of cyclin Y in the development and progression of cancer.
Cyclin Y affects many cellular processes via the WNT pathway
Wnt is an evolutionarily conserved signaling pathway that plays an important role in embryonic development and tissue homeostasis [Citation13]. Deregulated Wnt signaling may contribute to carcinogenesis in humans, and a growing body of research shows, that Wnt upregulation contributes to drug resistance. The Wnt pathway is divided into canonical and several alternative pathways. The canonical pathway depends on β-catenin. It regulates the activity of the T cell transcription factor (TCF) influencing processes such as embryogenesis, differentiation, survival, and proliferation of cells. Non-classical pathways operate independently of β-catenin and TCF. Their role has so far been poorly understood, but some literature reports indicate their importance in processes such as cytoskeleton rearrangement and neuronal migration [Citation14].
It has been found that in many pathological conditions, including neurodegenerative and metabolic diseases, as well as in various types of neoplasms, especially in non-small cell lung cancer, disturbances in the functioning of the Wnt pathway occur [Citation10,Citation15]. Literature data indicate that the Wnt signaling pathway plays an important role in the regulation of cellular processes such as cell proliferation and survival, embryogenesis, and cell differentiation. The abnormal course of the Wnt pathway may contribute to the maintenance of the population of neoplastic cells, which may also affect their drug resistance [Citation16]. When examining the components of the Wnt pathway, it has been reported that increasing or decreasing its substrates is associated with survival as well as increased proliferation of neoplastic cells, which affects the worse prognosis of patients with non-small cell lung cancer [Citation15]. The results of the research suggest that cyclin Y in the PFKT1/Cdk14 complex promotes the non-canonical pathway of the Wnt pathway by enhancing the expression of the Dvl2 and Naked1 proteins, which are substrates of this pathway. The participation of the cyclin Y/PFKT1 complex in the activation of Rho GTPases, which are targets of the non-canonical Wnt signaling pathway, has also been shown, which leads to actin polymerization in cells [Citation17]. Other reports also suggest that cyclin Y in healthy cells, together with Cdk14, affects the transduction of the Wnt pathway through the phosphorylation of LRP6. It has been shown that the cyclin Y-Cdk14 complex through phosphorylation of LRP6 prevents the degradation of β-catenin, which allows signaling in the Wnt pathway (). Phosphorylation and signaling of the Wnt receptor are maximal in the G2/M phase, as is the expression of cyclin Y, which may suggest a relationship between the cell cycle and the transcription process [Citation18].
Cyclin Y and autophagy
Autophagy, which is an evolutionarily conserved process occurring in all eukaryotic cells, is activated e.g. in response to nutrient deficiencies, damage from cellular toxins, and growth and differentiation-inducing factors [Citation19]. Under physiological conditions, it is found to a small extent in most tissues. It contributes to the adaptation of cells to stressful conditions and thus to their survival. The main types of autophagy are macroautophagy and microautophagy. Macroautophagy occurs with the formation of an autophagosome which, when fused with the primary lysosome, forms the autophagolysosome. Microautophagy is defined as the process of incorporation of cytoplasmic components through the invagination of the lysosomal membrane [Citation19].
There is evidence that this process is linked to disease states. Increased levels of autophagy have been reported in neurodegenerative Parkinson’s disease. On the other hand, a decrease in the intensity of this process has been proven in certain types of cancer or heart disease (Danone’s disease). The results of other studies suggest, however, that the autophagy process contributes to the increase in the survival of cancer cells under stressful conditions (ionizing radiation) by eliminating damaged organelles [Citation19]. The cyclin Y/CDK16 complex is an AMPK substrate and an autophagy effector. Cyclin Y/CDK16 induces autophagy which is dependent on the phosphorylation of S326 and requires ULK1 and Beclin1 which are necessary for the induction of macroautophagy. AMPK activates Cyclin Y/CDK16 to initiate the first step in autophagosome formation. A potential link with autophagy is further suggested by the function of CDK16 in the vesicular transport of actin cytoskeleton organization, as both of these processes are important for autophagy [Citation20–22].
Cyclin Y in neural function
The expression of cyclin Y and its significant influence has been described in non-dividing neuronal cells – hippocampal cells. Cho et al. in their research showed that cyclin Y is expressed not only in the hippocampus but also located in the postsynaptic domains of dendritic spines. The presence of cyclin Y may contribute to an increase in the complexity and variety of brain functions. It has been shown that cyclin Y can inhibit the movement of the AMPA receptor (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) toward the synapses and inhibit its exocytosis and thus block LTP. In addition, lowering the expression of cyclin Y results in an increase in LTP (long-term potentiation) in the hippocampal slices. The authors also suggest that the role of cyclin Y is largely unrelated to the cell cycle. Since cyclins were originally identified as proteins that oscillate during the cell cycle, and their expression depends on the phase of the cycle, taking into account the participation of cyclin Y in neurons of the Central Nervous System (CNS), which are post-mitotic cells unable to divide, this suggestion is can be considered close to the truth [Citation8]. Hwang et. al. concerning research and the role of cyclin Y in hippocampal cells showed that cyclin Y binds to filamentous actin and interferes with actin polymerization which is induced by LTP. Cyclin Y depolymerizes the actin-depolymerizing factor by blocking the activation of cofilin. As a result, this results in a structural impairment of LTP and a reduction in the number of plastic spines. The data presented suggest that cyclin Y inhibits LTP functionally and structurally via the cofilin-actin pathway [Citation23].
A year later, Joe et al. by studying RNA sequences, transcript analysis, and qRT-PCR, showed that many functions and pathways in neuronal cells are influenced by cyclin Y. Actin cytoskeleton, synaptic plasticity, focal adhesion, apoptosis, chemokine signaling pathway, extracellular matrix-receptor interaction, and even learning and memory, all these processes are influenced by the expression of cyclin Y [Citation12].
Cyclin Y in complex with CDK 14 regulates specific neuronal functions. High CDK 14 expression has been detected in post-mitotic brain cells, the cyclin Y/CDK 14 complex plays a key role in the polarized trafficking of presynaptic vesicles and elimination of synapses during nerve conduction [Citation24].
Another neuronal function performed by cyclin Y has been investigated by Hwang et. al. The results of their research provide information that cyclin Y changes the expression profile of many genes that are associated with neurodegenerative diseases and epilepsy. Studies in mice show that wild-type mice are less susceptible to kainic acid epilepsy than CCNY-knockout mice [Citation25].
Cyclin Y and spermatogenesis
The final stages of spermatogenesis differentiation are also influenced by cyclin Y, more specifically the cyclin Y/CDK 16 complex. Mikolcevic et al. showed that CDK 16 knockout mice were viable, and kinase deficiency did not affect meiosis, although CDK16 was required in the final stages of differentiation in spermatogenesis. The CDK 16 knockout mice tested had all cells in their testes at different stages of spermatogenesis. After careful examination of the sperm cells, many abnormalities in the structure and functioning of the cells were found. Among other things, there were: aberrations in the structure of the ring (i.e. elongation and thinning of the ring region), dyskinesia, i.e. a defect in the structure of spermatozoa, distortions in the sperm head, and also had an excess of residual cytoplasm. These defects impaired sperm function and contributed to infertility [Citation10].
The role of cyclin Y in the process of spermatogenesis was also investigated by Zi et. al. using mice and many advanced laboratory techniques, they compared Cyclin Y with the highly similar Cyclin Y Like 1 in the protein sequence, which is a poorly characterized protein with the highest levels of expression in the testes of mice. The authors showed that male CCNYL1 knockout mice were infertile, and examination of the sperm showed impaired mobility, a bent head, and aberrations in the structure of the ring. Whereas CCNY knockout females and males showed normal fertility [Citation15].
Cyclin Y in adipogenesis and lipid production
As mentioned earlier, cyclin Y in a complex with CDK 14 is involved in the signaling of the WNT pathway through the phosphorylation of the LRP6 protein. Importantly, it has been reported that the WNT signaling pathway plays an important role in regulating the adipogenesis process [Citation26,Citation27]. The available data may indicate an association of cyclin Y with adipogenesis. Confirmation of these speculations may be the research of An et. al. Researchers studied wild-type and CCNT -/- knockout mice, as well as hepatocytes and HepG2 cell lines. It was observed that CCNY-knockout mice, unlike wild-type mice, showed both lower body weight and reduced body fat content. It was also found that the metabolism was faster, the need for food, and the sensitivity of rodents to the reduction of caloric intake. On the other hand, HepG2 cells and hepatocytes with decreased expression of cyclin Y became insensitive to insulin. This suggests that cyclin Y is involved in the regulation of the hepatic insulin signaling pathway. Taken together, these results support a new relationship between CCNY and lipid metabolism and suggest that inhibiting CCNY may offer a therapeutic approach to obesity and diabetes [Citation16].
Cyclin Y in other cellular processes
Martinotti et al. investigated the role cyclin Y plays in inflammatory bowel disease (IBD). The materials used in the research were mice and cell lines: HTC116, HEK209T, SW48, and PC-3. Scientists initially suggested that cyclin Y may be essential for maintaining normal intestinal epithelial cell homeostasis (IEC) by promoting autophagy and the WNT/B-catenin signaling pathway in which it is involved. Following this lead, Martinotti et. al. investigated the extent to which the mucosa regenerates and what is the response to intestinal epithelial damage in CCNY -/- knockout mice and wild-type mice. Analysis of the results showed that there were no differences between the 2 types of mice in terms of cell proliferation and disease activity. In model IEC, the reduction of cyclin Y expression also did not affect cell proliferation, the WNT signaling pathway, and autophagy. The authors, therefore, concluded that cyclin Y does not contribute to the maintenance of normal intestinal epithelial homeostasis and is not involved in IBD [Citation28].
Kyselova et. al. investigated the possible expression of cyclin Y and its probable role in platelets. The studies confirmed the presence of cyclin Y in platelets and also showed that higher expression of cyclin Y occurs in the blood of healthy patients than in the blood of diabetic patients. Studies in mice have provided a wealth of information on the effects of cyclin Y ep expression on platelets and related processes. In CCNY -/- knockout mice, a reduced number of platelets and changes in their biogenesis were demonstrated, as well as decreased clotting and a longer bleeding time. Platelets were also shown to form filopodia, not lamellipodia, and their ability to spread was impaired. In addition, wild-type mice were mono-ubiquitination of cyclin Y and its translocation to the plasma membrane, where it regulated β3 tyrosine integrin phosphorylation and outward signaling using thrombin compared to CCNY -/- knockout mice [Citation29].
Cyclin Y in cancer
For a long time, progressive loss of cells growth control loss was the main factor contributing to the initiation of the carcinogenesis mechanism [Citation30]. It seems that cyclin Y (CCNY) has a significant role in tumorigenesis through interactions with different protein complexes involved in the Wnt pathway. For explanation, the Cyclin Y/Cdk14 complex could phosphorylate and activate the low-density lipoprotein receptor-related protein 6 (LRP6) which constitutes an important promoting regulator in the Wnt signaling pathway. LRP6 phosphorylation and Wnt signaling pathway are under the tight control of extracellular and intracellular cell cycle checkpoints and peak at the gap 2 phase/mitosis (G2/M) phase which results in uncontrolled proliferation of cells, including cancer cells. Recent literature data indicate that’s what CCNY overexpression is associated with poor prognosis manifested by increased proliferation of abnormal cells, suggesting that CCNY is implicated in tumor mass development and cancer metastasis. Due to the CCNY properties, this functional protein so could be defined as a novel prognostic marker and therapeutic target in diverse tumor types. The association between the high CCNY expression and the increased proliferative activity of neoplastic cells is observed in many neoplasms, including hepatocellular carcinoma, lung, breast, kidney, laryngeal and colorectal cancer, but also among glioma cells [Citation11,Citation31–34].
Moving on to the topic, liver cancer is the seventh most common tumor type in the world [Citation35]. Hepatocellular carcinoma (HCC) is the main type of this cancer and has several known causes such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, but also other factors include toxins and alcohol. Knowledge of the molecular pathways of HCC initiation and progression may allow for an efficient diagnosis of this cancer and the selection of appropriate treatment methods, thus reducing HCC mortality in society [Citation36]. In the case of hepatocellular carcinoma, studies conducted on the HuH7, SK-Hep1, HEP3B, HepG2, and L02 cell lines indicate significantly increased cancer cell proliferation, targeted migration, and reduced apoptosis percentage ratio. The study results based on determining metabolic activity assays, cell death analysis, and protein expression level evaluation indicate that the cause of the above-mentioned phenomena is higher CCNY level expression compared to normal liver cells. The high level of CCNY in neoplastic cells correlates with a decreased level of Bcl-2-associated X (Bax) protein expression, whose expression should accelerate the process of apoptosis by creating pores in the outer membrane of the mitochondria, thus increasing its permeability. The opposite effect was observed in the case of anti-apoptotic proteins, where particular attention was paid to the significant increase in the level of B-cell lymphoma 2 (Bcl-2) in cells, simultaneously accompanying the increased expression of CCNY. In summary, due to the characteristic protein profile of cancer cells, an elevated level of CCNY can inhibit the apoptosis of HCC cells [Citation11]. Apart from the CCNY overexpression, additional factors favoring the development of HCC is an abnormality in certain regulating pathways, including the Wnt signaling pathway, 53 protein/retinoblastoma protein (p53/pRB) relationship, and phosphoinositide 3-kinase/phosphatase and tensin homolog deleted on chromosome ten/protein kinase B/mechanistic target of rapamycin (PI3K/PTEN/PKB/mTOR) pathway. The literature data reports that HCC progression may also be associated with the formation of CCNY complexes with protein kinase 1 (PFTK1) also called cyclin-dependent kinases (CDK14). The CCNY and PFTK1 property implication enhances the key points of the Wnt signaling pathway regulated through segment polarity protein disheveled homolog 2 (DVL2) and naked cuticle 1 (NKD1) promoting an increased tumor cell proliferation profile and metastasis profile. The involvement of proteins from the Ras homologous – guanosine-5’-triphosphate hydrolase enzymes (Rho-GTPases) family indicates the participation of CCNY in the non-canonical Wnt signaling cascade consisting of the planar cell polarity (PCP) signaling pathway and the calcium signaling pathway. The activity of this cascade is primarily associated with increased cell migration, which promotes the phenomenon of metastasis. It is associated with the fact that Rho-GTPases are involved in regulating actin polymerization, but also reorganization of the actin cytoskeleton and stress fibers formation, consequently leading to cortactin recruitment, formation of invasive protrusions, promoted degradation of the extracellular matrix and consequently confers the motile phenotype of the tumor mass cells [Citation37]. The studies conducted on cell lines reflect the results of staining of paraffin sections of HCC patients showing a high correlation coefficient of the overexpression of CCNY with tumor size, invasion stage, distant and surrounding lymph nodes metastasis, and histological grade. Overall, the CCNY expression has been significantly higher in tumor tissue compared to normal tissue preparations [Citation38].
The overexpression of cyclin Y also has been found among lung cancer patients A similar association of strong CCNY overexpression correlating with tumorigenesis is observed within lung cancer cells. The results of two-hybrid screening indicate an interaction of CCNY with PFTK1 which not only enhances the PFTK1 kinase activity but also changes its intracellular location by recruiting PFTK1 to the plasma membrane. The analysis of the clinical material showed an association between CCNY overexpression with the histological subtype and tumor size. However, the high level of this protein does not correlate with the frequency of lymph node metastases and the clinical stage of the tumor. Thus, the level of CCNY expression may be significantly important, above all at the stage of NSCLC initiation. Histologically, NSCLC includes three of the most common types with similar characteristics, including squamous cell carcinoma (SCC), adenocarcinoma (AC), and large cell carcinoma (LCC), and CCNY overexpression in adenocarcinoma could result in significantly higher metastatic potential, as compared with squamous cell carcinoma [Citation39]. Moreover, the serum levels of CCNY were significantly increased among the patients with NSCLC, and also there was a correlation between CCNY protein and aggressive progression of both lymphatic and distant metastases [Citation40]. In addition, CCNY is characterized by a polymorphism referred to as the presence of this protein in two forms including cytoplasmic isoform (CCNYc) coding an N-terminal truncated protein, and membrane distribution isoform (CCNYm) coding a full-length 341 protein, localized by a myristoylation signal motif in the N-terminal domain. However, the CCNYc is this isoform that is responsible for the increased migration and invasive activity of abnormal cells. Mentioned migration and invasive activity could be associated with the formation of the CCNYc/PFTK1 complex responsible for regulating the polymerization of actin monomers and stress fibers formation via tropomyosin 4 (TPM4) and Ras homolog family member A (RhoA) which promotes cell metastasis as well as epithelial-mesenchymal transitions (EMTs) [Citation41,Citation42]. As a result of EMT induction, the cell begins to express EMT-specific transcription factors: Zinc Finger E-Box Binding Homeobox 1 and 2(Zeb1, Zeb2), Slug, Snail, and Twist proteins, which change the gene expression in this cell [Citation43]. This results in a decrease in the expression of epithelial markers (E-cadherin, claudins, occludins, type IV collagen, and laminin 1), with a simultaneous increase in mesenchymal markers (N-cadherin, laminin 5, fibronectin, α5β1 and αvβ6 integrin, vimentin, type I collagen) [Citation44]. On the other hand, a decrease in CCNY expression is sufficient to induce the opposite occurrence have been described as MET mesenchymal-epithelial transitions (MET) [Citation45]. Particularly noteworthy is vimentin, which, apart from its marker function in EMT, is also one of the target proteins of the Wnt signaling pathway. It is commonly considered a cytoskeleton protein, but it should be remembered that vimentin comes in two forms: attached to the outer surface of the cell and secreted into the extracellular space. Each of these forms plays a significant role in overall cellular functions. Surface vimentin is overexpressed in neoplastic cells and contributes to the progression of neoplasms, while the secretion form of this protein modulates inflammation. Research indicates that CCNY overexpression, notably CCNYc, enhances vimentin expression, which is critical for lung cancer progression and metastasis [Citation41].
Furthermore, a significantly increased in the CCNY expression is also observed within ovarian cancer cells [Citation46]. Ovarian cancer (OC) is considered a primary cancer of the reproductive organs, which ranks behind cervical cancer, but the lack of effective methods of early detection means that the death rate from OC is much higher than the mortality rate of endometrial and cervical cancer [Citation47]. The CCNY overexpression indicates association with a high level of proteins including c-Myc, cyclin D1, PFTK1, invasion-related molecule O-GlcNAc transferase (OGT), and tumor proliferation marker protein Ki-67 as well as overexpression of β-catenin colocalized in the cell nucleus with a simultaneous decrease in the cytoplasm level of this protein. As previously mentioned, high CCNY expression is associated with the noncanonical Wnt pathway, which engages the activity of PFTK1 protein and Rho GTPases activity which contributes to actin polymerization which affects the motility and migration of ovarian cancer cells. Additionally, a significant relationship was observed between CCNY overexpression and the clinical-pathological stage and lymph node metastases. Research reported an associated increase in CCNY expression with III and IV tumor stages and metastases to regional and distant lymph nodes which correlate with poor prognosis in the course of ovarian cancer [Citation46].
Less evidence for a link between high CCNY expression and tumor progression for breast cancer is observed, however, they could be found. It was presented that the immunohistochemical expression of CCNY is stronger in neoplastic tissues than in normal tissues. Also, all breast cancer cell lines showed a high level of the tested protein. The expression of cyclin Y in lines MDA-MB-231 and MCF-7 was reduced through the lentivirus that contained short hairpin ribonucleic acid shRNA [Citation48]. This treatment decreased the ability to form colonies and reduce the proliferation of cell lines. In addition, analyzing the cell cycle of the MDA-MB-231 cells, it was noticed that lowering the expression of CCNY causes the arrest of cells in the gap 0 phase/gap 1 phase (G0/G1 phase), and inhibits cell growth by cleavage of poly (ADP-ribose) polymerase (PARP) and caspase-3 depending on p53 and by activating the proteins: glycogen synthase kinase-3 beta (GSK3β) and Bcl-2-associated death promoter (Bad). Based on the presented research, scientists concluded that inhibition of cyclin Y by introducing a lentivirus containing shRNA could be a therapeutic option in breast cancer. Other scientists showed the effect of Cdk16 expression, activated by CCNY on triple-negative breast cancer (TNBC). Analysis of the results shows that cyclin-dependent kinase 16 (Cdk 16) is highly expressed in TNBC. The increased level of the tested protein increases the proliferation and migration of cancer cells, promotes tumor growth, and is correlated with the poor prognosis of patients. On the other hand, lowering the level of Cdk 16 expression significantly inhibits tumor progression [Citation48,Citation49]. Zhang et al. provided strong evidence that inhibition of CDK14 activity has great potential as a strategy in the treatment of TNBC. Using the FMF-04-159-2 CDK14 inhibitor on murine xenografts and patient-derived organoid model they showed that attenuating Wnt/β-catenin signaling results in suppression of TNBC progression and metastasis [Citation50].
In conclusion, the results of the presented research indicate a significant relationship between increased CCNY expression and tumor progression. The study analysis obtained so far indicate a similar mechanism of action of CCNY in differential cancer types, based on the Wnt signaling pathway, inducing increased proliferation and migration of cancer cells. Further investigation into this protein will probably help to more broadly understand the molecular mechanism underlying the action of CCNY on the cell cycle machinery which could allow using CCNY overexpression as a potential biomarker for a cancer diagnostic.
Conclusion
The presented data on the protein of the cyclin family – cyclin Y – suggest that in healthy cells cyclin Y plays an important role in the regulation of many processes for example adipogenesis, spermatogenesis, neuronal functions, as well as recently described involvement in the process of autophagy. Moreover, abnormal expression of cyclin Y contributes to the formation of various types of cancers. Increased expression is most often associated with increased proliferation, malignancy, and tumor metastasis. On the other hand, lowering the level of cyclin Y mainly inhibits the proliferation of cancerous cells. Therefore, understanding the molecular mechanisms of deregulation of cell cycle progression in cancer can provide important information on how normal cells become cancerous and how new treatment strategies can be designed. Therefore, it is evident that the study of the expression level of cyclin Y may be a target in the treatment of neoplastic diseases. Expression monitoring can help estimate the risk of cancer and allow it to be diagnosed quickly.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–3093.
- Child ES, Hendrychová T, McCague K, et al. A cancer-derived mutation in the PSTAIRE helix of cyclin-dependent kinase 2 alters the stability of cyclin binding. Biochim Biophys Acta. 2010;1803(7):858–864.
- Obaya AJ, Sedivy JM. Regulation of cyclin-Cdk activity in mammalian cells. Cell Mol Life Sci. 2002;59(1):126–142.
- Wood DJ, Endicott JA. Structural insights into the functional diversity of the CDK–cyclin family. Open Biol. 2018;8(9):180112.
- Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115.
- Liu D, Finley RL. Cyclin Y is a novel conserved cyclin essential for development in drosophila. Genet. 2010;184(4):1025–1035.
- Safran M, Rosen N, Twik M, et al. The GeneCards Suite. In: Abugessaisa I Kasukawa T editors. Practical Guide to Life Science Databases. Singapore: Springer Singapore; 2021pp. 27–56. cited 2022 Apr 28. InternetAvailable from. DOI:10.1007/978-981-16-5812-9_2
- Cho E, Kim D-H, Hur Y-N, et al. Cyclin Y inhibits plasticity-induced AMPA receptor exocytosis and LTP. Sci Rep. 2015;5(1):12624.
- Jiang M, Gao Y, Yang T, et al. Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 2009;583(13):2171–2178.
- Mikolcevic P, Sigl R, Rauch V, et al. Cyclin-Dependent Kinase 16/PCTAIRE kinase 1 is activated by Cyclin Y and is essential for spermatogenesis. Mol Cell Biol. 2012;32(4):868–879.
- Shi K, Ru Q, Zhang C, et al. Cyclin Y modulates the proliferation, invasion, and metastasis of hepatocellular carcinoma cells. Med Sci Monit. 2018;24:1642–1653.
- Joe I-S, Kim J-H, Kim H, et al. Cyclin Y-mediated transcript profiling reveals several important functional pathways regulated by Cyclin Y in hippocampal neurons. PLoS ONE. 2017;12(2):e0172547.
- Pond KW, Doubrovinski K, Thorne CA. Wnt/β-catenin signaling in tissue self-organization. Genes. 2020;11(8):939.
- Jung Y-S, Park J-I. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med. 2020;52(2):183–191.
- Zi Z, Zhang Z, Li Q, et al. Correction: cCNYL1, but not CCNY, cooperates with CDK16 to regulate spermatogenesis in mouse. PLoS Genet. 2019;15(3):e1008021.
- An W, Zhang Z, Zeng L, et al. Cyclin Y is involved in the regulation of adipogenesis and lipid production. PLoS ONE. 2015;10(7):e0132721.
- Sun T, Co NN, Wong N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem Biophys Res Commun. 2014;449(1):163–168.
- Wang X, Jia Y, Fei C, et al. Activation/proliferation-associated Protein 2 (Caprin-2) Positively Regulates CDK14/Cyclin Y-mediated Lipoprotein Receptor-related Protein 5 and 6 (LRP5/6) Constitutive Phosphorylation. J Biol Chem. 2016;291(51):26427–26434.
- Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14(2):207–215.
- Dohmen M, Krieg S, Agalaridis G, et al. AMPK-dependent activation of the Cyclin Y/CDK16 complex controls autophagy. Nat Commun. 2020;11(1):1032.
- Vervoorts J, Neumann D, Lüscher B. The CCNY (cyclin Y)-CDK16 kinase complex: a new regulator of autophagy downstream of AMPK. Autophagy. 2020;16(9):1724–1726.
- Gatica D, Klionsky DJ. New tricks of an old autophagy regulator: aMPK-dependent regulation of autophagy through CCNY (cyclin Y)-CDK16. Autophagy. 2020;16(6):973–974.
- Hwang H, Hur Y-N, Sohn H, et al. Cyclin Y, a novel actin-binding protein, regulates spine plasticity through the cofilin-actin pathway. Prog Neurobiol. 2021;198:101915.
- Ou C-Y, Poon VY, Maeder CI, et al. Two cyclin-dependent kinase pathways are essential for polarized trafficking of presynaptic components. Cell. 2010;141(5):846–858.
- Hwang H, Seo J, Choi Y, et al. Ccny knockout mice display an enhanced susceptibility to kainic acid-induced epilepsy. Pharmacol Res. 2020;160:105100.
- Kennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent Mechanisms. J Biol Chem. 2005;280(25):24004–24010.
- Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953.
- Molinas A, Heil S, Koch S. The candidate IBD risk gene CCNY is dispensable for intestinal epithelial homeostasis. Cells. 2021;10(9):2330.
- Kyselova A, Siragusa M, Anthes J, et al. Cyclin Y is expressed in platelets and modulates integrin outside-in signaling. Int J Mol Sci. 2020;21(21):8239.
- Chao DL, Sanchez CA, Galipeau PC, et al. Cell proliferation, cell cycle abnormalities, and cancer outcome in patients with barrett’s esophagus: a long-term prospective study. Clin Cancer Res. 2008;14(21):6988–6995.
- Casimiro MC, Crosariol M, Loro E, et al. Cyclins and cell cycle control in cancer and disease. Genes & Cancer. 2012;3(11–12):649–657.
- Liu D, Guest S, Finley RL. Why cyclin Y? a highly conserved cyclin with essential functions. Fly. 2010;4(4):278–282.
- Davidson G, Niehrs C. Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol. 2010;20(8):453–460.
- Xu Y, Wang Z, Wang J, et al. Lentivirus-mediated knockdown of cyclin Y (CCNY) inhibits glioma cell proliferation. Oncol Res Featuring Preclinical Clin Cancer Ther. 2009;18(8):359–364.
- Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6(2):104–111.
- Bouchard MJ, Navas-Martin S. Hepatitis B and C virus hepatocarcinogenesis: lessons learned and future challenges. Cancer Lett. 2011;305(2):123–143.
- Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes Migr. 2011;5(2):170–180.
- Chen L, Wang X, Cheng H, et al. Cyclin Y binds and activates CDK4 to promote the G1/S phase transition in hepatocellular carcinoma cells via Rb signaling. Biochem Biophys Res Commun. 2020;533(4):1162–1169.
- Hou J, Meng F, Chan LWC, et al. Circulating plasma microRNAs as diagnostic markers for NSCLC. Front Genet. 2016;7:193.
- Ma L, Gu M, Teng Y, et al. Establishing a detection method for CCNY: a potentially significant clinical investigative marker in NSCLC patients. OTT. 2019;12:921–932.
- Zhao X, Jiang M, Teng Y, et al. Cytoplasmic localization isoform of cyclin Y enhanced the metastatic ability of lung cancer via regulating tropomyosin 4. Front Cell Dev Biol. 2021;9:684819.
- Ribatti D, Tamma R, Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol. 2020;13(6):100773.
- Derynck R, Weinberg RA. EMT and cancer: more than meets the eye. Dev cell. 2019;49(3):313–316.
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–1437.
- Recondo G, Che J, Jänne PA, et al. Targeting MET dysregulation in cancer. Cancer Discov. 2020;10(7):922–934.
- Liu H, Shi H, Fan Q, et al. Cyclin Y regulates the proliferation, migration, and invasion of ovarian cancer cells via Wnt signaling pathway. Tumour Biol. 2016;37(8):10161–10175.
- Momenimovahed Z, Tiznobaik A, Taheri S, et al. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287–299.
- Yan F, Wang X, Zhu M, et al. RNAi-mediated downregulation of cyclin Y to attenuate human breast cancer cell growth. Oncol Rep. 2016;36(5):2793–2799.
- Li X, Li J, Xu L, et al. CDK16 promotes the progression and metastasis of triple-negative breast cancer by phosphorylating PRC1. J Exp Clin Cancer Res. 2022;41(1):149.
- Zhang M, Zhang L, Geng A, et al. CDK14 inhibition reduces mammary stem cell activity and suppresses triple negative breast cancer progression. Cell Rep. 2022;40(11):111331.