ABSTRACT
Metal responsive transcription factor 1 (MTF-1) is a zinc-dependent transcription factor involved in the development of pulmonary arterial hypertension (PAH), which is a life-threatening disease characterized by elevated pulmonary artery pressure and pulmonary vascular remodeling. However, little is known about the role and regulatory signaling of MTF-1 in PAH. This study aimed to investigate the effect and mechanism of MTF-1 on the proliferation of pulmonary arterial smooth muscle cells (PASMCs). Several techniques including intracellular-free zinc detected by fluorescent indicator-fluozinc-3-AM, western blot, luciferase reporter, and cell proliferation assay were conducted to perform a comprehensive analysis of MTF-1 in proliferation of PASMCs in PAH. Increased cytosolic zinc was shown in monocrotaline (MCT)-PASMCs and ZnSO₄-treated PASMCs, which led to overexpression and overactivation of MTF-1, followed by the up-regulation of placental growth factor (PlGF). Elevated MTF-1 and PlGF were observed in western blot, and high transcriptional activity of MTF-1 was confirmed by luciferase reporter in ZnSO4-treated cells. Further investigation of cell proliferation revealed a favorable impact of zinc ions on PASMCs proliferation, with the deletion of Mtf-1/Plgf attenuating ZnSO4-induced proliferation. Flow cytometry analysis showed that blockade of PKC signaling inhibited the cell cycle of MCT-PASMCs and ZnSO4-treated PASMCs. The Zinc/PKC/MTF-1/PlGF pathway is involved in the up-regulatory effect on the PASMCs proliferation in the process of PAH. This study provided novel insight into zinc homeostasis in the pathogenesis of PAHs, and the regulation of MTF-1 might be a potential target for therapeutic intervention in PAH.
1. Introduction
Pulmonary arterial hypertension (PAH) is a chronic and life-threatening disease characterized by a progressive increase in pulmonary arterial pressure (PAP), pulmonary vascular remodeling, and right heart failure. It is believed that the aberrant proliferation of pulmonary artery smooth muscle cells (PASMCs) primarily contributes to this remodeling. However, the underlying mechanisms responsible for PASMCs proliferation in PAH have not yet been completely elucidated. Multiple factors have been reported to be associated with the prevalence of PAH, whose development is primarily related to abnormal expression of BMPR2 and PTGIS, imbalance of vasoconstriction and relaxation, excessive proliferation of pulmonary artery endothelial cells (PAECs) and PASMCs, disorders of extracellular matrix metabolism, formation of thrombus in situ and fluctuation of trace elements (such as calcium, iron, or zinc ions) [Citation1–3]. Unfortunately, existing strategies for preventing and treating PAH are ineffective. Therefore, it is essential to find out novel targets or clues for treatment of PAH.
Basically, zinc is the second largest metal nutrient in human body after iron, and no matter cell growth, proliferation, differentiation or programmed cell death, free zinc ions, or protein-bound forms of zinc are involved [Citation4]. Due to its catalytic and structural roles within proteins, zinc is required by more than 300 enzymes and nearly 2000 transcription factors [Citation5], which are estimated to contain a zinc binding motif, including metalloenzymes and zinc finger proteins [Citation6]. Zinc is essential for cell proliferation, differentiation, and survival, and has been linked to various disorders owing to its involvement in neutralizing free radicals and antioxidant properties, which is regarded as “calcium in the 21st century”. Secondly, a dynamic equilibrium of zinc levels is required for the maintenance of normal physiological functions [Citation7], and disorders emerge when the balance is disturbed. Excessive cytoplasmic zinc has been demonstrated to contribute to tumorigenesis [Citation8], neurological disorders [Citation9], PAH [Citation10], male reproductive disorders, and cardiovascular diseases [Citation11]. Additionally, activities of phosphatases are reported to be suppressed by free zinc ions in cytoplasm, such as protein-tyrosine-phosphatase (PTPs) [Citation12], phosphodiesterase (PDEs), phosphatase, and tensin homologues (PTEN) [Citation13]. Furthermore, mitogen-activated protein kinase (MAPK) [Citation14], phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB, AKT), and protein kinase C (PKC) [Citation15] are directly or indirectly modulated by zinc ions.
Metal-regulatory transcription factor 1 (MTF-1), a cytoplasmic zinc sensor, is activated by the interaction of zinc ions with its six highly conserved zinc finger domains. In response to zinc, MTF-1 regulates approximately 1000 genes by binding to metal-response elements (MREs) in the 5’ regulatory region of genes [Citation16]. Numerous studies [Citation17,Citation18] have demonstrated that direct activation of MTF-1’s MRE-binding affinity by zinc ions is necessary for the activation of target protein expression, including placental growth factor (PlGF), a member of the vascular endothelial growth factor (VEGF) family that stimulates angiogenesis, proliferation, and migration of endothelial cells. Researches [Citation19,Citation20] have shown that PlGF participates in the reconstruction of hypoxic lung tissue, and serum PlGF levels are considerably elevated in PAH. In addition, the knockout of Plgf gene inhibits the promotion of hypoxia on cell proliferation.
In this study, we determined the involvement of zinc in PAH by examining the proliferation of PASMCs following monocrotaline (MCT) injection and ZnSO4 administration and further examined whether zinc activates MTF-1 in PASMCs via up-regulating its expression and transcriptional activity.
2. Materials and methods
2.1. Ethical statement
All these animal procedures are strictly in accordance with the recommendations in the “Guidelines for the Care and Use of Laboratory Animals (National Academy of Sciences, 2011)”. This study was approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University (Approval No. 2017–070, Fuzhou, China).
2.2. PAH-rat models
Twenty male Sprague-Dawley (SD) rats, weighing 150–180 g, purchased from Shanghai SLACCAS Laboratory Animal Co. Ltd., were randomly divided into two groups, including control group (Ctrl) and MCT-PAH group (MCT-PAH). As stated in our previous work, the PAH-rat model was newly established by intraperitoneal injection of 20 mg/kg MCT on the 1st and 8th day [Citation21,Citation22]. The control rats received the same volume of saline. All these operations conducted on rats were anesthetized with sodium pentobarbital and efforts to minimize the pain of the rats. Mean pulmonary arterial pressure (mPAP) and right ventricular hypertrophy index (RVHI, [RVHI = RV/(LV+S)]) were assessed at the 4th week as reported previously [Citation23].
2.3. Histological and immunohistochemical analysis
The lung tissues of the rats were collected simultaneously at 4th week after MCT injection. For histological and collagen analyses, the lung tissues of rats were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5 μm-thick sections, which were then stained with Hematoxylin & eosin (HE) staining. MTF-1 antibody (1:200, TP0707, HuaBio, China), PlGF antibody (1:200, sc -57402, Santa Cruz Biotechnology, USA), and proliferating cell nuclear antigen (PCNA) antibody (1:200, ab29, Abcam, USA) were used for immunohistochemistry. The sections were observed under the light microscope Nikon 80i Microscope (Nikon, Japan). This experiment was repeated five times independently. The WT% ([WT%=vessel wall thickness/total vascular diameter]) and WA% ([WA%=vessel wall area/total vascular area]), which were utilized to score the pulmonary vascular remodeling, were evaluated at the 4th week as reported previously [Citation22,Citation23].
2.4. Fluorescent immunohistochemistry
The protocol was described previously in our previous work [Citation24]. Briefly, the sections were deparaffinized, rehydrated, and incubated in 2% albumin for 1 h at room temperature to saturate nonspecific bindings. At this point, MTF-1 antibody (1:200, TP0707, HuaBio, China) and α-SAM (1:200, ABM0052, Abbkine Scientific, China) were added for overnight at 4°C. After incubation, the slides were washed 5 times in phosphate buffer saline (PBS) and incubated with donkey anti-rabbit-FITC (1:200, ab6761, Abcam, USA) and donkey anti-mouse-Tex-Red (1:200, ab6818, Abcam, USA) for 1 h at room temperature. After washing 5 times in PBS, the fluorescent nuclear stain 4’,6-diamidino-2-phenylindole (DAPI) (1 µg/mL; Cell Signaling Technology, USA) was added. The sections were observed under the light microscope Nikon 80i Microscope (Nikon, Japan). This experiment was repeated five times independently.
2.5. Cell culture and treatment
PASMCs were isolated from the pulmonary arteries using a previously described method [Citation21,Citation22], cultured in Dulbecco’s modified Eagle medium (DMEM, Hyclone, USA) containing 10% FBS (Gibco, Australia) and passaged with 0.25% trypsin (Hyclone, USA). The cultured dishes were kept in a moist atmosphere of 5% CO2 at 37°C. When the cells were 70–80% confluent, the medium was replaced with DMEM and starved for 24 h. Then cells were exposed to different treatments and interventions, respectively, including zinc sulfate solution (ZnSO4, 100 µM), Mtf-1/Plgf-siRNA, Mtf-1/Plgf-siRNA+ZnSO4 (100 µM). After incubation for indicated times, PASMCs were collected and tested.
2.6. Generation of polyclonal anti-MTF-1 antibody
MTF-1 antibody was generated by Hangzhou HuaBio. The synthetic peptide corresponding to rat MTF-1 last 14 amino acids (C-DSAKERAAGRRKGC) was used in immunizations (conjugated to keyhole limpet hemocyanin protein) and for antigen affinity purification of the antibody. The peptide sequence was confirmed to be MTF-1 specific using RStudio. The specificity of immunized rabbit serum containing anti-MTF-1 antibody was confirmed by ELISA or immunoblotting with rat PASMCs lysates. A single band at about 70 kDa was visible in the immunoblots.
2.7. Cell immunofluorescence staining
PASMCs were digested with 0.25% trypsin and grown on 22 mm2 coverslips in 12-well plates. Immunofluorescence staining was described previously [Citation24]. After washing once with PBS, slides were fixed with 4% formaldehyde in PBS for 10 min at room temperature. Cells were rinsed with PBS, treated with 0.2% Triton X-100 on ice for 10 min, and then blocked with 5% nonfat milk for 30 min at room temperature. Cells were then incubated with 2 µg/mL anti-α-SAM (ABM0052, Abbkine Scientific, China) or anti-MTF-1 (TP0707, HuaBio, China) in blocking solution overnight. Next, the cells were washed three times with PBS and incubated with 20 µg/mL CY3 conjugated affinipure goat anti-rabbit IgG (BA1032, Boster Biological Technology, China) in PBS for 2 h at room temperature. They were then washed three times with PBS before being incubated with DAPI in PBS for 5 min. Specimens were mounted in 90% glycerol, sealed with nail polish oil, and observed under a confocal microscope Zeiss LSM780 (Zeiss, Germany). This experiment was repeated five times independently.
2.8. Cell proliferation assay
For the determination of proliferation, Cell Counting Kit-8 (NCM Biotech, China) was used. In brief, PASMCs were seeded into 96-well plates. After 24 h, the cells were transfected or treated with different treatments and interventions, respectively. When testing, 10 µL of Cell Counting Kit-8 reagent was added to each well, incubated at 37°C for 3 h, and then the absorbance at 450 nm was measured on a microplate reader (Biotek, USA). The proliferation rate was calculated by the absorbance ratio of the treated group relative to that of the control group. Besides, PASMCs proliferation was measured using EdU Proliferation Assay Kit (Beyotime Biotechnology, China) as well [Citation21], in which proliferative cells were shown by labeled with EdU [red], whereas the nuclei were stained with Hoechst33342 [blue]. Cells were viewed and photographed using the fluorescent microscopy (Nikon, Japan). Meanwhile, the cell cycle distribution was detected by flow cytometry analysis. The PASMCs were seeded into 6-well plates. After 24 h, the cells were transfected or treated with different treatments and interventions, respectively. Subsequently, the cells were stained with propidium iodide (PI, Heruibio, HRF0032, China) (20 μg/mL PI, 100 μg/mL RNase A in PBS). The cell cycle was analyzed by flow cytometry (Beckman, USA). Furthermore, the expression of PCNA was determined by western blotting. Each experiment was repeated five times independently.
2.9. Cell scratch assay
Horizontal lines were evenly drawn on the bottom of six-well plates at a distance of 0.5–1 cm using a ruler and a marking pen, with at least five lines passing through every well. The cells were then seeded into six-well plates at a density of 5 × 105 cells/well and cultured overnight in DMEM containing 10% FBS. Next, a sterile 200 μL pipette tip was used to make scratches perpendicular to the horizontal lines. Scratch distances were measured and recorded under an optical microscope at the 0 h and 48 h time intervals, respectively. Moreover, images were collected under an inverted microscope to observe cell migration in each group. The cell migration rate was calculated using the following equation: cell migration=(scratch distance at 0 h−scratch distance at 48 h)/scratch distance at 0 h × 100%. This experiment was repeated five times independently.
2.10. Mtf-1/Plgf- siRNA transfection
Cells were transfected overnight with 5 µmol siRNA (GenePharma, China) against Mtf-1 and Plgf, or negative siRNA as a control, using OPTI-MEM and Lipofectamine RNAiMAX (Invitrogen Life Technologies, USA) according to the previous protocol [Citation25]. Cells were cultivated at 37°C for 12 h to observe cell status, then replaced OPTI-MEM with a complete medium for 24 h and detected MTF-1 and PlGF expression by western blot after 48 h. This experiment was repeated five times independently.
Table 1. siRNA sequences of Mtf-1 and Plgf.
2.11. Dual-luciferase reporter assay
The Plgf promoter was cloned and constructed into the pGl3-Basic vectors by ClonExpress II One-Step Cloning Kit (Vazyme, USA). The MTF-1 binding sites (MREs) in the Plgf promoter were predicted by JASPAR (http://jaspar.genereg.net/). The mutative metallic response element (MUT-MREs) was performed with the Quick-Change Site-Directed Mutagenesis Kit (Vazyme, USA) (5’-TAGTCGATCAGTTGGACTTA-3’). All plasmids were sequenced for verification in a commercial company (Sangon Biotech, China). HEK293T cells were plated in 24-well plates with high glucose DMEM (4.5 g/L) and then transfected with different plasmids using Lipofectamine 2000 Transfection Reagent (Invitrogen Life Technologies, USA). The Dual-Luciferase Reporter Assay System (Yeasen BioTech, China) was used to assay luciferase activity. Relative luciferase activity = fluorescence intensity of firefly/fluorescence intensity of Renilla. This experiment was repeated five times independently.
2.12. Measurement of intracellular zinc using the fluorescent indicator
The concentration of free intracellular zinc was measured in PASMCs by fluorescent indicator, fluozin-3-AM as previously described [Citation24]. Cells cultured on glass coverslips were placed inside a culture chamber and exposed to different interventions. Cells were loaded with 1 µM FluoZin-3-AM (Invitrogen Life Technologies, USA) in PBS at 37 °C for 60 min. Before fluorescence measurements, cells were washed in PBS three times to remove any dye that was nonspecifically associated with the cell surface, and then incubated for a further 30 min. For fluorescence detection, a Zeiss LSM780 confocal (Zeiss, Germany) was employed to acquire fluorescent images. Fluozin-3-AM has been extensively characterized to measure free intracellular zinc in living cells using microscopy (excitation/emission: 494 nm/516 nm) and has an affinity constant for zinc of 15 nM. This experiment was repeated three times independently.
2.13. Cytoplasmic and nuclear protein extraction
Control PASMCs, ZnSO4-treated PASMCs, and MCT-PAH PASMCs were cultured in 10 cm dishes. Cytoplasmic and nuclear proteins were extracted by the Nuclear Protein Extraction kit (Solarbio, China). PASMCs were ruptured by plasma protein extraction reagent, and cytoplasmic proteins and nuclei were separated by centrifugation. The nuclear proteins were then extracted by nuclear extract reagent. Cytoplasmic and nuclear MTF-1 expressions were detected by Western blot analysis and were standardized with β-actin (1:2000, sc -47778, Santa Cruz Biotechnology, USA) and Lamin B (1:1000, sc -374015, Santa Cruz Biotechnology, USA), respectively. This experiment was repeated five times independently.
2.14. Protein extraction and western blotting
Frozen rat tissues (lungs) and cell pellets were homogenized in RIPA buffer (Sigma, USA) supplemented with protease inhibitor (Roche, Switzerland). Mini-PROTEAN TGX Precast Gels (Bio-rad, USA) was used in western blotting following the manufacturer’s suggestions. Blots were incubated overnight at 4 °C with anti-MTF-1 (1:500, TP0707, HuaBio, China), anti-PlGF (1:500, sc -57402, Santa Cruz Biotechnology, USA), anti-p-PKCζ and anti-t-PKC (1:500, ET1611–78 and ER64620, HuaBio, China), anti-PCNA (1:500, ab29, Abcam, USA), and anti-β-actin (1:2000, sc -47778, Santa Cruz Biotechnology, USA) respectively. The HRP-conjugated secondary anti-mouse antibody and anti-rabbit antibody were applied for 1 h at room temperature following with incubation with the enhanced chemiluminescent substrate. Proteins were detected by Clarity western ECL substrate (Meilunbio, China). Optical densities of individual bands were measured using Image J software and protein expressions were standardized with β-actin. This experiment was repeated five times independently.
2.15. Statistical analysis
Data are expressed as mean ± standard deviation. Most of the experiments were repeated five times independently. Graphpad prism 8 (Graphpad Software Inc., San Diego, CA) was used for the statistical analysis. A one-way analysis of variance (ANOVA), followed by an LSD (Least Significant Difference) test was used to compare the means among multiple groups. Differences with P values <0.05 were considered to be statistically significant.
3. Results
3.1. Increased free cytosolic zinc in MCT-PASMCs and ZnSO4-treated PASMCs
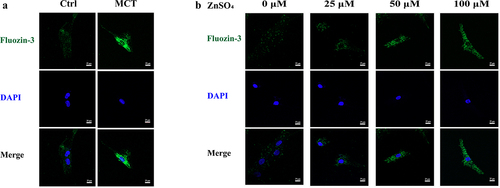
To examine the concentration of zinc in MCT-PASMCs and ZnSO4-treated PASMCs, the fluorescence intensity of fluozin-3-AM in PASMCs was measured. The relative fluorescence intensity of fluozin-3-AM was higher in PASMCs isolated from MCTPAH rats (MCT) than in control (Ctrl) (). Furthermore, increased intracellular-free zinc ions were observed qualitatively in the medium supplemented with ZnSO4 ().
Figure 1. Representative zinc probe fluorescence images in PASMCs. a Elevated intensity of fluorescent indicator which reflects intracellular free zinc levels in MCT-PASMCs compared with control (n = 3). b Elevated intensity of fluorescent indicator which reflects intracellular free zinc levels in PASMCs in response to increased ZnSO4 concentration in medium (n = 3). Ctrl: control; MCT: PASMCs isolated from MCT-PAH rats. Scale bar = 25 μm.
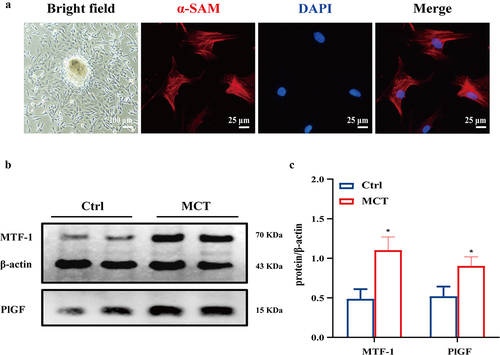
3.2. Enhanced MTF-1 expression in MCTPAH rats
To address the possible role of MTF-1 in PAH, MTF-1, and PlGF expression in the lungs and PASMCs of MCT-PAH rats were determined. MCT-induced PAH was characterized by elevated mPAP and RVHI (P < 0.05) (). It was showed that walls of pulmonary arterioles gradually thickened as PAH developed, which manifested as a significant increment in WT% and WA%, key parameters of vascular remodeling (P < 0.05) ()). Further, western blotting was analyzed and demonstrated that MTF-1 and PlGF expressions in lung tissues of MCT-PAH rats were obviously increased in comparison with those of normal control rats (P < 0.05) (). Immunohistochemistry was consistent with the observations, which showed a small amount of brownish yellow pigments in the intracellular space in lung tissues of control rats; however, a large amount of brownish yellow filament, small-strip, and flake-like was staining in the intracellular space of the rats in MCT-PAH groups (). Furthermore, fluorescent immunohistochemical investigations revealed the similar result that demonstrated higher expression of MTF-1 [green] was shown in the pulmonary arteries of MCT-PAH rats ().
Figure 2. Enhanced MTF-1 expression in lung tissues of MCT-PAH rats. a,b Construction of MCT-PAH model in rats. MCT-induced PAH was characterized by elevated mPAP and RVHI (n = 8). Scale bar = 50 μm. c-e Pulmonary arterioles wall thickened and increment of WT% and WA% in MCT-PAH rats (n = 8). Scale bar = 50 μm. f,g The elevated expressions of MTF-1 and PlGF in lung tissues of MCT-PAH rats (n = 5). h More positive cells were found in MCT-PAH pulmonary arteries, including MTF-1, PlGF and PCNA (n = 8). Scale bar = 100 μm. i Higher expression of MTF-1 in MCT-PAH pulmonary arteries. α-SAM [red], MTF-1 [green] and DAPI [blue] (n = 5). Scale bar = 50 μm. Ctrl: control; MCT-PAH: MCT induced PAH. *P<0.05 vs. Ctrl. The arrow points to the pulmonary artery.
![Figure 2. Enhanced MTF-1 expression in lung tissues of MCT-PAH rats. a,b Construction of MCT-PAH model in rats. MCT-induced PAH was characterized by elevated mPAP and RVHI (n = 8). Scale bar = 50 μm. c-e Pulmonary arterioles wall thickened and increment of WT% and WA% in MCT-PAH rats (n = 8). Scale bar = 50 μm. f,g The elevated expressions of MTF-1 and PlGF in lung tissues of MCT-PAH rats (n = 5). h More positive cells were found in MCT-PAH pulmonary arteries, including MTF-1, PlGF and PCNA (n = 8). Scale bar = 100 μm. i Higher expression of MTF-1 in MCT-PAH pulmonary arteries. α-SAM [red], MTF-1 [green] and DAPI [blue] (n = 5). Scale bar = 50 μm. Ctrl: control; MCT-PAH: MCT induced PAH. *P<0.05 vs. Ctrl. The arrow points to the pulmonary artery.](/cms/asset/4f3e3134-1abf-46c3-b543-917afc1e47f5/kccy_a_2205209_f0002_oc.jpg)
The identification and purity confirmation of primary PASMCs were executed by cell immunofluorescence staining of α-SMA (). Consistent with the results in lung tissues, MTF-1 and PlGF expressions were considerably increased in MCT-PASMCs (P < 0.05) ().
3.3. Increased MTF-1 expression and its transcriptional activity in ZnSO4-PASMCs
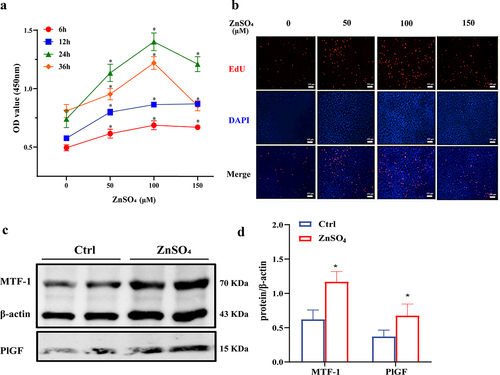
To compare the expression of MTF-1 and PlGF among control- and ZnSO4 -PASMCs, western blotting was performed. As shown in , CCK8 assay revealed that OD values were elevated in a time-dose-dependent manner in ZnSO4-PASMCs and treatment with 24 h of 100 μM ZnSO4 was the most optimal (P < 0.05). Similar results were observed in EdU assay (). Based on the above pilot test, the concentration of 100 μM ZnSO4 for 24 h was used in subsequent experiments. In line with the findings in MCT-PASMCs, higher expressions of MTF-1 and PlGF in ZnSO4-PASMCs were observed (P < 0.05) ().
Figure 4. The effect of moderate ZnSO4 on PASMCs proliferation. a,b CCK8 and EdU assay showed PASMCs proliferated in a time-dose manner in ZnSO4 group and treatment with 24 h of 100 μM ZnSO4 was the most optimal (n = 8, 5). Scale bar = 100 μm. c,d The elevated expression of MTF-1 and PlGF in ZnSO4-treated PASMCs (n = 5). Ctrl: control; ZnSO4: ZnSO4-treated PASMCs. *P<0.05 vs. 0 μM at the same time.
3.4. Nuclear localization of MTF-1 induced by MCT injection and ZnSO4 stimulation
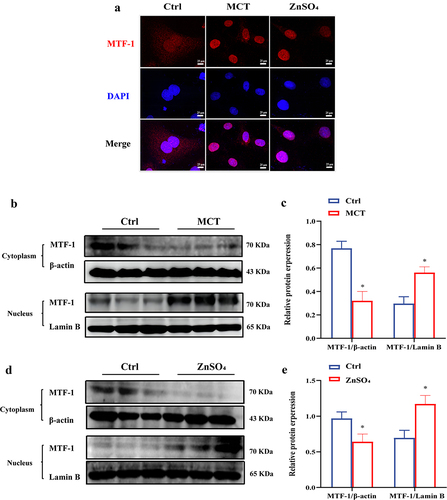
To investigate the cellular distribution and translocation of MTF-1 in MCT-PASMCs and untreated PASMCs in response to ZnSO4 stimulation, extraction of cytoplasmic and nuclear proteins and immunofluorescence staining were carried out. In untreated PASMCs, MTF-1 localized predominantly to the cytoplasm, while localized to nucleus in MCT-PASMCs and ZnSO4-treated PASMCs (). In addition, western blotting showed that significant nuclear translocation of MTF-1 was observed in MCT-PASMCs and ZnSO4-treated PASMCs (P < 0.05) ()). The data were confirmed by immunofluorescence staining, which demonstrated that in untreated PASMCs, MTF-1 resided in the cytoplasm while transferred to the nucleus in MCT-PASMCs and ZnSO4-treated PASMCs.
Figure 5. Nuclear localization of MTF-1 in MCT-PASMCs and ZnSO4-treated PASMCs. a Immunofluorescence staining showed MTF-1 localized to nucleus in MCT-PASMCs and ZnSO4-treated PASMCs (n = 3). Scale bar = 25 μm. b-e Reduced cytoplasmic MTF-1 and increased nuclear MTF-1 in MCT-PASMCs and ZnSO4-treated PASMCs (n = 5). Ctrl: control; MCT: PASMCs isolated from MCT-PAH rats; ZnSO4: ZnSO4-treated PASMCs. *P<0.05 vs. Ctrl.
3.5. Confirmation of the functional binding of MTF-1 to MREs on Plgf promoters in response to ZnSO4
To confirm the binding of MTF-1 to the putative MREs on Plgf promoters, dual-luciferase reporter assay was performed. HEK293T cells were transiently transfected with expression vectors encoding MTF-1, WT-Plgf vector (WT), MUT-Plgf vector (MUT), and pRL-TK. In , ZnSO4 addition elevated the relative luciferase activities in HEK293T+ WT; however, no significant elevation was observed in HEK293T and HEK293T + MUT. Moreover, decreased transcriptional activity of MTF-1 on MUT-MREs Plgf promoters was displayed compared to WT-MREs Plgf promoters, indicating that expression of PlGF was MREs-dependent (P < 0.05) and regulated by zinc ions (P < 0.05).
Figure 6. Role of ZnSO4 in regulation of MTF-1-mediated transcription of PlGF. a Base-pair binding between MTF-1 and MREs within Plgf 3′-untranslated regions (UTRs). b Luciferase assays of Plgf 3′-UTR constructs with intact and mutated sequences for MTF-1 in HEK293T cells. Elevated transcriptional activity of MTF-1 on intact Plgf promoters in response to ZnSO4, but not significantly on the mutated promoter (n = 8). HEK293T: HEK293T without transfection of any plasmid; HEK293T+WT: HEK293T with transfection of wild-type promoter; HEK293T+MUT: HEK293T with transfection of mutative promoter; Ctrl: control; ZnSO4: 100 μM ZnSO4 treatment for 24 h. *P<0.05 vs. wild-type promoter without treatment, #P<0.05 vs. wild-type promoter with treatment of ZnSO4, nsP>0.05.
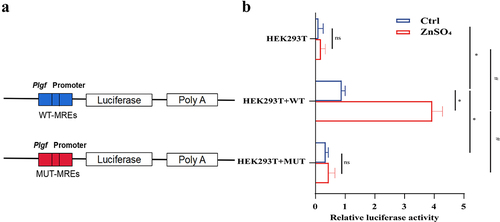
3.6. Loss of MTF-1 and PlGF reduced MCT and ZnSO4 induced PASMCs proliferation
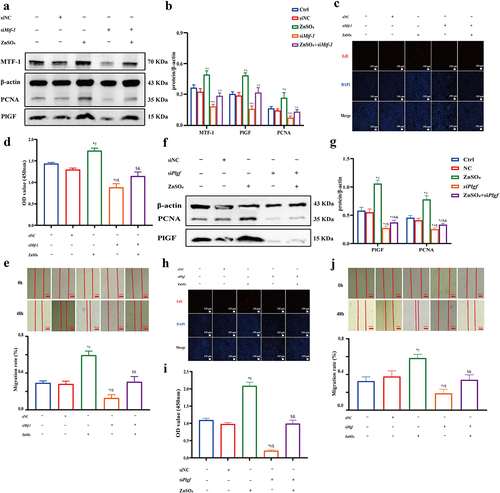
To verify the role of MTF-1 and PlGF in PASMCs proliferation, siRNA was used to knock down Mtf-1 and Plgf. PASMCs were transfected with either scrambled (siNC) or siRNA, then stimulated with ZnSO4 and further western blotting, EdU, CCK8, and scratch assay were performed. As shown in , knocking down Mtf-1 and Plgf down-regulated the expressions of MTF-1, PlGF, and PCNA in MCT-PASMCs (P < 0.05). Similar results of proliferation of PASMCs with knocking down Mtf-1 and Plgf were lower than untreated PASMCs were revealed by EdU and CCK8 assay (P < 0.05) (), and results of scratch assay showed that PASMCs migration was significantly diminished in the Mtf-1/Plgf-siRNA treated cells compared with that in the control cells (P < 0.05) (,j)). Similarly, ZnSO4 administration ameliorated the siRNA-induced decline in cell proliferation (all P < 0.05) ()).
Figure 7. The negative effect of Mtf-1/Plgf-siRNA on MCT-PASMCs proliferation. a,b Loss of Mtf-1 downregulated the expressions of MTF-1, PlGF and PCNA in MCT-PASMCs (n = 5). c,d EdU and CCK8 assay revealed knocking down Mtf-1 reduced MCT-PASMCs proliferation (n = 5, 8). e Scratch assay showed that Mtf-1-siRNA abated MCT-PASMCs migration (n = 5). f,g Loss of Plgf downregulated the expressions of PlGF and PCNA in MCT-PASMCs (n = 5). h,i EdU and CCK8 assay revealed knocking down Plgf reduced MCT-PASMCs proliferation (n = 5, 8). j Scratch assay showed that Plgf-siRNA abated MCT-PASMCs migration (n = 5). Ctrl: control; MCT: PASMCs isolated from MCT-PAH rats; NC: negative control. *P<0.05 vs. Ctrl, #P<0.05 vs. MCT, $P<0.05 vs. MCT+NC. EdU assay scale bar = 100 μm, scratch assay scale bar = 100 μm.
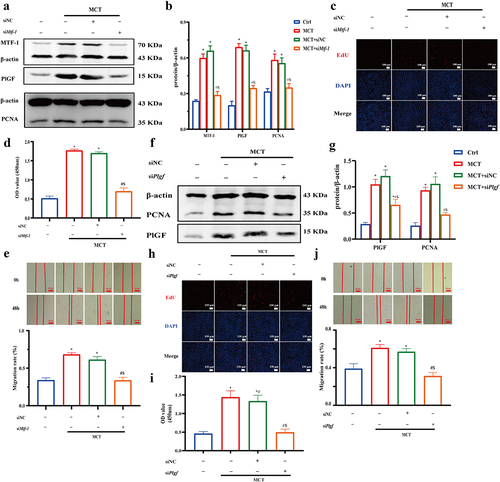
Figure 8. The negative effect of Mtf-1/Plgf-siRNA on ZnSO4-treated PASMCs proliferation. a,b Loss of Mtf-1 downregulated the expressions of MTF-1, PlGF and PCNA in ZnSO4-treated PASMCs (n = 5). c,d EdU and CCK8 assay revealed knocking down Mtf-1 reduced ZnSO4-treated PASMCs proliferation (n = 5, 8). e Scratch assay showed that Mtf-1-siRNA abated ZnSO4-treated PASMCs migration (n = 5). f,g Loss of Plgf downregulated the expressions of PlGF and PCNA in ZnSO4-treated PASMCs (n = 5). h,i EdU and CCK8 assay revealed knocking down Plgf reduced ZnSO4-treated PASMCs proliferation. (n = 5, 8). j Scratch assay showed that Plgf-siRNA abated ZnSO4-treated PASMCs migration (n = 5). Ctrl: control; ZnSO4: ZnSO4-treated PASMCs; NC: negative control. *P<0.05 vs. Ctrl, #P<0.05 vs. NC, $P<0.05 vs. ZnSO4, &P<0.05 vs. siMtf-1/Plgf. EdU assay scale bar = 100 μm, scratch assay scale bar = 100 μm.
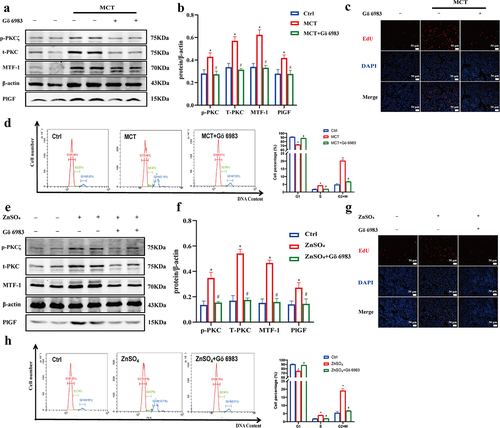
3.7. Gö 6983 inhibits PKC signaling pathway activation to reduce PlGF expression mediated by MTF-1
Gö 6983 has been reported as a pan-inhibitor against for several isoforms of PKC [Citation26]. To determine whether the PKC signaling pathway participates in regulating MTF-1 activity and PlGF expression, we pretreated PASMCs with Gö 6983 for 30 min. Elevated levels of phosphorylation PKCζ (p-PKCζ), total PKC (t-PKC), MTF-1, and PlGF were displayed in both MCT-PASMCs and ZnSO4-treated PASMCs (P < 0.05). However, it was shown that Gö 6983 suppressed PKC activation as indicated by the decreased ratio of p-PKCζ to β-actin. Meanwhile, lower expressions of MTF-1 and PlGF were also observed, demonstrating that positive effects of zinc ions on MTF-1 and PlGF expressions were offset by Gö 6983 (P < 0.05) (). Moreover, similar results were found to be related to regulatory cell proliferation (,g)). Furthermore, the inhibition of cell growth by Gö 6983 was reflected by changes in the cell cycle. Gö 6983 in PASMCs significantly increased the percentage of cells in G1 phase and decreased the percentage of cells in the G2/M phase (P < 0.05) (,h)), demonstrating that the pro-proliferative role of PKC signaling pathway in PASMCs proliferation.
Figure 9. Gö 6983 inhibited PKC signaling pathway activation to reduce PlGF expression mediated by MTF-1. a,b The negative effect of Gö 6983 on p-PKCζ, t-PKC, MTF-1 and PlGF levels in MCT-PASMCs (n = 5). c,d EdU and flow cytometry showed Gö 6983 decreased MCT-PASMCs proliferation (n = 5). e,f The negative effect of Gö 6983 on p-PKCζ, t-PKC, MTF-1 and PlGF levels in ZnSO4-treated PASMCs (n = 5). g,h EdU and flow cytometry showed Gö 6983 decreased ZnSO4-treated PASMCs proliferation (n = 5). Ctrl: control; MCT: PASMCs isolated from MCT-PAH rats; ZnSO4: ZnSO4-treated PASMCs. *P<0.05 vs. Ctrl, #P<0.05 vs. MCT/ZnSO4. EdU assay scale bar = 100 μm.
4. Discussion
Previously, Zrt-like, IRT-like protein 12 (ZIP12), a transporter of zinc ions on the cell membrane, was found to be overexpressed in PAHs resulting from MCT injection [Citation27], and intracellular-free zinc level was elevated in MCT-PASMCs [Citation24], which was in line with our research. In this paper, we presented evidence that MTF-1 played a vital role in the development of PAH. MCT administration and ZnSO4 incubation enhanced intracellular-free zinc ion concentration, leading to elevated MTF-1 expression, rapid nuclear translocation of MTF-1, and increased occupancy of MREs in Plgf promoter, which boosted PlGF expression. Thus, by up-regulating the PlGF expression, MTF-1 effectively promoted PASMCs proliferation in response to zinc ions. Furthermore, the attendance of zinc/PKC/MTF-1 signaling pathway in cell proliferation was confirmed. Taken together, our findings strongly support the hypothesis that MTF-1 functions as an essential downstream mediator of zinc/PKC-induced cell proliferation by virtue of its involvement in up-regulating PlGF expression. However, it is still unknown how PKC affects the transcriptional activity of MTF-1, and phosphorylation identification of MTF-1 should be applied for further investigation.
Cellular zinc ions primarily work as a catalytic or structural protein co-factor to generate a large number of binding configurations [Citation28]. Recent studies have highlighted its dynamic role as a second messenger in cellular metabolic [Citation29] and transcriptional regulation [Citation30]. Zinc is the most abundant intracellular metal ion found in the cytoplasmic vesicle organelles and nuclei of mammalian cells [Citation31], whereas intracellular pool of loosely bound zinc ions available for biological exchange is in the picomolar range and almost all zinc is tightly bound to proteins, and a small imbalance can be catastrophic for human health due to the vast number of biological dysfunctions. Accordingly, mechanisms contributing to dysfunctional zinc signaling are suggested to be associated with metabolic disease states, such as cancer [Citation32], cardiovascular disease [Citation33], Alzheimer’s disease [Citation34], and diabetes [Citation35]. Therefore, maintaining the dynamic equilibrium of zinc ions is essential for appropriate physiological processes.
MTF-1, extensively expressed in humans and rodents, is a pluripotent transcriptional regulator located in both cytoplasm and nucleus and has been highly conserved throughout evolution [Citation36]. This constitutively expressed transcription factor is activated upon diverse stress stimuli, notably metal load but also oxidative stress and hypoxia [Citation37]. MTF-1 is mostly cytoplasmic in resting cells, but translocates to the nucleus upon stimulation. Interestingly, MTF-1 null-mutant mice die in utero due to degeneration of the embryonic hepatocytes, suggesting that MTF-1 also serves a developmental role [Citation38]. Numerous researches have indicated that MTF-1 is overexpressed in lungs, colorecta, breast, and cervical cancers, implying a vital effect on malignancy [Citation39]. MTF-1 is also a biomarker for predicting the recurrence of advanced-stage head and neck Carcinoma [Citation40]. Furthermore, MTF-1 was identified as an essential transcription factor in osteoarthritis pathogenesis [Citation41], and previous studies showed that it acted as a positive regulator of PlGF expression in human embryonic kidney HEK293T cells and human choriocarcinoma BeWo cells under hypoxia conditions [Citation42], which is partially consistent with our findings. In contrast, a study conducted by Yajima [Citation43] revealed that MTF-1 adversely regulated Plgf transcription in HaCaT cells. Nevertheless, the involvement of MTF-1 in PAH remains uncertain. To our knowledge, this is the first time we have reported an association between MTF-1 and PAH.
In the present study, MTF-1 responded rapidly to the presence of heavy metal by moving into the nucleus where it directed zinc fingers to MREs to stimulate transcription of PlGF. Several processes, including nuclear translocation, DNA binding, and MTF-1 transcriptional activation, have been revealed to be necessary for metal- and stress-inducible transcription [Citation44]. It is worth noting that the majority of MTF-1 resides in the cytoplasm of resting cells, but moves toward the nucleus after exposure to zinc [Citation45]. A putative MRE motif (5’-TGCACACTTCCAG-3’) containing the conserved core sequence TGCRCNC and a GC-rich sequence [Citation46] was identified in the Plgf promoter region at positions 354 and 341 bp. The dual luciferase reporter assay revealed a direct connection between MTF-1 and PlGF, suggesting that the endogenous MTF-1 protein is recruited to the putative Plgf binding sites. Exposure of the nuclear localization signal (NLS) in the first zinc finger guided MTF-1 toward the nucleus when free zinc ions in the cytoplasm bind to the zinc fingers, as revealed by Yajima I et al. [Citation43]. In addition, the data by Chen [Citation47] and Zhang et al. [Citation5] delineated that zinc-induced MTF-1 expression by functionally binding on HSE site of Mtf-1 promoter and that MTF-1 expression levels corresponded with intracellular zinc concentration. On the other hand, it was reported that MTF-1 was not responsive to zinc in melanoma [Citation48]. However, potential transcriptional regulatory mechanisms of MTF-1 remained unclear. To date, the role of phosphorylation on the regulation of MTF-1 activation is among the best understood [Citation49], including PKC, PI3K/AKT, c-Jun N-terminal kinase (JNK), casein kinase-2 (CK2), and protein tyrosine kinase (PTK) [Citation50]. Furthermore, an analysis of MTF-1 motifs identified multiple evolutionarily conserved phosphorylation sites, including 11 PKC sites, 13 CK1 sites, and 1 PTK site. Nevertheless, the MTF-1 phosphorylation sites in PAH have not been thoroughly elucidated. In contrast, it has been proposed that specific dephosphorylation of MTF-1 is responsible for transcription activation [Citation51].
Furthermore, MTF-1 recruits different co-regulators, such as specificity protein 1 (SP1), p300/CREB binding protein (p300/CBP) [Citation52] and HIF-1α to coordinated target gene expression after binding DNA. Dubé et al. [Citation53] found that MTF-1 contributed to HIF-1α activity and conversely, HIF-1α enhanced MTF-1 activity. Under hypoxic condition, the involvement of an NF-κB/MTF-1 complex in the transcription of Plgf [Citation54]. It has been proposed that the downstream targets play important roles in embryogenesis, metal homeostasis, tumor progression, oxidative stress, and hypoxia signaling. Importantly, inhibition of MTF-1 increases the synthesis of tumor suppressor factor Kruppel-like factor 4 (KLF4) [Citation55], resulting in the down-regulation of cyclin D1, blocking cell cycle progression and proliferation. Our results suggested that the possible function of MTF-1 in PAH warranted further investigation.
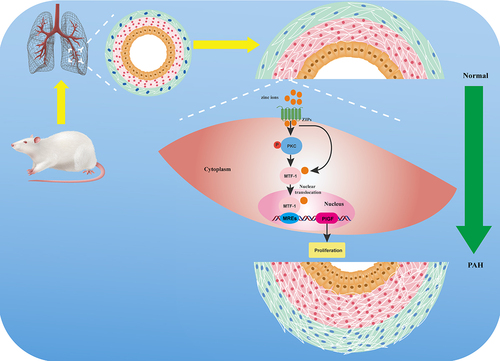
Collectively, it was established that the zinc/PKC/MTF-1 axis in PASMCs might form a cascade that promoted up-regulation of the critical effector, PlGF, thereby leading to proliferation (). In the future, a detailed investigation of the specific modified residues in MTF-1 following exposure to metals or other stressors and the characterization of post-translational modifications of MTF-1 would be necessary for a comprehensive understanding of stress-mediated gene regulation. Our findings support the notion that local zinc ion depletion, suppression of zinc ion influx, and inhibition of MTF-1 expression and activity in lung tissue would be effective therapeutic approaches for the treatment of PAH.
Figure 10. A schematic diagram of molecular mechanism underlying MTF-1 mediated proliferation of PASMCs. Extracelluar elevated zinc ions enter the cytoplasm through ZIPs, resulting in the binding of zinc ions to zinc fingers in MTF-1 and phosphorylation of PKC. Binding of zinc ions leads to NSL exposure, which leads to nuclear translocation of MTF-1 and MTF-1 transcriptionally promotes PlGF expression through the activation of PKC, thus, promotes PASMCs proliferation. MTF-1: metal-regulatory transcription factor 1; PASMCs: pulmonary arterial smooth muscle cells; ZIPs: Zrt-like, IRT-like proteins; PKC: protein kinase C; MREs: metal-response elements; PlGF: placental growth factor.
Acknowledgements
The authors thank the participants and participating physicians from The First Affiliated Hospital of Fujian Medical University, China.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Additional information
Funding
References
- Reyes PA, Gu M, Grubert F, et al. Remodeling of active endothelial enhancers is associated with aberrant gene-regulatory networks in pulmonary arterial hypertension. Nat Commun. 2020;11(1):1673.
- Wang XJ, Xu XQ, Sun K, et al. Association of rare PTGIS variants with susceptibility and pulmonary vascular response in patients with idiopathic pulmonary arterial hypertension. JAMA Cardiol. 2020;5(6):677–684.
- Spiekerkoetter E, Kawut SM, de Jesus Perez VA. New and emerging therapies for pulmonary arterial hypertension. Annu Rev Med. 2019;70:45–59.
- Baltaci AK, Yuce K, Mogulkoc R. Zinc metabolism and metallothioneins. Biol Trace Elem Res. 2018;183:22–31.
- Zhang R, Zhao G, Shi H, et al. Zinc regulates primary ovarian tumor growth and metastasis through the epithelial to mesenchymal transition. Free Radic Biol Med. 2020;160:775–783.
- Ollig J, Kloubert V, Taylor KM, et al. B cell activation and proliferation increase intracellular zinc levels. J Nutr Biochem. 2019;64:72–79.
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342–1365.
- Krizkova S, Ryvolova M, Hrabeta J, et al. Metallothioneins and zinc in cancer diagnosis and therapy. Drug Metab Rev. 2012;44(4):287–301.
- Lovell MA. A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer’s disease. J Alzheimers Dis. 2009;16(3):471–483.
- Kambe T, Hashimoto A, Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci. 2014;71(17):3281–3295.
- Yao J, Hu P, Zhang DF. Associations between copper and zinc and risk of hypertension in US adults. Biol Trace Elem Res. 2018;186:346–353.
- Bellomo E, Massarotti A, Hogstrand C, et al. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics. 2014;6(7):1229–1239.
- Zhao T, Huang Q, Su Y, et al. Zinc and its regulators in pancreas. Inflammopharmacology. 2019;27(3):453–464.
- Nimmanon T, Ziliotto S, Morris S, et al. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics. 2017;9(5):471–481.
- Slepchenko KG, Holub JM, Li YV. Intracellular zinc increase affects phosphorylation state and subcellular localization of protein kinase C delta(δ). Cell Signal. 2018;44:148–157.
- Hogstrand C, Zheng D, Feeney G, et al. Zinc-controlled gene expression by metal-regulatory transcription factor 1 (MTF1) in a model vertebrate, the zebrafish. Biochem Soc Trans. 2008;36(Pt 6):1252–1257.
- Viola G, Lindert U, Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta. 2012;1823(9):1416–1425.
- O’Shields B, McArthur AG, Holowiecki A, et al. Inhibition of endogenous MTF-1 signaling in zebrafish embryos identifies novel roles for MTF-1 in development. Biochim Biophys Acta. 2014;1843(9):1818–1833.
- Van BT, Etienne I, Cunningham F, et al. The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog Retin Eye Res. 2019;69:116–136.
- Albonici L, Giganti MG, Modesti A, et al. Multifaceted role of the placental growth factor (PlGF) in the antitumor immune response and cancer progression. Int J Mol Sci. 2019;20(12):2970.
- Wang J, Lian G, Luo L, et al. Role of 20-hydroxyeicosatetraenoic acid in pulmonary hypertension and proliferation of pulmonary arterial smooth muscle cells. Pulm Pharmacol Ther. 2020;64:101948.
- Zhuang W, Lian G, Huang B, et al. Pulmonary arterial hypertension induced by a novel method: twice-intraperitoneal injection of monocrotaline. Exp Biol Med (Maywood). 2018;243(12):995–1003.
- Xie L, Lin P, Xie H, et al. Effects of atorvastatin and losartan on monocrotaline-induced pulmonary artery remodeling in rats. Clin Exp Hypertens. 2010;32(8):547–554.
- Xiao G, Lian G, Wang T, et al. Zinc-mediated activation of CREB pathway in proliferation of pulmonary artery smooth muscle cells in pulmonary hypertension. Cell Commun Signal. 2021;19(1):103.
- Huang J, Xie LD, Luo L, et al. Silencing heat shock protein 27 (HSP27) inhibits the proliferation and migration of vascular smooth muscle cells in vitro. Mol Cell Biochem. 2014;390(1–2):115–121.
- Li M, Zhang X, Li C, et al. Galanin receptor 2 is involved in galanin-induced analgesic effect by activating PKC and CaMKII in the nucleus accumbens of inflammatory pain rats. Front Neurosci. 2021;14:593331.
- Ye C, Lian G, Wang T, et al. The zinc transporter ZIP12 regulates monocrotaline-induced proliferation and migration of pulmonary arterial smooth muscle cells via the AKT/ERK signaling pathways. BMC Pulm Med. 2022;22(1):111.
- Kocyła A, Adamczyk J, Krężel A. Interdependence of free zinc changes and protein complex assembly - insights into zinc signal regulation. Metallomics. 2018;10:120–131.
- Passerini A, Andreini C, Menchetti S, et al. Predicting zinc binding at the proteome level. BMC Bioinf. 2007;8:39.
- Krezel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem. 2006;11:1049–1062.
- Tuncay E, Bitirim VC, Durak A, et al. Hyperglycemia-induced changes in ZIP7 and ZnT7 expression cause Zn2+ release from the sarco(endo)plasmic reticulum and mediate ER stress in the heart. Diabetes. 2017;66(5):1346–1358.
- To PK, Do MH, Cho JH, et al. Growth modulatory role of Zinc in prostate cancer and application to cancer therapeutics. Int J Mol Sci. 2020;21(8):2991.
- Begum F, Me HM, Christov M. The role of Zinc in cardiovascular disease. Cardiol Rev. 2022;30:100–108.
- Rivers AJ, Tapia VS, White CS, et al. Zinc status alters alzheimer’s disease progression through NLRP3-dependent inflammation. J Neurosci. 2021;41(13):3025–3038.
- Meika F, Samir S. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal. 2010;13(10):1549–1573.
- Höckner M, Dallinger R, Stürzenbaum SR. Metallothionein gene activation in the earthworm(Lumbricus rubellus). Biochem Biophys Res Commun. 2015;460:537–542.
- Bonaventura P, Benedetti G, Albarède F, et al. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14(4):277–285.
- Günes C, Heuchel R, Georgiev O, et al. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. Embo J. 1998;17(10):2846–2854.
- Shi Y, Amin K, Sato BG, et al. The metal-responsive transcription factor-1 protein is elevated in human tumors. Cancer. 2010;9(6):469–476.
- Pavón MA, Parreño M, Téllez-Gabriel M, et al. CKMT1 and NCOA1 expression as a predictor of clinical outcome in patients with advanced-stage head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1392–1403.
- Kim JH, Jeon J, Shin M, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156(4):730–743.
- Nishimoto F, Sakata M, Minekawa R, et al. Metal transcription factor-1 is involved in hypoxia-dependent regulation of placenta growth factor in trophoblast-derived cells. Endocrinology. 2009;150(4):1801–1808.
- Yajima I, Kumasaka MY, Ohnuma S, et al. Arsenite-mediated promotion of anchorage-independent growth of HaCaT cells through placental growth factor. J Invest Dermatol. 2015;135:1147–1156.
- Saydam N, Adams TK, Steiner F, et al. Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: identification of signal transduction cascades that control metal-inducible transcription. J Biol Chem. 2002;277(23):20438–20445.
- Saydam N, Georgiev O, Nakano MY, et al. Nucleo-cytoplasmic trafficking of metal-regulatory transcription factor 1 is regulated by diverse stress signals. J Biol Chem. 2001;276(27):25487–25495.
- Giedroc DP, Chen X, Apuy JL. Metal response element (MRE)-binding transcription factor-1 (MTF-1): structure, function, and regulation. Antioxid Redox Signal. 2001;3:577–596.
- Chen GH, Lv W, Xu YH, et al. Functional analysis of MTF-1 and MT promoters and their transcriptional response to zinc (Zn) and copper (Cu) in yellow catfish Pelteobagrus fulvidraco. Chemosphere. 2020;246:125792.
- Burian Z, Ladanyi A, Barbai T, et al. Selective inhibition of HIF1alpha expression by ZnSO4 has antitumoral effects in human Melanoma. Pathol Oncol Res. 2019;26(2):673–679.
- Andrews GK. Cellular zinc sensors: mTF-1 regulation of gene expression. Biometals. 2001;14:223–237.
- Chen L, Ma L, Bai Q, et al. Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. J Biol Chem. 2014;289(32):22413–22426.
- Lin MC, Liu YC, Tam MF, et al. PTEN interacts with metal-responsive transcription factor 1 and stimulates its transcriptional activity. Biochem J. 2012;441(1):367–377.
- Ogra Y, Suzuki K, Gong P, et al. Negative regulatory role of Sp1 in metal responsive element-mediated transcriptional activation. J Biol Chem. 2001;276(19):16534–16539.
- Dubé A, Harrisson JF, Saint-Gelais G, et al. Hypoxia acts through multiple signaling pathways to induce metallothionein transactivation by the metal-responsive transcription factor-1 (MTF-1). Biochem Cell Biol. 2011;89(6):562–577.
- Cramer M, Nagy I, Murphy BJ, et al. NF-kappaB contributes to transcription of placenta growth factor and interacts with metal responsive transcription factor-1 in hypoxic human cells. Biol Chem. 2005;386(9):865–872.
- Kindermann B, Döring F, Budczies J, et al. Zinc-sensitive genes as potential new target genes of the metal transcription factor-1 (MTF-1). Biochem Cell Biol. 2005;83(2):221–229.