ABSTRACT
Objective: Human body models have the potential to better describe the human anatomy and variability than dummies. However, data sets available to verify the human response to impact are typically limited in numbers, and they are not size or gender specific. The objective of this study was to investigate the use of model morphing methodologies within that context.
Methods: In this study, a simple human model scaling methodology was developed to morph two detailed human models (Global Human Body Model Consortium models 50th male, M50, and 5th female, F05) to the dimensions of post mortem human surrogates (PMHS) used in published literature. The methodology was then successfully applied to 52 PMHS tested in 14 impact conditions loading the abdomen. The corresponding 104 simulations were compared to the responses of the PMHS and to the responses of the baseline models without scaling (28 simulations). The responses were analysed using the CORA method and peak values.
Results: The results suggest that model scaling leads to an improvement of the predicted force and deflection but has more marginal effects on the predicted abdominal compressions. M50 and F05 models scaled to the same PMHS were also found to have similar external responses, but large differences were found between the two sets of models for the strain energy densities in the liver and the spleen for mid-abdomen impact simulations. These differences, which were attributed to the anatomical differences in the abdomen of the baseline models, highlight the importance of the selection of the impact condition for simulation studies, especially if the organ location is not known in the test.
Conclusions: While the methodology could be further improved, it shows the feasibility of using model scaling methodologies to compare human models of different sizes and to evaluate scaling approaches within the context of human model validation.
Introduction
Human body models (HBM) based on the finite element (FE) methods have the potential to better describe the human anatomy and its variability than physical car crash dummies. Recent commercial model families such as the THUMS (from Toyota Motor Corporation and Toyota Central R&D) and the GHBMC (from the Global Human Body Model Consortium) are available for a few sizes that are the same as the dummies (e.g., 50th percentile male, M50; 5th female, F05; 95th male or M95). However, a better description of the variability seems now within reach using nonlinear interpolation methods, which can be used to transform a baseline model into multiple sizes (as reviewed in Jolivet et al. Citation2015). Recent examples of such model scaling approaches include Vavalle et al. (Citation2014) for the GHBMC and Hwang et al. (Citation2016) for the THUMS.
The diversity of models raises the question of their validation. Data sets available to verify the human response to impact are typically limited in numbers, and are not size or gender specific. Response (curve) scaling techniques based on simple mechanical models are classically used to normalize experimental responses for use with dummies (e.g.,
Eppinger et al. Citation1984; Mertz Citation1984). These methods have sometimes been used to normalize a model response to a 50th percentile male corridor (e.g., analysis of a M95 model performance in Vavalle et al. Citation2014). However, with the availability of HBM scaling approaches, the HBM could be directly scaled to the reference for which the experimental data are available, removing the need for the strong assumptions associated with simplified response scaling techniques. This approach could also be useful to evaluate the performance of HBM scaling approach.
Recently, Davis et al. (Citation2016) scaled the GHBMC F05 model to the dimensions of the GHBMC M50 to compare its response to 50th percentile male corridors. However, these reference corridors were built using simplified response scaling techniques. Using a methodology that could be used to generate large numbers of HBMs by scaling, Hwang et al. (Citation2016) scaled the THUMS to the dimensions of two post mortem human surrogates (PMHS) for which experimental data were available. They found that the scaled HBM responses were closer to the PMHS than the baseline HBM. However, only two PMHS and corresponding models were used in the study, making it difficult to conclude with certainty and to quantify the improvement.
Within that context, the objectives of this study were to (1) develop a methodology to globally scale/morph HBMs to the dimensions of numerous PMHS used in previous study, (2) investigate the effect of scaling on the abdomen external response, and (3) investigate the effect on organ-level metric. This study was conducted as part of the development and validation process of the GHBMC F05 model.
Methods
Models and simulation conditions
The models used for the current study are the GHBMC models M50 v4.3 (commercial version, Global Human Body Model Consortium, USA) and F05 v2.2. The F05 model is a development version. Overviews of the models can be found in their respective manuals and in publications (e.g., Vavalle et al. Citation2013; Davis et al. Citation2016). Both models are based on imaging data sets collected on human volunteers with dimensions close to the targeted percentiles (e.g., statures of 1750 mm and 1499 mm for the M50 and the F05, respectively). Corresponding model masses are 77 kg for the M50 and 51 kg for the F05. The models have around 1.3 million nodes for more than 2 million elements. They also share similar modeling principles: the same organs and structures are represented using similar numerical modeling assumptions (e.g., element type and formulation) and, for the abdomen at least, the same material properties. The abdominal solid organs are represented by tetrahedral meshes, while the hollow organs are represented by linear airbag fluids. Anatomical relationships (tied, sliding, etc.) are also represented using contacts or continuous meshes.
For the current study, 14 loading conditions from 6 studies were used (). These conditions were selected to try to cover the main loading modes encountered in an automotive environment. They include free and fixed back tests, with belt or bar loading the mid or upper abdomen, in frontal, oblique, or side directions. The model was not repositioned for any of the simulations, but the impact direction was tilted such that the impactor is perpendicular to the overall orientation of the trunk as in the tests (e.g., 22 degrees for the M50 in a mid-abdomen impact). The model was left free to translate in the free back conditions, while it was fixed using SPC (Single Point Constraint) in the fixed back ones. No gravity was used. Impactors and seats were rigid while belts were deformable (50 mm by 2 mm, elastic properties: 1.11 GPa, ν = 0.3). Initial velocities were applied to the impactor except for belt configurations where the belt force time history was applied. Average stimulus values (e.g., average impactor initial velocity, average force time history) were used for each condition with the baseline models.
Table 1. Summary of the setups, conditions, and number of PMHS used for the simulations in the current study.
Model morphing methodology
Each model was then morphed to approximate the dimensions and masses of PMHS used in the reference studies from . As complete anthropometries are typically not available in the published studies, height, mass, and a relevant depth (e.g., depth at location of impact) were used to estimate other anthropometric dimensions using the ANSUR anthropometric database (n = 3982, Gordon et al. Citation1989). These three inputs were selected as these are consistently available in all reference studies, allowing use of the same methodology for all subjects. ANSUR subjects were selected first by gender and then by closest weight and depth (after isotropic scaling to the PMHS stature). When several subjects were within 2 mm for depth and 1 kg for weight of the PMHS (after stature scaling), their characteristics were averaged. Then a network of control points was defined on the F05 and M50 models (). Fifteen anthropometric dimensions from ANSUR (Anthropometric Survey of U.S. Army Personnel) were used (listed in ). These are external circumferences, depths, or breadths measured in sections of the body. They were represented on the models by control points defined on the skin. Circumferences (e.g., arm, thigh or neck) were estimated using ellipses based on four control points. In the trunk sections (e.g., chest, abdomen), lateral, anterior, and posterior points corresponding to depth and breadth were complemented by intermediate points for which the positions on the scaled models were calculated by natural cubic spline interpolation. In order to describe the overall stature change, internal points were added along the spine and limbs, and external points were added at the extremities (head, feet, and hands). Combined, this led to a network of 126 control points. To adjust to the targeted PMHS, the control points were first scaled linearly with stature, and then adjusted using the 15 anthropometric dimensions. The arms were also moved away from the body when needed to accommodate a wider torso.
Figure 1. Scaling workflow with illustration of the network of control points and ANSUR anthropometric dimensions used to drive transformation (top) and examples of resulting scaled female or male models (at the bottom from left to right: Viano Run12, Lamielle MHA155, Kremer O09, Hardy GI8, Kremer O08, Foster A2). The network of control points includes both points selected to describe the stature (e.g., head, limbs, spine, in blue) and sections corresponding to anthropometric dimensions.
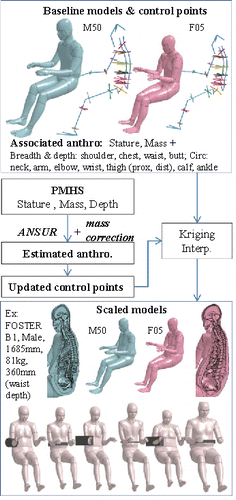
The models were then transformed by Kriging interpolation based on these control points (as in Jolivet et al. (Citation2015) for the child model). Only the node positions were updated by Kriging, and parameters such as densities, thickness, and sections were not modified. In order to limit the errors on the scaled model mass, a corrective factor was applied to the anthropometric dimensions (except the relevant depth) prior to scaling. The corrective factor was determined by regression based on the preliminary scaling of a first set of 15 subjects (Appendix A-1; see online supplement). Illustrations of the methodology along with a few transformed models are provided in .
Scaled models simulations
Both M50 and F05 were transformed to correspond to 52 PMHS from the reference studies in , and corresponding simulations were run with the same boundary conditions as the baseline but using the exact value of the stimulus (e.g., exact value of initial velocity corresponding to each PMHS tested with a bar). Some subjects tested in the reference studies were not used because no plausible anthropometry could be found based on the known variables (n = 1 for Cavanaugh et al. Citation1986, subject A24, 1870 mm, i.e., 25% taller than the F05, 257 mm abdominal depth or 41% thicker, 45 kg or 12% lighter).
Data analysis
The simulations (28 baselines, 104 scaled models) were run in Ls-Dyna Version 7.1 MPP (LSTC, Livermore, CA). Output metrics included the force, the deflection, and, when possible, the abdominal compression (deflection divided by abdominal thickness) and the abdominal soft tissue compression (deflection divided by thickness between skin and spine at the level of impact). For the tests, as the soft tissue thickness is not available, it was taken as the average of the thickness measured on the corresponding scaled models. Outputs were compared using peak values and CORA scores (CORA software version 3.61, Partnership for Dummies and Biomechanics, Germany). For test results, curves were used without scaling. For the CORA scores, corridor and cross-correlation methods were used for the baseline simulations (comparison between model and all curves). CORA provides scores ranging between 0 (no correlation) and 1 (perfect match) for each of the computed metrics. Paired comparisons were also made between tests and simulations (peak and cross correlation in CORA). For paired comparisons with scaled models, simulation results were directly compared with the test on the corresponding PMHS. For paired comparisons with the baselines, as only one simulation was run in each condition, the same baseline response was compared to each PMHS tested in the corresponding condition. CORA time intervals were always reduced to the duration of the simulation and set individually in the CORA settings. The whole curves were used for analysis (overlap of 100% between experiment and simulation, or INT_MIN = 1.00), which means that no phase shift was allowed (phase correlation metric was not computed). When the force was applied to the model (i.e., belt loading cases), the force was not used in the assessment. Finally, to prepare for injury analyses, the strain energy densities (SED) in the liver and spleen were analysed.
Results
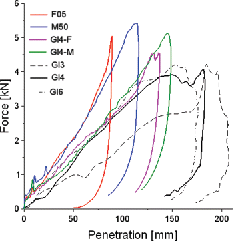
All 104 scaled models could run without the need for manual corrections. Using the PMHS as the reference, the mean absolute mass error for the scaled models was 0.9 kg. A summary of all values used to define the scaled models, and the corresponding model masses is provided in Appendix A-2 (see online supplement). Out of 132 simulations, 19 terminated with errors (7 baselines, 12 scaled) but after sufficient duration to analyze the results. The errors generally occurred in strenuous loading cases (e.g., 6 baselines errors on the smaller F05 model) and they were negative volumes or Nan (not a number) in the abdomen or in the neck. An example of simulation results for one of the conditions is shown in . While scaling seemed to improve the response for both male and female models in many simulations (example in ), there were also cases for which scaling degraded it (example of the female model in Appendix A-3; see online supplement).
Figure 2. Example of simulation: Hardy et al. (Citation2001) mid-abdomen bar impact at 6 m/s scaled to the subject GI4. Another example is provided in Appendix A-3 (see online supplement).
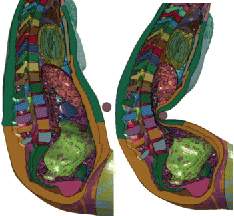
Figure 3. Example of response: Hardy et al. (Citation2001) mid-abdomen bar impact at 6 m/s scaled to the subject GI4 baselines and models scaled to PMHS GI4 (corresponding to ). CORA scores are provided in Appendix A-4 (e.g., the M50 deflection CORA size changed with scaling from 0.42 to 0.67; see online supplement). Compression curves and another example are provided in Appendix A-3.
When considering all conditions, baseline model's average global CORA rating (combination of corridor method using force and displacement) was similar for the M50 (0.58) and F05 (0.60). Overall, the models (baseline or scaled) captured well the overall shape of the response curves, and the responses of the male and female models scaled to the same dimensions were very similar. The CORA shape (progression) mean scores were above 0.97 for both baseline and scaled models (cross-correlation method). When comparing to the baseline, the effect of model scaling was mostly prominent on the deflection, followed by the force. For the scaled models, the size CORA scores averaged 0.75, 0.61, 0.60, and 0.60 for the force, deflection, and two compressions, respectively. Corresponding baselines scored 0.69, 0.51, 0.61, and 0.60. Differences were significant for force and displacement (p < 10−7) but not for compressions (p > 0.25). The F05 scored slightly higher than the M50 for both baseline (e.g., force size: 0.72 vs 0.67) and morphed models (e.g., force size: 0.78 vs. 0.72). In setups where force was applied, displacement-related scores were typically lower than for the impactor-type conditions (e.g., 0.5 for compression in belt test vs. 0.66 for impactor tests). A complete table with all shape and size CORA scores against all PMHS results is provided in Appendix A-4 (see online supplement).
Responses were also analyzed in terms of peak values by using linear regressions (curves are provided in Appendix A-5; see online supplement). Results were found to be consistent with the size CORA metric. Model scaling helped increase the percentage of force variance that could be predicted by both F05 and M50 models (e.g., from R² = 76% to 84% for the F05). The largest R-squared increase attributed to scaling was for the F05 deflection (from 39% to 61%). The increase was not as large for the M50 (from 53% to 66%), perhaps due to less difference between the M50 and PMHS (Table A-1; see online supplement). The effect of scaling was more limited for the compression metrics, and scaling actually seemed to reduce the R-squared (e.g., M50 from 70% to 65%). Scaling had a limited effect on the time of peak force for both F05 and M50 (e.g., R² of 0.70 and 0.72 for the F05), but it had more effect on the time of peak deflection for the F05 (R² going from 0.54 to 0.64 with scaling). For the M50 time of peak deflection, the R² decreased with scaling (from 0.56 to 0.48). Except for the deflection and time to peak deflection in the F05, the regression curves computed with baseline or scaled models (which could be similar to transfer functions for dummies) were almost the same.
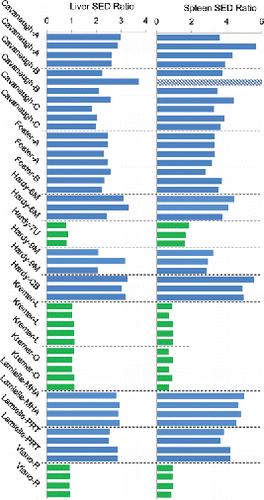
Finally the ratios of SED in the liver and the spleen between pairs of scaled female and male models corresponding to the same PMHS were computed (). While the material properties, external dimensions, masses, and impact conditions are the same for paired models, the ratios were high in many setups; that is, the SED in the F05 scaled was much larger than in the M50 scaled. Variations within setups were more limited. When looking more closely at the results, all conditions for which the loading was applied directly to the mid abdomen (whether by a bar or a belt) were associated with large SED ratios. Other conditions involving the upper abdomen or for which the loading was more distributed had ratios closer to 1.
Figure 4. Ratio of strain energy density (SED) in the liver and spleen of the M50 and F05 scaled to the same PMHS (paired simulations, F05/M50). The colors aim to highlight the contrast between the test setups. The value for the bar that is cross-hatched is 13, but the axis was limited to 6 to improve legibility.
Discussion and conclusions
In this study, 66 pairs of simulations (52 scaled, 14 baselines) were run to study the abdominal response of two human body models designed to represent different percentiles of the population (50th male and 5th female). In the absence of suitable validation data sets specific to these percentiles, a methodology was developed to scale the models to estimated PMHS dimensions. It was successfully applied to 52 PMHS from 6 studies (times two models), enabling simulations without manual model corrections.
The method requires only a few metrics that are almost always available in reference studies (stature, mass, depth of the impacted body region) and could be expanded to other regions. Based on these metrics, a plausible (in the sense of the ANSUR anthropometric database) set of anthropometric dimensions is predicted and then used to drive the model transformation using Kriging interpolation. This approach is very simplified and many improvements should be considered in the future. First, the scaling uses the ANSUR database, in which the subjects are both younger and likely more fit than the population used in the experiment (muscle mass and distribution). Also, the lack of muscle tone in PMHS may have affected the abdominal geometry once in position (e.g., Howes et al. Citation2013). The mass correction scheme (respecting the impact depth measured in the test position on the PMHS and modulating the circumferences outside this region of interest) may have helped, but using data sources more in line with the experimental population (elderly and PMHS) would better address this limitation. Also, instead of selecting the closest subjects in the ANSUR database, methods such as the one proposed by Parkinson and Reed (Citation2010) could be used to predict the most likely set of dimensions or to sample a likely population respecting arbitrary constraints. This would allow integrating additional anthropometric constraints when they are available in the reference studies, studying the sensitivity of the response to geometrical parameters that were not measured on the PMHS, and assessing (statistically) the improvement brought by additional parameters. Preliminary work is ongoing to implement this approach and release it in an Open Source tool (PIPER tool, www.piper-project.eu). Another important limitation is that the approach does not currently account for a priori knowledge related to the internal geometry or to possible internal–external relationships. The only constraint used in the current study was the assumption of linear scaling near the spine. The fact that skeletal parameters were not considered in the scaling may have contributed to some of the mismatches (and relatively low CORA size scores) in some loading configurations. This could be particularly true for the ribcage, as it is involved in upper abdomen loading. Also, the amount of fat (subcutaneous or visceral) was not considered in the scaling, which may be especially an issue for the thoraco-abdominal response. This may have been mitigated by the fact that the PMHS populations only included 3 obese specimens and had an average body mass index (BMI) of 23.7 ± 3.7, while the baseline models had BMIs of 25.1 and 22.7 for the M50 and F05, respectively. The realism of the scaling could be improved for example by accounting for volume or positional constraints on the soft organs (e.g., Beillas et al. Citation2009 and Parenteau et al. Citation2013 for liver volume and position), skeletal characteristics (through landmark or statistical shape models as in Hwang et al. Citation2016), and by using internal–external relationships (e.g., Bertrand et al. Citation2009 for skin to bone measurements, Holcombe and Wang Citation2014 for subcutaneous fat thickness). Material parameters and other local properties (e.g., shell thicknesses) could also be scaled if they can be correlated to parameters that are provided in experimental studies. However, it should be remembered that with current models and continuous interpolation methods (as in the current study, and Hwang et al. Citation2016; Jolivet et al. Citation2015; Vavalle et al. Citation2014), it may not be possible to respect all geometrical constraints while maintaining an acceptable element quality. For example, Vavalle et al. (Citation2014) scaled the GHBMC M50 to a 95th percentile male target built using magnetic resonance imaging (MRI) data but the target spleen volume could not be matched in order to maintain element quality. Several baseline models (or parameterized models describing different anatomical topologies) may be required to describe some of the human variability, which cannot be obtained by continuous interpolation techniques. This may be illustrated by the fact that some of the results derived from the two baseline models used in the current study are very different (this point is discussed in detail later). For now, some of these limitations may have been mitigated by the facts that the region of interest (the abdomen) is mostly composed of soft tissues for which the mechanical properties are not known to be gender or age dependent (e.g., for the solid organs) and that the skeleton may not be the main determinant of the response.
The simulation results that can be obtained with the proposed methodology allow several types of observations. First, models' performances can be compared for the same set of sizes without the use of simplified response scaling approaches. In the current study, the F05 scaled models were found to respond similarly to the M50 scaled models (high correlations on peak data in particular). When compared to test results, the F05 was found to score slightly better than the M50 (CORA scores and peaks). While there are no obvious reasons for this observation when considering the similarities between the models, this type of result may be useful to evaluate the suitability of a given model combined with a scaling approach to represent a part of the population. Second, the quantification of the experimental variance that can be explained by a combination of a model and scaling assumptions allows comparing of the performance of scaling methodologies. For example, in the current study, model scaling increased the percentage of peak force variance explained by the model. However, simple geometrical normalization techniques (calculation of compression and soft tissue compression) seemed to perform as well as or sometimes better than model scaling for displacement-related outputs. The performance of other scaling approaches should be tested in the future. In particular, different curve scaling approaches could be applied consistently to all experimental results (e.g., scaling the PMHS response to the baseline model sizes) and then compared between each other and also with the scaled models. This could help in selecting scaling approaches to be used for future model validation efforts. Also, it can also be noticed that even without scaling, the model captures a significant part of the force peak force variance. As all test conditions are combined in the analyses, this may suggest that the impact condition/stimulus may be its main determinant. A more detailed analysis could be attempted by normalizing the variance for each test condition, but the limited number of specimens for each condition (sometimes only two or three) is likely to make the analysis difficult. Also, only the average stimulus was used with the baseline models in each of the condition. While it is a common approach in model validation, a full analysis repeating the simulations with the stimulus of each PMHS test would be useful to understand what part of the variance can be explained by small variations of input. Another limitation in the current study is the use of simple linear regression models to investigate the part of the experimental variance that a model can explain. While this could be useful to build transfer functions, the use of linear regressions may be inappropriate for compression metrics, as these have physical limitations making them nonlinear by definition (the impactor displacement is limited by the spine, resulting in visible plateaux on the compression plots of ). Other prediction models should be investigated.
Regarding the CORA scores, only relative comparisons could be made as only some of the metrics were calculated (e.g., assuming no phase shift) and it is believed that this approach differs too much from the ISO/TS 18571 total rating to be able to use its corresponding goodness scale. In the current study, a CORA phase shift was not allowed as it could have been limited by the time intervals available for the signals (e.g., some of the loads do not come back to zero in the experimental sources, and some of the simulations do not terminate successfully). It was believed that this approach could help obtaining consistent results from CORA and that it could be a reasonable limitation since the loading conditions corresponded to single loading events with a beginning of loading already aligned between test and simulation. However, the phase could be still relevant as part of the assessment of the impact, as it may be related for example to abdominal depth or subcutaneous fat thickness. Computation of the phase and ISO rating should be considered in the future after examining each test condition in detail to ensure consistent results.
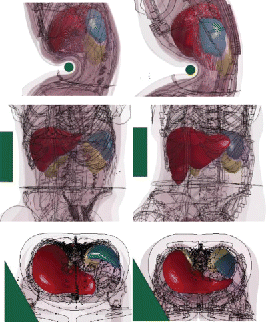
Another finding of the study was that despite the similarities of the human models, their responses at the organ level were found to differ widely, with much larger SED predicted by the F05 model when loading was applied to the mid abdomen. This may be explained by the anatomical differences between the models: The F05 liver is more caudal and less covered by the ribcage than the M50 liver. As a consequence, the M50 liver does not seem to be engaged in mid-abdomen impacts while the F05 is (). These results, which are in line with those of Le Ruyet et al. (Citation2016) on the sensitivity of the SED to the relative location between the organ and the impactor, highlight the importance of the internal geometry when organ level response is concerned. Similar observations could be made for the spleen (). This high sensitivity of the organ response to the impact conditions, combined with the fact that the organ position and possible engagement are not known in most tests of the literature, suggests that setups may not be suitable to assess the injury prediction capability of human models or to attempt developing model-based injury risk curves. For the time being, setups with the lowest model-to-model sensitivity (mainly upper abdomen or distributed loading) should be prioritized for that purpose. This also suggests that, considering the assumptions of current morphing techniques, the use of several baseline models (or parametrized models) may be important to better understand the sensitivity of the response to anatomical variations.
Figure 5. Differences of liver (red) and spleen (blue) shapes and positions for the M50 (left) and the F05 (right). In mid-abdomen impacts from Hardy et al. (Citation2001), the bar is below the M50 liver but engages the F05 liver (top, models scaled to the PMHS GI4). In the Kremer et al. (Citation2011) oblique impact, the impactor engages both livers (middle: models scaled to FBL08, bottom: baselines). The spleen is more posterior and more distant to the mid abdomen in the M50 than in the F05, which is likely to affect its engagement in mid-abdomen and side impact (top and bottom).
In summary, this study demonstrates the feasibility of global morphing to study the effects of scaling within the context of HBM validation and to compare different models against the same relatively large set of PMHS. In the future, models and scaling approaches may have to be evaluated jointly for their ability to represent the human variability (joint model and scaling method validation), and the current study may be a step in that direction.
APPENDIX.docx
Download MS Word (1,018.8 KB)Funding
Funding for this study was provided by the Global Human Body Models Consortium, LLC (GHBMC).
References
- Beillas P, Lafon Y, Smith FW. The effects of posture and subject-to-subject variations on the position, shape and volume of abdominal and thoracic organs. Stapp Car Crash J. 2009;53:127.
- Bertrand S, Kojadinovic I, Skalli W, Mitton D. Estimation of external and internal human body dimensions from few external measurements. J Musculoskeletal Res. 2009;12(04):191–204.
- Cavanaugh JM, Nyquist GW, Goldberg SJ, King AI. Lower abdominal tolerance and response. In: Proceedings of the 30th Stapp Car Crash Conference, San Diego, CA, October 27–29, 1986:41–63.
- Davis ML, Koya B, Schap JM, Gayzik FS. Development and full body validation of a 5th percentile female finite element model. Stapp Car Crash J. 2016;60:509–544.
- Eppinger RH, Marcus JH, Morgan RM. Development of dummy and injury index for NHTSA's thoracic side impact protection research program. SAE Technical Paper 840885; 1984.
- Foster CD, Hardy WN, Yang KH, King AI, Hashimoto S. High-speed seatbelt pretensioner loading of the abdomen. Stapp Car Crash J. 2006;50:27–51.
- Gordon CC, Bradtmiller B, Churchill T, et al. 1988 Anthropometric survey of U.S. army personnel: Methods and summary statistics. Technical report NATICK / TR-89 / 044. Natick, MA: U.S. Army Natick Research, Development, and Engineering Center; 1989. Data downloaded from http://mreed.umtri.umich.edu/mreed
- Hardy WN, Schneider LW, Rouhana SW. Abdominal impact response to rigid-bar, seatbelt, and airbag loading. Stapp Car Crash J. 2001;45:1–31.
- Holcombe SA, Wang SC. Subcutaneous fat distribution in the human torso. In: Proceedings of the International IRCOBI Conference, Berlin, Germany, September 10–12, 2014.
- Howes MK, Hardy WN, Beillas P. The effects of cadaver orientation on the relative position of the abdominal organs. Ann. Adv. Automot. Med. 2013;57:209–224.
- Hwang E, Hu J, Chen C, Klein KF, Miller CS, Reed MP, Hallman JJ. Development, evaluation, and sensitivity analysis of parametric finite element whole-body human models in side impacts. Stapp Car Crash J. 2016;60:473–508.
- Jolivet E, Lafon Y, Petit P, Beillas P. Comparison of Kriging and moving least square methods to change the geometry of human body models. Stapp Car Crash J. 2015;59:337–357.
- Kremer MA, Gustafson HM, Bolte JH, Stammen J, Donnelly B. Pressure-based abdominal injury criteria using isolated liver and full-body post-mortem human subject impact test. Stapp Car Crash J. 2011;55:317–350.
- Lamielle S, Vezin P, Verriest JP, Petit P, Trosseille X, Vallancien G. 3D deformation and dynamics of the human cadaver abdomen under seatbelt loading. Stapp Car Crash J. 2008;52:267–294.
- Le Ruyet A, Berthet F, Rongiéras F, Beillas P. Effect of abdominal loading location on liver motion: Experimental Assessment using ultrafast ultrasound imaging and simulation with a human body model. Stapp Car Crash J. 2016;60:25–57.
- Mertz, H. A procedure for normalizing impact response data. SAE Technical Paper 840884; 1984.
- Parenteau CS, Ehrlich P, Ma L, Su GL, Holcombe S, Wang SC. The quantification of liver anatomical changes and assessment of occupant liver injury patterns. Stapp Car Crash J. 2013;57:267–283.
- Parkinson M, Reed MP. Creating virtual user populations by analysis of anthropometric data. Int J Ind Ergon. 2010;40:106–111.
- Vavalle NA, Moreno DP, Rhyne AC, Stitzel JD, Gayzik FS. Lateral impact validation of a geometrically accurate full body finite element model for blunt injury prediction. Ann Biomed Eng. 2013;41(3):497–512.
- Vavalle NA, Schoell SL, Weaver AA, Stitzel JD, Gayzik, FS. Application of radial basis function methods in the development of a 95th percentile male seated FEA model. Stapp Car Crash J. 2014;58:361–384.
- Viano DC, Lau IV, Asbury C, King AI, Begeman PC. Biomechanics of the human chest, abdomen, and pelvis in lateral impact. In: Proceedings of the 33rd Annual Meeting of the Association for the Advancement of Automotive Medicine, Baltimore, MD, October 1989:367–382.
