ABSTRACT
Objective/Background: Insomnia and depression are disorders that affect many perinatal women and that often are interrelated. The present study aimed to examine concurrent and prospective associations between mid-pregnancy insomnia and depression during mid-pregnancy and 8 weeks postpartum. Furthermore, differences in depression and in the sleep-related characteristics insomnia, chronotype, and sleep efficiency were explored between the two time points (mid-pregnancy versus 8 weeks postpartum), and between primiparous and multiparous participants.
Participants/Methods: The study was part of the Norwegian population-based Depression and Anxiety in the Perinatal Period (DAPP) prospective cohort study. Among 539 women that were recruited for participation when receiving a routine ultrasound examination, we analyzed data from hospital birth records and questionnaire responses from pregnancy week 17 and postpartum week 8. We used the Edinburgh Postnatal Depression Scale to measure depression. The Bergen Insomnia Scale, the reduced Horne-Östberg Morningness-Eveningness Questionnaire, and three questions from the Pittsburgh Sleep Quality Index were used to measure the sleep-related characteristics.
Results: Mid-pregnancy insomnia was significantly associated with concurrent depression (p < .001), but not with postpartum depression (p = .288), in a linear mixed model with adjustment for several reproductive and psychosocial variables. Sleep efficiency was reduced from mid-pregnancy to postpartum (from 88% to 77%), and primiparous women reported less efficient sleep than multiparous women after childbirth.
Conclusions: The results indicate that mid-pregnancy insomnia may be a marker for concurrent depression but not a predictor of postpartum depression. Future research should examine the extent to which treatment of insomnia from mid-pregnancy on reduces both perinatal insomnia and depression.
Introduction
Insomnia is the most prevalent among the sleep disorders, affecting more women than men (Bei et al., Citation2015; Morin & Benca, Citation2012). Pregnant women frequently experience insomnia, with rates ranging between 12% and 38% during early gestation (Facco et al., Citation2010; Okun et al., Citation2015), and rising to 60% in the third trimester (Sivertsen et al., Citation2015). Insomnia is considered an independent disorder (American Psychiatric Association, Citation2013) but can co-occur with other disorders, such as depression (Riemann et al., Citation2017). Estimated to be present in 6.5%–19% of perinatal women (Gavin et al., Citation2005), depression was reportedly equally prevalent during the antenatal and postpartum periods (Milgrom et al., Citation2008), or even more prevalent antenatally (Underwood et al., Citation2016), which suggests that many cases of postpartum depression likely develop during pregnancy (Gavin et al., Citation2005). Indeed, the recognition of that relationship is reflected in changes in the DSM-5, which now specifies peripartum onset of depression, instead of only postpartum onset (American Psychiatric Association, Citation2013). In non-perinatal populations, insomnia has been found to predict depression (Hertenstein et al., Citation2018). Thus, it is conceivable that insomnia and depression are associated during the perinatal period.
The negative implications that postpartum depression can have for the mother–infant attachment (Field, Citation2010), as well as for children’s behavioral (Avan et al., Citation2010) and health outcomes (Murray et al., Citation2011) are well known, but also antenatal depression is associated with disturbances in child development (Pearson et al., Citation2013). Even sub-clinical maternal depression has been linked to adverse outcomes during childhood, which justifies emphasis on the need for early perinatal identification across different levels of severity (Kingston et al., Citation2018). Depressed pregnant women are not only underdiagnosed (Byatt et al., Citation2012), but also reluctant to seek help (Byatt et al., Citation2016), both of which imply that identifying variables that can reveal antenatal symptoms of depression might be an effective strategy to detect women who require additional care throughout the perinatal period (Fisher et al., Citation2016). Moreover, antenatal depression is an important risk factor for postpartum depression (Wisner et al., Citation2013).
Disturbances in sleep during pregnancy are associated with several adverse health outcomes, including increased risk of gestational diabetes (Cai et al., Citation2017), binge eating disorder (Chang et al., Citation2010), preterm birth (Okun et al., Citation2011), longer labor, and the need for cesarean delivery (Chang et al., Citation2010). Postnatally, sleep disturbances among mothers are associated with reduced neurobehavioral performance, including impaired daytime attentiveness (Insana et al., Citation2013). At the same time, primiparous and multiparous women experience different perinatal challenges (Krieg, Citation2007). Given these possible outcomes, the need for longitudinal studies on changes in perinatal sleep (Sedov et al., Citation2018) that also focus on differences between primiparous and multiparous women (Franco-Sena et al., Citation2018), as well as additional research into the relationship between sleep and depression during the perinatal period (Kamysheva et al., Citation2010), has been highlighted.
The few studies that previously have explored associations between insomnia specifically and depression during the perinatal period are characterized by cross-sectional designs (Dørheim et al., Citation2012; Swanson et al., Citation2011), recruitment from psychiatric settings (Swanson et al., Citation2011), limited availability of data regarding covariates (Marques et al., Citation2011; Swanson et al., Citation2011), and assessments late in pregnancy (Dørheim et al., Citation2014; Marques et al., Citation2011; Pietikäinen et al., Citation2018). Late-pregnancy insomnia was associated with postpartum depression at 8 weeks (Dørheim et al., Citation2014) and 3 months (Marques et al., Citation2011) postpartum only when unadjusted for previous depression. Consequently, insomnia as late as during the final gestational phase may represent a prodrome, or an early symptom, of recurrent depression after childbirth instead of an independent risk factor for postpartum depression (Sivertsen et al., Citation2012). Given that cognitive behavioral treatment, which is the recommended first-line treatment for chronic insomnia, typically requires 4–8 sessions (Riemann et al., Citation2017), we wanted to examine symptoms of insomnia and depression at a point in time during pregnancy from which such treatment interventions can be completed before delivery. To our knowledge, this prospective study of 539 perinatal women from the general population is the first to examine the relationship between antenatal insomnia and perinatal depression from as early on as mid-pregnancy.
The primary aim of this study was to examine concurrent and prospective associations between mid-pregnancy insomnia and depression during mid-pregnancy and 8 weeks postpartum, while adjusting for several previously identified risk factors for both insomnia and perinatal depression (Lancaster et al., Citation2010; Robertson et al., Citation2004; Singareddy et al., Citation2012). We hypothesized that mid-pregnancy insomnia is associated with concurrent and postpartum depression. Furthermore, differences in depression and in the sleep-related characteristics insomnia, chronotype, and sleep efficiency were explored between the two time points (mid-pregnancy versus 8 weeks postpartum), and between primiparous and multiparous participants.
Methods
Design and study procedure
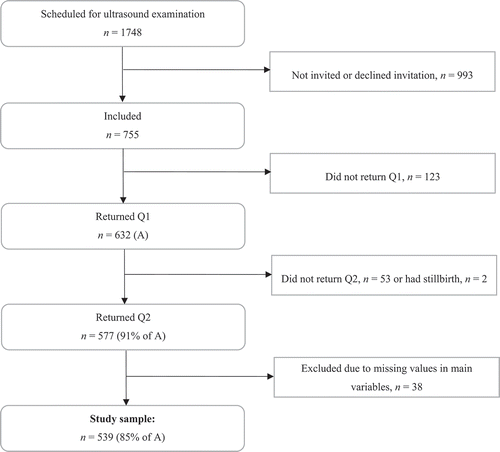
Our study was part of the Depression and Anxiety in the Perinatal Period (DAPP) Study, a prospective cohort study targeting all women scheduled to deliver at Ålesund Hospital in Norway. The hospital serves a population of roughly 100,000 people, among whom an average of 1300 women give birth each year. To collect data, we administered questionnaires during mid-pregnancy (i.e., pregnancy week 17) (Q1) and during week 8 postpartum (Q2), the results of which we linked to hospital birth records. illustrates the procedure of the study.
Figure 1. The Depression and Anxiety in the Perinatal Period (DAPP) study: overview of the study procedure
Study sample
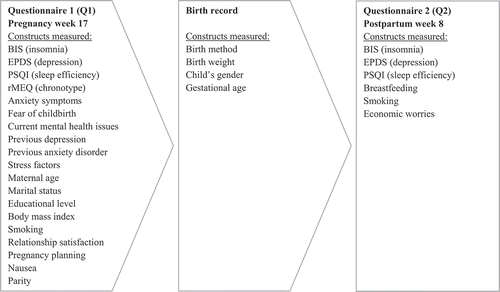
The public antenatal care program in Norway provides a routine ultrasound examination, free of charge, at gestational week 17. Our study’s target population was all women who received that ultrasound examination, provided that they were at least 18 years of age and able to answer a questionnaire in Norwegian. From November 2015 to April 2017, a total of 1748 women came for this examination at Ålesund Hospital, of whom 755 (43%) provided written consent to participate and were included in the study. Possible reasons for nonparticipation were refusal of invitation, time constraints, or receiving the examination during the evening when recruiting personnel could be absent. Ultimately, the study sample consisted of 539 women who returned both questionnaires and submitted data regarding all variables included in our primary analysis. They represented 85% of the women who returned the first questionnaire. presents details about the study sample. The DAPP Study was approved by the Regional Committees for Medical and Health Research Ethics (approval number 2014/1480/REC South East).
Measures
Sleep measures
To measure insomnia during mid-pregnancy (Q1) and postpartum (Q2), participants completed the Bergen Insomnia Scale (BIS), which includes six items corresponding to the DSM-IV-TR diagnostic criteria for insomnia (American Psychiatric Association, Citation2000). The score of each item ranges from 0 to 7, which reflects the number of days per week during the last month when respondents experienced various symptoms of insomnia. The first four items measure sleep impairment according to DSM-IV-TR criterion A for insomnia, whereas the last two assess daytime sleepiness or tiredness and dissatisfaction with sleep according to DSM-IV-TR criterion B. The BIS provides a total score on a continuous scale as well as a dichotomous score for the presence or absence of insomnia. Individuals were characterized as having insomnia if they reported ≥3 days per week on any of the first four items combined with ≥3 days per week on any of the last two items. Absence of insomnia is referred to as normal sleep (Wassing et al., Citation2018). The BIS demonstrates good psychometric properties (Pallesen et al., Citation2008), and has been used in several studies involving surveys of pregnant women (Dørheim et al., Citation2014; Osnes et al., Citation2019).
We included three questions from the Pittsburgh Sleep Quality Index (PSQI) in both Q1 and Q2 to measure the average point of time when the women went to bed and woke up, as well as their average total amount of sleep during the previous month (Buysse et al., Citation1989). The PSQI has been validated for use among pregnant women (Jomeen & Martin, Citation2007). From those data, we calculated habitual sleep efficiency as time spent sleeping divided by time spent in bed. When calculations of sleep efficiency exceeded 100% due to self-reports (n = 12 in Q1 and n = 5 in Q2), we recorded sleep efficiency as 100%.
Only Q1 included the reduced Horne-Östberg Morningness-Eveningness Questionnaire (rMEQ) (Horne & Östberg, Citation1976), which measures chronotype, i.e., circadian phase preference in terms of having an evening (“night owl”) or a morning (“early bird”) preference (Sharkey et al., Citation2013). The rMEQ consists of five questions yielding total scores from 4 to 25; a lower score indicates “eveningness” and a higher score “morningness”. Scores on the rMEQ can be divided into five categories: definitely evening type (score <8), moderately evening type (score 8–11), neither type (score 12–17), moderately morning type (score 18–21), and definitively morning type (score >21) (Adan & Almirall, Citation1991). Due to low n in the two extreme categories, the five categories of the rMEQ were merged into three categories for some analyses: evening type (score <12), neither type (score 12–17), and morning type (score >17).
Depression measures
To gauge perinatal depression, the questionnaires both during mid-pregnancy (Q1) and postpartum (Q2) included the Edinburgh Postnatal Depression Scale (EPDS), a 10-item scale assessing symptoms of depression during the previous 7 days (Cox & Sagovsky, Citation1987). The score for each item ranges from 0 to 3, and a higher sum score indicates more symptoms. A cutoff of ≥10 affords good psychometric properties for the diagnosis of postpartum depression (Eberhard-Gran et al., Citation2001), has been used among pregnant women (Eberhard-Gran et al., Citation2004), and was also the cutoff we used to define perinatal depression. Although the EPDS was developed for postpartum depression screening, it also shows good psychometric properties and is validated for use during pregnancy (Bergink et al., Citation2011). Additionally, we constructed a 9-item EPDS subscale that excluded item 7 regarding sleep difficulty (i.e., “I have been so unhappy that I have had difficulty sleeping”).
The Lifetime Major Depression Scale, which measures previous depression based on DSM-IV criteria (Kendler et al., Citation1993), was included in Q1. It comprises five items concerning sadness, appetite change, lack of energy, self-blame, and poor concentration, to be answered “Yes” or “No”. Having had at least three symptoms simultaneously for a minimum of two consecutive weeks was defined as a previous depression. The scale shows high reliability for measuring lifetime history of major depression (Kendler et al., Citation1993).
Anxiety measures
Symptoms of anxiety was measured during mid-pregnancy (Q1) and postpartum (Q2) using the anxiety dimension SCL-A (i.e., the first ten items) from the Hopkins Symptom Checklist-25 (SCL-25) (Derogatis et al., Citation1974). Referring to the past 7 days, the score for each item ranges from 1 to 4, and higher scores indicate more severe symptomatology. The Norwegian version of the SCL-25 has been validated against the criteria of the International Classification of Diseases (ICD) for anxiety and depression (Sandanger et al., Citation1998), and the SCL-A shows good psychometric properties and reliable construct measurement (Veijola et al., Citation2003). Anxiety was defined as having a SCL-A score ≥18 (Osnes et al., Citation2019).
In Q1, participants reported whether a health professional had ever diagnosed them with an anxiety disorder (i.e., generalized anxiety disorder, social phobia, specific phobia, panic disorder, agoraphobia, obsessive-compulsive disorder, or post-traumatic stress disorder) (American Psychiatric Association, Citation2000). If participants reported having been diagnosed with one or more of those disorders, we categorized them as having had an anxiety disorder.
Reproductive measures
In the mid-pregnancy questionnaire (Q1), participants reported whether they had planned their pregnancy and whether they had experienced a problematic degree of nausea during the first trimester. We asked participants whether they had previously given birth, and if so, we characterized them as multiparous. Also, in Q1, we used 6 factors from the 33-item Wijma Delivery Expectancy/Experience Questionnaire version A (W-DEQ) (Wijma et al., Citation1998), which measures fear of childbirth (Garthus-Niegel et al., Citation2011). From hospital birth records, we retrieved data regarding emergency and elective cesarean sections, gestational age, and infant gender and birth weight. In the postpartum questionnaire (Q2), we asked participants whether they were breastfeeding, to be answered “Yes” (i.e., either exclusively or partly) or “No”.
Psychosocial measures
The mid-pregnancy questionnaire (Q1) contained the Relationship Satisfaction Scale, a 10-item, modified and shortened version of Mehrabian’s Marital Satisfaction Scale (Blum & Mehrabian, Citation1999), assessing partners’ closeness, problems, happiness, responsiveness, plans for quitting the relationship, satisfaction with the relationship, disagreements, feeling lucky with the other partner, views on child rearing, and thoughts regarding the other partner’s satisfaction. The score for each item ranges from 1 to 4, and greater total scores indicated lower satisfaction with the relationship. The instrument has been used in previous studies conducted in Norway (Osnes et al., Citation2019; Røsand et al., Citation2011).
In Q1, we measured stress factors experienced during the last year with ten items selected from life event scales (Coddington, Citation1972; Swearingen & Cohen, Citation1985). The items included marital separation or divorce, severe problems in the relationship with one’s partner, conflicts with family members, traffic accident, fire or theft, loss of a close relative, and other crises or difficulties in life. The score for each item ranged from 1 (not so difficult) to 3 (very difficult) (Coddington, Citation1972).
Q1 included a question regarding whether the woman considered herself to currently have mental health issues, to be answered either “Yes” or “No”.
From both questionnaires, we retrieved information regarding sociodemographic factors and current smoking, the latter to be answered “Yes” or “No”. In Q1, we asked for each participant’s age, marital status (i.e., “married or cohabitating”, “has partner but live separately”, or “single”), level of education, and weight and height (to calculate body mass index). In the postpartum questionnaire (Q2), we asked each woman whether she frequently worried about her personal economic situation.
Statistical analyses
Continuous data are presented as mean and standard deviation (SD) for normally distributed data, and additionally as median with 25th and 75th percentiles for non-normally distributed data. Given the large sample size, t-tests were used to analyze between-group differences for both normally and non-normally distributed data. Data were assessed for normal distribution by visual inspection of Q-Q plots. Categorical data are presented as frequency and percentage, and differences in proportions were examined using the Chi-square test. Paired-samples t-tests and McNemar’s tests were used to test differences in mean scores and proportions, respectively, before versus after delivery. The Kruskal-Wallis test was used to analyze differences in postpartum (Q2) depression levels among sleep groups. Cronbach’s alpha values were calculated to test the internal validity of psychometric scales.
Linear mixed models were used to study the associations between depression, measured during mid-pregnancy (Q1) and 8 weeks postpartum (Q2), and insomnia, anxiety, previous depression, and reproductive and psychosocial variables. All covariates were measured in Q1, except for frequent economic worries, for which the value in Q2 was used as a proxy for the unmeasured value in Q1. We tested for interactions between the time points (Q1 and Q2) and some chosen covariates (i.e., insomnia, previous depression, symptoms of anxiety, stress factors in the last year, fear of childbirth, multiparity, and current mental health issues), and we included the interactions in the final model if statistically significant. Random intercepts for women were used to account for intra-women correlations.
The EPDS distributions in Q1 and Q2 were positively skewed and were first adjusted to range from 1 and then log-transformed before the linear mixed model analyses. Missing values for the covariates were handled by case-wise deletion. We performed additional linear mixed model analyses with the shifted (to range from 1) and log-transformed 9-item EPDS variable.
The relationship dissatisfaction variable was answered only by women who were married, cohabitating or in a relationship. To include also the single women in the linear mixed model analyses (n = 11), we performed the analyses both with and without this variable.
Spearman’s rho (rs) was used to measure bivariate correlations between non-normally distributed continuous variables, and was, in addition to the Pearson correlation coefficient and Kendall’s tau-b, used to investigate whether any pair of independent variables were too highly correlated to be jointly entered into the regression models. The variance inflation factor (VIF) was also used to rule out multicollinearity.
The significance level was set to 0.05. The p-values of the univariate tests were adjusted for multiple testing by the Holm-Bonferroni method. Data analyses were performed using the SPSS statistical software package, version 25 (SPSS Inc., Chicago, IL, USA), and the STATA statistical software package, version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
Study population characteristics
The study population comprised 539 women, whose sociodemographic characteristics, information regarding chronotype and birth outcomes, as well as data from the Medical Birth Registry of Norway (Norwegian Institute of Public Health, Citation2017) appear in . Participants were between 20 and 48 years old. The gestational age of offspring at birth was between 28 and 42 weeks, and birth weight ranged from 915 to 5320 grams. After returning the mid-pregnancy questionnaire (Q1), 55 study participants dropped out of the study (). Scores of insomnia, depression, and anxiety in Q1, as well as age and level of education, did not significantly differ among participants who dropped out of the study and those who remained. We excluded the data of 38 women with missing values for explanatory variables used in the linear mixed model analyses (). The excluded women did not differ significantly from the study sample concerning age or educational level, or insomnia, depression, and anxiety scores during mid-pregnancy (Q1) or postpartum (Q2).
Table 1. Sociodemographic, chronotype, and birth outcome characteristics of the study sample and data from the Medical Birth Registry of Norway in 2017
Prevalence
Insomnia was present among 59.7% (n = 322) of study participants during mid-pregnancy (Q1) and 55.2% (n = 297) postpartum (Q2). A proportion of 38.3% (n = 206) experienced insomnia both in Q1 and Q2. Cronbach’s alpha for the BIS was 0.81 in Q1 and 0.75 in Q2. When the BIS item regarding non-restorative sleep was excluded (American Psychiatric Association, Citation2013), the rates of insomnia were 45.8% (n = 247) in Q1 and 48.1% (n = 259) in Q2.
The prevalence of depression defined as an EPDS score ≥10 was 9.8% (n = 53) in Q1 and 9.6% (n = 52) in Q2, and 3.3% (n = 18) of study participants were depressed both in Q1 and Q2. In Q1, the women with an EPDS score ≥10 had a mean score 13.2 (SD 3.1), while in Q2, those with an EPDS score ≥10 had a mean score 13.0 (SD 2.6). Cronbach’s alpha for the EPDS was 0.83 in Q1 and 0.84 in Q2. In Q1 and Q2, 8.7% (n = 47) and 8.4% (n = 45) of participants, respectively, exhibited concurrent insomnia and depression. A medium, positive correlation was found between the BIS and the EPDS in both Q1 (rs = 0.41; p < .001) and Q2 (rs = 0.39; p < .001).
Linear mixed model analyses for depression
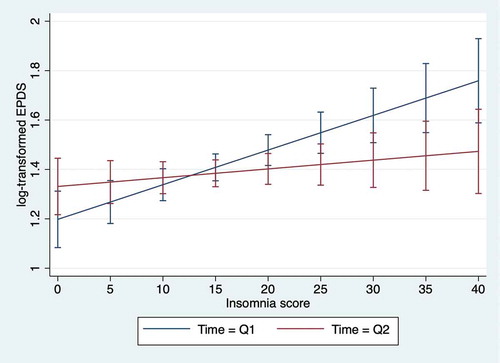
presents the association between mid-pregnancy insomnia and perinatal depression from the linear mixed model with the log-transformed EPDS in mid-pregnancy (Q1) and 8 weeks postpartum (Q2) as the dependent variable. The association with insomnia in Q1 was significant for concurrent depression in Q1 (p < .001), but not for postpartum depression in Q2 (p = .288), although the estimated association was positive for both. The interaction with the time points (Q1 and Q2) showed that insomnia in Q1 was more strongly associated with concurrent depression than with postpartum depression (p = .006). The estimated marginal means of depression in Q1 and Q2 as a function of insomnia in Q1 are illustrated in .
Table 2. Concurrent and prospective associations between mid-pregnancy insomnia and perinatal depression. Results from a linear mixed model, with depression measured by the log-transformed Edinburgh Postnatal Depression Scale (EPDS) in pregnancy week 17 (Q1) and postpartum week 8 (Q2) as the dependent variable. Covariates were measured in Q1a.
Figure 3. The association between mid-pregnancy insomnia and perinatal depression
Insomnia (p = .006) and anxiety (p < .001) were the only variables that showed significant interaction with the time points (Q1 and Q2) among the values tested for interactions with time (i.e., insomnia, previous depression, symptoms of anxiety, stress factors in the last year, fear of childbirth, multiparity, and current mental health issues) in the linear mixed model. The results including those two interaction terms and all the covariates are shown in Supplemental Table 1. The interaction between parity and insomnia was not statistically significant (p = .359).
In order to include the single women, we also performed a linear mixed model analysis including the variable concerning marital status instead of the variable measuring dissatisfaction with the relationship. Being married or cohabitating was not significantly associated with perinatal depression (p = .306).
To examine whether chronotype impacted the association between mid-pregnancy insomnia and perinatal depression, we performed an additional linear mixed model analysis including the chronotype variable (Q1, n = 533) with three categories (morning type, n = 80; neither type, n = 334; evening type, n = 119) and its interaction with mid-pregnancy insomnia. This interaction was not statistically significant (p = .633).
We additionally performed a linear mixed model analysis using the log-transformed 9-item EPDS (excluding item 7: “I have been so unhappy that I have had difficulty sleeping”) in Q1 and Q2 as the dependent variable, which caused only small variations with regard to estimated coefficients and p-values. Insomnia in Q1 was still significantly associated with concurrent depression (p < .001), but not with postpartum depression (p = .320). A normal approximation was found to be reasonable for residual distributions from the linear mixed model analyses.
Differences in sleep-related characteristics and depression
shows summary values for the variables of sleep-related characteristics and depression before (Q1) and after (Q2) childbirth for the entire study cohort. Neither continuous nor dichotomous variables for insomnia or depression showed significant differences across the perinatal period. Sleep efficiency declined from 88% during pregnancy to 77% after delivery (p < .001), and the frequency of nights with difficulty maintaining sleep doubled in the postpartum period [mean in Q1: 2.2 vs. mean in Q2: 4.1; p < .001]. In contrast, difficulty initiating sleep [mean in Q1: 2.1 vs. mean in Q2: 1.6; p < .001] and daytime impairment [mean in Q1: 2.5 vs. mean in Q2: 1.7; p < .001] significantly declined after delivery. We also performed Wilcoxon signed rank tests in addition to paired samples t-tests for non-normally distributed variables, and the results did not deviate much with respect to p-values.
Table 3. Sleep and depression before (Q1) and after (Q2) childbirth (n = 539a)
presents a comparison of primiparous (n = 211) and multiparous (n = 328) study participants. Primiparous participants had significantly lower scores for rMEQ-measured chronotype during mid-pregnancy (indicating greater “eveningness”), spent more time in bed during mid-pregnancy and postpartum, and had less efficient sleep after (but not before) delivery than multiparous participants. In addition to t-tests, we performed Mann Whitney U-tests for non-normally distributed variables, with minor differences in results.
Table 4. Comparison of sleep and depression characteristics between primiparous (n = 211) and multiparous (n = 328) study participantsa.
A Kruskal-Wallis test revealed a significant difference in postpartum (Q2) depression levels among four different sleep groups (G1, n = 206: insomnia in Q1 and Q2; G2, n = 115: insomnia in Q1 and normal sleep in Q2; G3, n = 91: normal sleep in Q1 and insomnia in Q2; G4, n = 126: normal sleep in Q1 and Q2, p < .001). G1 and G3 had higher median postpartum depression scores (both median = 4.0) than G2 and G4 (both median = 2.0).
Discussion
In this first prospective study to examine associations between mid-pregnancy insomnia specifically and perinatal depression, mid-pregnancy insomnia was significantly associated with concurrent depression, but, although positively associated, not significantly so with postpartum depression. We found high rates of insomnia and depression that were stable across the perinatal period. Approximately 60% of study participants experienced symptoms of insomnia mid-pregnancy and 55% postpartum, while 10% experienced symptoms of depression both mid-pregnancy and postpartum. Moreover, sleep efficiency declined from 88% to 77% during the perinatal period, and primiparous mothers had less efficient sleep than multiparous mothers after delivery.
About one in 10 women experienced symptoms of perinatal depression, which aligns with previous results using a similar EPDS cutoff score (Eberhard-Gran et al., Citation2004). The cohort exhibited significant change of neither scores nor rates of depression across the perinatal period, which supports that many cases of postpartum depression probably develop during pregnancy (Gavin et al., Citation2005). Peripartum depression poses serious health-related consequences, not least for the infants (Kingston et al., Citation2018). Therefore, it seems plausible to claim that detecting markers to help health professionals reveal symptoms and initiate treatment of early antenatal depression is important.
Our findings show that mid-pregnancy insomnia, being significantly associated with concurrent symptoms of depression, may be a marker for antenatal depression – especially since women with depression are liable to consider reporting symptoms of insomnia to be less stigmatizing or shameful than reporting symptoms of depression (Christensen et al., Citation2016). Additionally, insomnia, a disorder for which there are effective non-pharmacological treatment methods, was highly prevalent during mid-pregnancy. Insomnia during pregnancy was previously found to be associated with perinatal anxiety (Osnes et al., Citation2019; Citation2020); screening for insomnia may thus represent an efficient tool for detecting pregnant women at risk of developing both mood and anxiety disorders. Consequently, implementation of screening procedures to detect insomnia along with the existing screening procedures for perinatal depression may be warranted.
The participants with insomnia during both mid-pregnancy and postpartum, or who developed insomnia postpartum, had higher levels of postpartum depression compared with the participants with normal sleep during both mid-pregnancy and postpartum or who recovered from insomnia after mid-pregnancy. Furthermore, antenatal insomnia is reported as a risk factor for experiencing insomnia postpartum (Dørheim et al., Citation2014). Hence, since cognitive behavioral therapy for insomnia (CBT-I) is not only associated with reduced symptoms of both insomnia and depression (Ballesio et al., Citation2017; Manber et al., Citation2019), but also reportedly safe and effective during pregnancy (Manber et al., Citation2018; Tomfohr-Madsen et al., Citation2017), it might improve symptoms of perinatal insomnia and depression when provided from mid-pregnancy on to women with insomnia, but more research on that topic remains necessary.
Our results concerning associations between insomnia and depression did not differ significantly between primiparous and multiparous women, which suggests that parity plays a limited role in the interaction of perinatal sleep and mood (Coo Calcagni et al., Citation2012). Neither did the variables regarding symptoms of depression differ significantly according to parity. A previous study conducted in Norway also revealed that rates of depression were similar in terms of parity for mothers up to 35 years of age, whereas older primiparous women were more depressed (Glavin et al., Citation2009).
Rates of perinatal insomnia are in accordance with those of previous studies (Mindell et al., Citation2015), and were stable throughout the perinatal period, which corroborates the findings of a large survey conducted in Norway (Sivertsen et al., Citation2015). Our perinatal rates of insomnia were far higher than the prevalence estimates of 12%–17% among women in the same age group in Norway’s general population (Pallesen et al., Citation2014). Nevertheless, among middle-aged and older women, insomnia has been found to occur more frequently in women than in men (Bei et al., Citation2015), and women with perinatal insomnia have also been identified at high risk of experiencing insomnia 2 years later (Sivertsen et al., Citation2015). Consequently, it has been questioned whether perinatal insomnia can become chronic (Sivertsen et al., Citation2015).
Sleep duration and sleep efficiency decreased from mid-pregnancy to the postpartum period, while the number of nights with difficulty maintaining sleep increased, all of which aligns with the results of previous surveys (Dørheim et al., Citation2014; Richter et al., Citation2019). Our finding of a postpartum sleep efficiency of 77% is comparable with results from two studies conducted in Norway (Dørheim et al., Citation2014; Dorheim et al., Citation2009), and lower than the recommended sleep efficiency of >85% (Bastien, Citation2011). Considering the irregular sleep patterns among infants (Hysing et al., Citation2014), as well as new maternal responsibilities even during the nighttime, disruptions in sleep continuity throughout the night are not surprising. Moreover, postpartum decreases in estrogen and progesterone levels have been linked to difficulties with staying asleep (Lee et al., Citation2000). However, the frequency of experiencing daytime impairment and difficulty initiating sleep significantly declined postnatally, possibly due to the added responsibility of caring for the infant during the daytime, causing less rumination about how sleep impacts day-to-day functioning, and a greater accumulated sleep pressure when going to bed (Riemann et al., Citation2015).
Rates of perinatal insomnia did not differ significantly between primiparous and multiparous women, although sleep efficiency was lower among primiparous mothers after childbirth, as reported elsewhere (Coo Calcagni et al., Citation2012; Dørheim et al., Citation2014; Lee et al., Citation2000). Reduced sleep efficiency can reflect the onset of the maternal role, which entails a higher level of insecurity and arousal during the night than among multiparous mothers (Waters & Lee, Citation1996). Moreover, multiparous women had a significantly higher sum score for rMEQ-measured chronotype during pregnancy, indicating greater “morningness”, and spent less time in bed, than primiparous women. That tendency could be due to the responsibilities of caring for older children, which promotes a stricter schedule regarding both going to and getting out of bed.
Our study had several methodological limitations. First, data on depression and sleep-related characteristics, including insomnia, were based on self-reported instruments. A diagnosis of insomnia requires trouble with sleeping to occur despite adequate opportunities to sleep, but we did not survey participants regarding such opportunities. Second, we did not assess their sleep behavior pre-pregnancy. Third, less than one-half of the women who received ultrasound examinations at the hospital ultimately participated, although we did not record reasons why – that is, whether non-participants declined to participate or did not receive an invitation. Fourth, we invited only Norwegian-speaking women to participate, which might limit the generalizability of our findings. Rates of perinatal depression are higher among women with immigrant or minority status (Falah-Hassani et al., Citation2015). At the same time, the burden of psychiatric illness that occurs along with insomnia reportedly differs among people of various ethnic backgrounds (Kalmbach et al., Citation2016).
Our study had various strengths, as well. To our knowledge, this was the first prospective cohort study to examine associations between mid-pregnancy insomnia specifically and symptoms of perinatal depression. Additional strengths of the study were its large sample size and that participants were targeted for inclusion upon attending a routine examination received by most pregnant women in Norway, which suggests a low first wave selection bias. Moreover, the response rate for the last questionnaire was exceptionally high (91%), and the women who participated did not differ much from either the drop-outs or the women excluded from the sample in terms of important variables. We were able to adjust for several potential confounding factors, and the instrument measuring insomnia (i.e., BIS) has corresponded well with objective measures of sleep, including polysomnography (Pallesen et al., Citation2008).
Conclusion
Our study revealed that, after adjustment for several known risk factors for insomnia and perinatal depression, mid-pregnancy insomnia was significantly associated with concurrent depression. Thus, insomnia could function as an important marker for depression in pregnant women, aiding healthcare workers in identifying those who require extra follow-up. Although little evidence was found for an association between mid-pregnancy insomnia and postpartum depression, researchers should investigate whether the detection and subsequent treatment of insomnia during pregnancy can lower rates of perinatal insomnia and depression. Furthermore, six in ten women experienced insomnia during mid-pregnancy, while depression was present among 10%, both during mid-pregnancy and postpartum. The study participants, especially primiparous ones, experienced less efficient sleep after childbirth than during pregnancy.
Supplemental Material
Download MS Word (15.5 KB)Acknowledgments
We thank all the women who participated in the DAPP study. We also thank Janita Skogeng, Sissel Hjelle, Elin Hansen Ytterbø, Caroline Nekstad Jensen, Else Smørdal Lillestøl, Nasim Zanjan, and the other involved staff at the Department of Obstetrics and Gynecology at Ålesund Hospital for their contributions to the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Adan, A., & Almirall, H. (1991). Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Personality and Individual Differences, 12(3), 241–253. https://doi.org/10.1016/0191-8869(91)90110-W
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR. https://doi.org/10.1176/appi.books.9780890423349.5847
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. https://doi.org/10.1176/appi.books.9780890425596.744053
- Avan, B., Richter, L. M., Ramchandani, P. G., Norris, S. A., & Stein, A. (2010). Maternal postnatal depression and children ’ s growth and behaviour during the early years of life: Exploring the interaction between physical and mental health. Archives of Disease in Childhood, 95(9), 690–695. https://doi.org/10.1136/adc.2009.164848
- Ballesio, A., Aquino, M. R. J. V., Lombardo, C., Riemann, D., Johann, A. F., Kyle, S. D., … Spiegelhalder, K. (2017). The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: A systematic review and network meta-analysis. Sleep Medicine Reviews, 37, 114–129. https://doi.org/10.1016/j.smrv.2017.01.006
- Bastien, C. H. (2011). Insomnia: Neurophysiological and neuropsychological approaches. Neuropsychology Review, 21(1), 22–40. https://doi.org/10.1007/s11065-011-9160-3
- Bei, B., Coo, S., Baker, F. C., & Trinder, J. (2015). Sleep in Women: A review. Australian Psychologist, 50(1), 14–24. https://doi.org/10.1111/ap.12095
- Bergink, V., Kooistra, L., Lambregtse-van den Berg, M. P., Wijnen, H., Bunevicius, R., van Baar, A., & Pop, V. (2011). Validation of the Edinburgh depression scale during pregnancy. Journal of Psychosomatic Research, 70(4), 385–389. https://doi.org/10.1016/j.jpsychores.2010.07.008
- Blum, J. S., & Mehrabian, A. (1999). Personality and temperament correlates of marital satisfaction. Journal of Personality, 67(1), 93–125. https://doi.org/10.1111/1467-6494.00049
- Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., Kupfer, D. J., III, & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4
- Byatt, N., Simas, T. A. M., Lundquist, R. S., Johnson, J. V., & Ziedonis, D. M. (2012). Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. Journal of Psychosomatic Obstetrics and Gynecology, 33(4), 143–161. https://doi.org/10.3109/0167482X.2012.728649
- Byatt, N., Xiao, R. S., Dinh, K. H., & Waring, M. E. (2016). Mental health care use in relation to depressive symptoms among pregnant women in the USA. Archives of Women’s Mental Health, 19(1), 187–191. https://doi.org/10.1007/s00737-015-0524-1
- Cai, S., Tan, S., Gluckman, P. D., Godfrey, K. M., Saw, S.-M., Teoh, O. H., … Gooley, J. J. (2017). Sleep quality and nocturnal sleep duration in pregnancy and risk of gestational diabetes mellitus. Sleep, 40(2), 5–12. https://doi.org/10.1093/sleep/zsw058
- Chang, J. J., Pien, G. W., Duntley, S. P., & Macones, G. A. (2010). Sleep deprivation during pregnancy and maternal and fetal outcomes: Is there a relationship? Sleep Medicine Reviews, 14(2), 107–114. https://doi.org/10.1016/j.smrv.2009.05.001
- Christensen, H., Batterham, P. J., Gosling, J. A., Ritterband, L. M., Griffiths, K. M., Thorndike, F. P., … Mackinnon, A. J. (2016). Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): A randomised controlled trial. The Lancet Psychiatry, 3(4), 333–341. https://doi.org/10.1016/S2215-0366(15)00536-2
- Coddington, R. D. (1972). The significance of life events as etiologic factors in the diseases of children. I-A survey of professional workers. Journal of Psychosomatic Research, 16(1), 7–18. https://doi.org/10.1016/0022-3999(72)90018-9
- Coo Calcagni, S., Bei, B., Milgrom, J., & Trinder, J. (2012). The relationship between sleep and mood in first-time and experienced mothers. Behavioral Sleep Medicine, 10(3), 167–179. https://doi.org/10.1080/15402002.2012.668147
- Cox, J. L., & Sagovsky, J. M. H. R. (1987). Detection of postnatal depression development of the 10-item Edinburgh postnatal depression scale. British Journal of Psychiatry, 150(6), 782–786. https://doi.org/10.1192/bjp.150.6.782
- Derogatis, L. R., Lipman, R. S., Rickels, K., Uhlenhuth, E. H., & Covi, L. (1974). The Hopkins Symptom Checklist (HSCL): A self-report symptom inventory. Behavioral Science, 19(1), 1–15. https://doi.org/10.1002/()1099-1743
- Dørheim, S. K., Bjorvatn, B., & Eberhard-Gran, M. (2012). Insomnia and depressive symptoms in late pregnancy: A population-based study. Behavioral Sleep Medicine, 10(3), 152–166. https://doi.org/10.1080/15402002.2012.660588
- Dørheim, S. K., Bjorvatn, B., & Eberhard-Gran, M. (2014). Can insomnia in pregnancy predict postpartum depression? A longitudinal, population-based study. PloS One, 9(4), e94674. https://doi.org/10.1371/journal.pone.0094674
- Dorheim, S. K., Bondevik, G. T., Eberhard-Gran, M., & Bjorvatn, B. (2009). Sleep and depression in postpartum women: A population-based study. Sleep, 32(7), 847–855. https://doi.org/10.1093/sleep/32.7.847
- Eberhard-Gran, M., Eskild, A., Tambs, K., Schei, B., & Opjordsmoen, S. (2001). The Edinburgh postnatal depression scale: Validation in a Norwegian community sample. Nordic Journal of Psychiatry, 55(2), 113–117. https://doi.org/10.1080/08039480151108525
- Eberhard-Gran, M., Tambs, K., Opjordsmoen, S., Skrondal, A., & Eskild, A. (2004). Depression during pregnancy and after delivery: A repeated measurement study. Journal of Psychosomatic Obstetrics and Gynecology, 25(1), 15–21. https://doi.org/10.1080/01674820410001737405
- Facco, F. L., Kramer, J., Ho, K. H., Zee, P. C., & Grobman, W. A. (2010). Sleep disturbances in pregnancy. Obstetrics and Gynecology, 115(1), 77–83. https://doi.org/10.1097/AOG.0b013e3181c4f8ec
- Falah-Hassani, K., Shiri, R., Vigod, S., & Dennis, C.-L. (2015). Prevalence of postpartum depression among immigrant women: A systematic review and meta-analysis. Journal of Psychiatric Research, 70, 67–82. https://doi.org/10.1016/j.jpsychires.2015.08.010
- Field, T. (2010). Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant Behavior and Development, 33(1), 1–6. https://doi.org/10.1016/j.infbeh.2009.10.005
- Fisher, S. D., Wisner, K. L., Clark, C. T., Sit, D. K., Luther, J. F., & Wisniewski, S. (2016). Factors associated with onset timing, symptoms, and severity of depression identified in the postpartum period. Journal of Affective Disorders, 203, 111–120. https://doi.org/10.1016/j.jad.2016.05.063
- Franco-Sena, A. B., Kahn, L. G., Farias, D. R., Ferreira, A. A., Eshriqui, I., Figueiredo, A. C. C., … Kac, G. (2018). Sleep duration of 24 h is associated with birth weight in nulli- but not multiparous women. Nutrition, 55–56, 91–98. https://doi.org/10.1016/j.nut.2018.03.050
- Garthus-Niegel, S., Størksen, H. T., Torgersen, L., Von Soest, T., & Eberhard-Gran, M. (2011). The Wijma delivery expectancy/experience questionnaire - a factor analytic study. Journal of Psychosomatic Obstetrics and Gynecology, 32(3), 160–163. https://doi.org/10.3109/0167482X.2011.573110
- Gavin, N. I., Gaynes, B. N., Lorh, K. N., Meltzer-Brody, S., Gartlehner, G., & Swinson, T. (2005). Perinatal depression: A systematic review of prevalence and incidence. Obstetrics and Gynecology, 106(5), 1071–1083. https://doi.org/10.1097/01.AOG.0000183597.31630.db
- Glavin, K., Smith, L., & Sørum, R. (2009). Prevalence of postpartum depression in two municipalities in Norway. Scandinavian Journal of Caring Sciences, 23(4), 705–710. https://doi.org/10.1111/j.1471-6712.2008.00667.x
- Hertenstein, E., Feige, B., Gmeiner, T., Kienzler, C., Spiegelhalder, K., Johann, A., … Baglioni, C. (2018). Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Medicine Reviews, 43, 96–105. https://doi.org/10.1016/j.smrv.2018.10.006
- Horne, J. A., & Östberg, O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110.
- Hysing, M., Harvey, A. G., Torgersen, L., Ystrom, E., Reichborn-Kjennerud, T., & Sivertsen, B. (2014). Trajectories and predictors of nocturnal awakenings and sleep duration in infants. Journal of Developmental and Behavioral Pediatrics, 35(5), 309–316. https://doi.org/10.1097/DBP.0000000000000064
- Insana, S. P., Williams, K. B., & Montgomery-Downs, H. E. (2013). Sleep disturbance and neurobehavioral performance among postpartum Women. Sleep, 36(1), 73–81. https://doi.org/10.5665/sleep.2304
- Jomeen, J., & Martin, C. (2007). Assessment and relationship of sleep quality to depression in early pregnancy. Journal of Reproductive and Infant Psychology, 25(1), 87–99. https://doi.org/10.1080/02646830601117308
- Kalmbach, D. A., Pillai, V., Arnedt, J. T., & Drake, C. L. (2016). DSM-5 insomnia and short sleep: Comorbidity landscape and racial disparities. Sleep, 39(12), 2101–2111. https://doi.org/10.5665/sleep.6306
- Kamysheva, E., Skouteris, H., Wertheim, E. H., Paxton, S. J., & Milgrom, J. (2010). A prospective investigation of the relationships among sleep quality, physical symptoms, and depressive symptoms during pregnancy. Journal of Affective Disorders, 123(1–3), 317–320. https://doi.org/10.1016/j.jad.2009.09.015
- Kendler, K., Neale, M., Kessler, R., Heath, A., & Eaves, L. (1993). The lifetime history of major depression in women. Reliability of diagnosis and heritability. Archives of General Psychiatry, 50(11), 863–870. https://doi.org/10.1001/archpsyc.1993.01820230054003
- Kingston, D., Kehler, H., Austin, M. P., Mughal, M. K., Wajid, A., Vermeyden, L., … Giallo, R. (2018). Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PloS One, 13(4), 1–19. https://doi.org/10.1371/journal.pone.0195365
- Krieg, D. B. (2007). Does motherhood get easier the second-time around? Examining parenting stress and marital quality among mothers having their first or second child. Parenting, 7(2), 149–175. https://doi.org/10.1080/15295190701306912
- Lancaster, C. A., Gold, K. J., Flynn, H. A., Yoo, H., Marcus, S. M., & Davis, M. M. (2010). Risk factors for depressive symptoms during pregnancy: A systematic review. American Journal of Obstetrics and Gynecology, 202(1), 5–14. https://doi.org/10.1016/j.ajog.2009.09.007
- Lee, K. A., Zaffke, M. E., & McEnany, G. (2000). Parity and sleep patterns during and after pregnancy. Obstetrics and Gynecology, 95(1), 14–18. https://doi.org/10.1016/S0029-7844(99)00486-X
- Manber, R., Bei, B., Simpson, N., Asarnow, L., & Rangel, E. (2018). Cognitive behavioral therapy is effective for insomnia during pregnancy: A randomized controlled trial in an ethnically diverse sample. Sleep, 41(suppl_1), A154. https://doi.org/10.1093/sleep/zsy061.404
- Manber, R., Bei, B., Simpson, N., Asarnow, L., Rangel, E., Sit, A., & Lyell, D. (2019). Cognitive behavioral therapy for prenatal insomnia. Obstetrics & Gynecology, 1. https://doi.org/10.1097/AOG.0000000000003216
- Marques, M., Bos, S., Soares, M. J., Maia, B., Pereira, A. T., Valente, J., … Azevedo, M. H. (2011). Is insomnia in late pregnancy a risk factor for postpartum depression/depressive symptomatology? Psychiatry Research, 186(2–3), 272–280. https://doi.org/10.1016/j.psychres.2010.06.029
- Milgrom, J., Gemmill, A. W., Bilszta, J. L., Hayes, B., Barnett, B., Brooks, J., … Buist, A. (2008). Antenatal risk factors for postnatal depression: A large prospective study. Journal of Affective Disorders, 108(1–2), 147–157. https://doi.org/10.1016/j.jad.2007.10.014
- Mindell, J. A., Cook, R. A., & Nikolovski, J. (2015). Sleep patterns and sleep disturbances across pregnancy. Sleep Medicine, 16(4), 483–488. https://doi.org/10.1016/j.sleep.2014.12.006
- Morin, C. M., & Benca, R. (2012). Chronic insomnia. The Lancet, 379(9821), 1129–1141. https://doi.org/10.1016/S0140-6736(11)60750-2
- Murray, L., Arteche, A., Fearon, P., Halligan, S., Goodyer, I., & Cooper, P. (2011). Maternal postnatal depression and the development of depression in offspring up to 16 years of age. Journal of the American Academy of Child and Adolescent Psychiatry, 50(5), 460–470. https://doi.org/10.1016/j.jaac.2011.02.001
- Norwegian Institute of Public Health. (2017). Medical birth registry of Norway. Retrieved from http://statistikkbank.fhi.no/mfr/
- Okun, M. L., Buysse, D. J., & Hall, M. H. (2015). Identifying insomnia in early pregnancy: Validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. Journal of Clinical Sleep Medicine, 11(6), 645–654. https://doi.org/10.5664/jcsm.4776
- Okun, M. L., Schetter, C. D., & Glynn, L. M. (2011). Poor sleep quality is associated with preterm birth. Sleep, 34(11), 1493–1498. https://doi.org/10.5665/sleep.1384
- Osnes, R. S, Eberhard-Gran, M, Follestad, T, Kallestad, H, Morken, G, & Roaldset, J.O. (2020). Mid-pregnancy insomnia is associated with concurrent and postpartum maternal anxiety and obsessive-compulsive symptoms: a prospective cohort study. Journal Of Affective Disorders, 266(April), 319-326. doi: 10.1016/j.jad.2020.01.140
- Osnes, R. S., Roaldset, J. O., Follestad, T., & Eberhard-Gran, M. (2019). Insomnia late in pregnancy is associated with perinatal anxiety: A longitudinal cohort study. Journal of Affective Disorders, 248(April), 155–165. https://doi.org/10.1016/j.jad.2019.01.027
- Pallesen, S., Bjorvatn, B., Nordhus, I. H., Sivertsen, B., Hjornevik, M., & Morin, C. M. (2008). A new scale for measuring insomnia: The Bergen insomnia scale. Percept. Mot. Skills, 107(3), 691–706. https://doi.org/10.2466/PMS.107.1.691-706
- Pallesen, S., Sivertsen, B., Nordhus, I. H., & Bjorvatn, B. (2014). A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Medicine, 15(2), 173–179. https://doi.org/10.1016/j.sleep.2013.10.009
- Pearson, R. M., Evans, J., Kounali, D., Lewis, G., Heron, J., Ramchandani, P. G., … Stein, A. (2013). Maternal depression during pregnancy and the postnatal period risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry, 70(12), 1312–1319. https://doi.org/10.1001/jamapsychiatry.2013.2163
- Pietikäinen, J. T., Polo-Kantola, P., Pölkki, P., Saarenpää-Heikkilä, O., Paunio, T., & Paavonen, E. J. (2018). Sleeping problems during pregnancy—a risk factor for postnatal depressiveness. Archives of Women’s Mental Health. https://doi.org/10.1007/s00737-018-0903-5
- Richter, D., Krämer, M. D., Tang, N. K., Montgomery-Downs, H. E., & Lemola, S. (2019). Long-term effects of pregnancy on sleep satisfaction and duration of first-time and experienced mothers and fathers. Sleep, 42(4). https://doi.org/10.1093/sleep/zsz015
- Riemann, D., Baglioni, C., Bassetti, C., Bjorvatn, B., Dolenc Groselj, L., Ellis, J. G., … Spiegelhalder, K. (2017). European guideline for the diagnosis and treatment of insomnia. Journal of Sleep Research, 26(6), 675–700. https://doi.org/10.1111/jsr.12594
- Riemann, D., Nissen, C., Palagini, L., Otte, A., Perlis, M. L., & Spiegelhalder, K. (2015). The neurobiology, investigation, and treatment of chronic insomnia. The Lancet Neurology, 14(5), 547–558. https://doi.org/10.1016/S1474-4422(15)00021-6
- Robertson, E., Grace, S., Wallington, T., & Stewart, D. E. (2004). Antenatal risk factors for postpartum depression: A synthesis of recent literature. General Hospital Psychiatry, 26(4), 289–295. https://doi.org/10.1016/j.genhosppsych.2004.02.006
- Røsand, G. M. B., Slinning, K., Eberhard-Gran, M., Røysamb, E., & Tambs, K. (2011). Partner relationship satisfaction and maternal emotional distress in early pregnancy. BMC Public Health, 11(1), 161. https://doi.org/10.1186/1471-2458-11-161
- Sandanger, I., Moum, T., Ingebrigtsen, G., Dalgard, O. S., Sørensen, T., & Bruusgaard, D. (1998). Concordance between symptom screening and diagnostic procedure: The Hopkins symptom checklist-25 and the composite international diagnostic interview I. Social Psychiatry and Psychiatric Epidemiology, 33(7), 345–354. https://doi.org/10.1007/s001270050064
- Sedov, I. D., Cameron, E. E., Madigan, S., & Tomfohr-Madsen, L. M. (2018). Sleep quality during pregnancy: A meta-analysis. Sleep Medicine Reviews, 38, 168–176. https://doi.org/10.1016/j.smrv.2017.06.005
- Sharkey, K. M., Pearlstein, T. B., & Carskadon, M. A. (2013). Circadian phase shifts and mood across the perinatal period in women with a history of major depressive disorder: A preliminary communication. Journal of Affective Disorders, 150(3), 1103–1108. https://doi.org/10.1016/J.JAD.2013.04.046
- Singareddy, R., Vgontzas, A. N., Fernandez-Mendoza, J., Liao, D., Calhoun, S., Shaffer, M. L., & Bixler, E. O. (2012). Risk factors for incident chronic insomnia: A general population prospective study. Sleep Medicine, 13(4), 346–353. https://doi.org/10.1016/j.sleep.2011.10.033
- Sivertsen, B., Hysing, M., Dørheim, S. K., & Eberhard-Gran, M. (2015). Trajectories of maternal sleep problems before and after childbirth: A longitudinal population-based study. BMC Pregnancy and Childbirth, 15(1), 1–8. https://doi.org/10.1186/s12884-015-0577-1
- Sivertsen, B., Salo, P., Mykletun, A., Hysing, M., Pallesen, S., Krokstad, S., … Øverland, S. (2012). The bidirectional association between depression and insomnia: The HUNT study. Psychosomatic Medicine, 74(7), 758–765. https://doi.org/10.1097/PSY.0b013e3182648619
- Swanson, L. M., Pickett, S. M., Flynn, H., & Armitage, R. (2011). Relationships among depression, anxiety, and insomnia symptoms in perinatal women seeking mental health treatment. Journal of Women’s Health, 20(4), 553–558. https://doi.org/10.1089/jwh.2010.2371
- Swearingen, E. M., & Cohen, L. H. (1985). Measurement of adolescents’ life events: The junior high life experiences survey. American Journal of Community Psychology, 13(1), 69–85. https://doi.org/10.1007/BF00923260
- Tomfohr-Madsen, L. M., Clayborne, Z. M., Rouleau, C. R., & Campbell, T. S. (2017). Sleeping for two: An open-pilot study of cognitive behavioral therapy for insomnia in pregnancy. Behavioral Sleep Medicine, 15(5), 377–393. https://doi.org/10.1080/15402002.2016.1141769
- Underwood, L., Waldie, K., D’Souza, S., Peterson, E. R., & Morton, S. (2016). A review of longitudinal studies on antenatal and postnatal depression. Archives of Women’s Mental Health, 19(5), 711–720. https://doi.org/10.1007/s00737-016-0629-1
- Veijola, J., Jokelainen, J., La, K., Joukamaa, M., Kokkonen, P., & Ja, M. (2003). The Hopkins symptom checklist-25 in screening DSM-III-R axis-I disorders. Nordic Journal of Psychiatry, 57(2), 119–123. https://doi.org/10.1080/08039480310000941
- Wassing, R., Benjamins, J. S., Talamini, L. M., Schalkwijk, F., & Van Someren, E. J. W. (2018, December). Overnight worsening of emotional distress indicates maladaptive sleep in insomnia. Sleep, 1–8. https://doi.org/10.1093/sleep/zsy268
- Waters, M. A., & Lee, K. A. (1996). Differences between primigravidae and multigravidae mothers in sleep disturbances, fatigue, and functional status. Journal of Nurse-midwifery, 41(5), 364–367. https://doi.org/10.1016/S0091-2182(96)00049-3
- Wijma, K., Wijma, B., & Zar, M. (1998). Psychometric aspects of the W-DEQ; A new questionnaire for the measurement of fear of childbirth. Journal of Psychosomatic Obstetrics and Gynaecology, 19(2), 84–97. https://doi.org/10.3109/01674829809048501
- Wisner, K. L., Sit, D. K. Y., McShea, M. C., Rizzo, D. M., Zoretich, R. A., Hughes, C. L., … Hanusa, B. H. (2013). Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry, 70(5), 490–498. https://doi.org/10.1001/jamapsychiatry.2013.87