Abstract
Often considered an aggravating but otherwise benign component of chronic obstructive pulmonary disease (COPD), airway mucus hypersecretion is now recognised as a potential risk factor for an accelerated loss of lung function in COPD and is a key pathophysiological feature in many patients, particularly those prone to respiratory tract infection. Consequently, it is important to develop drugs that inhibit mucus hypersecretion in these susceptible patients. Conventional therapy including anticholinergics, β2-adrenoceoptor agonists, alone or in combination with corticosteroids, mucolytics and macrolide antibiotics are not entirely or consistently effective in inhibiting airway mucus hypersecretion in COPD. Novel pharmacotherapeutic targets are being investigated, including inhibitors of nerve activity (e.g., BKCa channel activators), tachykinin receptor antagonists, epoxygenase inducers (e.g., benzafibrate), inhibitors of mucin exocytosis (e.g., anti-MARCKS peptide and Munc-18B blockers), inhibitors of mucin synthesis and goblet cell hyperplasia (e.g., EGF receptor tyrosine kinase inhibitors, p38 MAP kinase inhibitors, MEK/ERK inhibitors, hCACL2 blockers and retinoic acid receptor-α antagonists), inducers of goblet cell apoptosis (e.g., Bax inducers or Bcl-2 inhibitors), and purinoceptor P2Y2 antagonists to inhibit mucin secretion or P2Y2 agonists to hydrate secretions. However, real and theoretical differences delineate the mucus hypersecretory phenotype in COPD from that in other hypersecretory diseases of the airways. More information is required on these differences to identify therapeutic targets pertinent to COPD which, in turn, should lead to rational design of anti-hypersecretory drugs for specific treatment of airway mucus hypersecretion in COPD.
Introduction
Airway mucus hypersecretion was steadily demoted to an aggravating but otherwise benign component of chronic obstructive pulmonary disease (COPD) Citation[1&2]. Thus, although included in earlier descriptions of COPD Citation[3&4], the term “mucus hypersecretion” is omitted from more recent definitions Citation[5&6]. However, new epidemiological studies demonstrate that mucus is far from innocent. Consequently, airway mucus hypersecretion is now recognised as a potential risk factor for an accelerated loss of lung function in COPD and is a key pathophysiological feature in many patients (). The inclusion of recommendations for mucolytic (i.e., mucus “lysis”) therapy in the latest guidelines for clinical management of COPD Citation[6], in contrast to the lack of such recommendations in guidelines of only 4 years ago Citation[5], is testament to our rapidly changing view of the role of mucus in the pathophysiology of COPD.
Figure 1 Airway mucus hypersecretion in COPD. A) Gross pathology. Luminal mucus (M) partially blocking an extrapulmonary bronchus in a cigarette smoker with chronic sputum production. B) Histopathology: Luminal mucus (M) occluding a small airway in a patient with COPD.
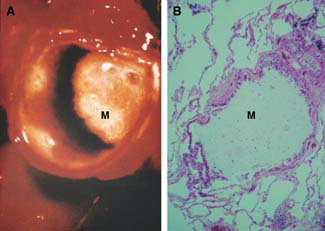
Mucus hypersecretion (commonly associated with the term chronic bronchitis) is 1 of 3 pathophysiological entities that comprise COPD, the other 2 being chronic bronchiolitis (often termed small airways disease) and emphysema (characterised by alveolar destruction and airspace enlargement) Citation[5]Citation[7]. The relative contribution of each component to pathophysiology varies between patients, with the impact of mucus hypersecretion on clinical symptoms varying accordingly. In many patients, airway hypersecretion has clinical significance, for example in patients with low lung function or who are prone to chest infections Citation[8] (). Consequently, it is important to understand the pathophysiology of mucus hypersecretion in COPD. This in turn should allow identification of therapeutic targets and rational development of pharmacotherapeutic drugs. It should be noted, however, that mucus hypersecretion in response to airway irritation, inflammation or infection is a physiologically protective response, with a balance between beneficial excess secretion and detrimental secretion retention. However, with continued hypersecretion there is presumably a point after which the secretions become excessive and, instead of being protective, become detrimental to airway homeostasis. Consequently, drugs that inhibited the excess secretion but retained the “normal” protective amount of secretion might have benefit over drugs that dried up secretions completely.
Figure 2 Putative schemas for airway mucus pathophysiology in COPD. A) Impact of mucus hypersecretion on lung function. B) ‘Vicious circle’ of mucus hypersecretion and bacterial infection.
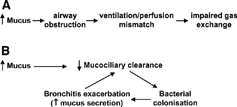
The present article: 1) assesses the contribution of airway mucus hypersecretion and impaired mucociliary clearance to pathophysiology of the “bronchitic” component of COPD, 2) considers the epidemiology and changing view of the clinical impact of mucus hypersecretion in COPD, and 3) briefly discusses current therapy and outlines potential novel therapy for this condition. To set these issues in context, the following section gives a condensed summary of airway mucus, mucins and mucin (MUC) genes.
Airway Mucus, Mucins and MUC Genes
In healthy individuals, a film of slimy liquid overlies and protects the airway surface Citation[9]. The liquid is often referred to as “mucus” and is a complex, non-homogeneous, dilute (1–2%) aqueous solution of salts, enzymes and anti-enzymes, oxidants and antioxidants, bacterial products, antibacterial agents, cell-derived mediators and proteins, plasma-derived mediators and proteins, and cell debris such as DNA. The mucus forms a bilayer comprising an upper gel layer and a lower sol layer. A thin layer of surfactant appears to separate the gel and sol Citation[10&11]. Cilia beat in the sol layer, often termed periciliary liquid. Inhaled particles are trapped in the gel layer and, by transportation on the tips of beating cilia, are removed from the airways, a process termed mucociliary clearance. Airway mucus requires an optimal combination of viscosity and elasticity for efficient ciliary interaction. Viscoelasticity is conferred primarily by high molecular weight mucous glycoproteins, termed mucins, which comprise up to 2% by weight of the mucus Citation[12]. Airway mucins are primarily produced in, and secreted by, goblet cells in the surface epithelium Citation[13] and by mucous cells in the submucosal glands Citation[14]. Mature mucins are long, thread-like molecules composed of monomers joined end-to-end by disulphide bridges. These threads form a “tangled network” Citation[15] that contributes to the formation of the mucus gel. The mucin monomers comprise a highly glycosylated linear peptide sequence, termed “apomucin,” that is encoded by specific mucin (MUC) genes. Nineteen human MUC genes are reported to date, namely MUC1, 2, 3A, 3B, 4, 5AC, 5B, 6–9, 11–13 and 16–20 Citation[16-21]. Although a number of these genes are expressed in the airways Citation[12], it is the MUC5AC and MUC5B gene products that are the major gel forming mucins in normal respiratory tract secretions Citation[12], although MUC2 may be upregulated in COPD (see below) ().
Figure 3 Putative differences in airway mucus pathophysiology between COPD and asthma. Compared with normal, in COPD there is increased luminal mucus, goblet cell hyperplasia, submucosal gland hypertrophy (with an increased proportion of mucous to serous acini), an increased ratio of mucin (MUC) 5B (low-charge glycoform, lcgf) to MUC5AC, small amounts of MUC2, and respiratory infection (possibly due to reduced bacterial enzymatic shield from reduced serous cell number). Pulmonary inflammation includes macrophages and neutrophils. In asthma, there is increased luminal mucus, epithelial fragility, marked goblet cell hyperplasia, submucosal gland hypertrophy (although without an increased mucous to serous ratio), tethering of mucus to goblet cells, and plasma exudation. Airway inflammation includes T lymphocytes and eosinophils. Many of these differences require more data from greater numbers of subjects.
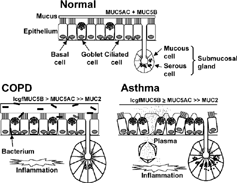
Mucus Hypersecretory Phenotype in COPD
Airway mucus hypersecretion in COPD has characteristic pathophysiological features. A number of these features, for example sputum production and goblet cell hyperplasia, are common to other hypersecretory respiratory diseases, for example asthma and cystic fibrosis (CF). Other features appear to be associated specifically with COPD (see below). Differences in mucus pathophysiology between COPD and asthma have been discussed previously Citation[22], and are summarised in . Presumably, differences in the pulmonary inflammatory “profile” of COPD and asthma (the former essentially a macrophage-driven neutrophilia, the latter a Th2 lymphocyte-driven eosinophilia) Citation[23&24] underlie the differences in hypersecretory phenotype between these two conditions.
Sputum production of up to 100 ml per day in many patients is associated with excessive mucus in the airway lumen ( and ) Citation[25-27]. The increased mucus is associated with goblet cell hyperplasia () Citation[25&26]Citation[28] and submucosal gland hypertrophy () Citation[25&26]Citation[29&30]. Of particular note is that the gland mucous cells are markedly increased relative to the serous cells Citation[31] (see below for pathophysiological implications of this change in gland mucous cell-serous cell ratio). This is in contrast to asthma where the glands, albeit hypertrophied, are morphologically normal. Gland size in COPD correlates with amount of luminal mucus and daily sputum volume Citation[29]Citation[32]. Although not necessarily causal, the latter observation suggests a significant relationship between gland hypertrophy and mucus hypersecretion in COPD.
Figure 4 Airway mucus hypersecretion in COPD. Amount of luminal mucus (mucus occupying ratio, MOR) is significantly greater in both central and distal airways of patients who die with a diagnosis of chronic bronchitis compared with those with emphysema or controls without respiratory disease. L, luminal perimeter; Sb1, size of bronchus before computer-based image analysis conversion to Sb2 (Br, bronchial radius); Sm, size of stained area of mucus. Redrawn after Ref. Citation[26].
![Figure 4 Airway mucus hypersecretion in COPD. Amount of luminal mucus (mucus occupying ratio, MOR) is significantly greater in both central and distal airways of patients who die with a diagnosis of chronic bronchitis compared with those with emphysema or controls without respiratory disease. L, luminal perimeter; Sb1, size of bronchus before computer-based image analysis conversion to Sb2 (Br, bronchial radius); Sm, size of stained area of mucus. Redrawn after Ref. Citation[26].](/cms/asset/8d0c5e49-a759-4231-8976-46934aec8ad1/icop_a_121792_uf0004_b.gif)
Figure 5 Airway submucosal gland hypertrophy in COPD. The percentage of the airway wall occupied by the glands (G) is significantly greater in the airways of patients who die with a diagnosis of chronic bronchitis compared with those with emphysema or controls without respiratory disease. Redrawn after Ref. Citation[26].
![Figure 5 Airway submucosal gland hypertrophy in COPD. The percentage of the airway wall occupied by the glands (G) is significantly greater in the airways of patients who die with a diagnosis of chronic bronchitis compared with those with emphysema or controls without respiratory disease. Redrawn after Ref. Citation[26].](/cms/asset/71fe667f-d969-49de-8c6e-98cb9fb68dc3/icop_a_121792_uf0005_b.gif)
The above features of hypersecretion are not common to all patients with COPD. Not all patients expectorate, and there is overlap in gland size with healthy non-smokers, and also between sputum producers and non-producers Citation[28]Citation[30]Citation[33-35]. Goblet cell hyperplasia is not noted in all patients () Citation[26]Citation[31]. Interestingly, although goblet cell hyperplasia is associated with degree of airway inflammation, gland size is not Citation[28]. Thus, although considered general features of COPD, mucus hypersecretion and its associated pathophysiological abnormalities do not characterise all patients.
Figure 6 Airway goblet cell mucus in COPD: lack of goblet cell hyperplasia? The percentage of the airway epithelium staining for mucus is not significantly different to that in the airways of patients who die with a diagnosis of chronic bronchitis compared with those with emphysema or controls without respiratory disease. Redrawn after Ref. Citation[26].
![Figure 6 Airway goblet cell mucus in COPD: lack of goblet cell hyperplasia? The percentage of the airway epithelium staining for mucus is not significantly different to that in the airways of patients who die with a diagnosis of chronic bronchitis compared with those with emphysema or controls without respiratory disease. Redrawn after Ref. Citation[26].](/cms/asset/89fb7ea8-7c92-4cdb-9c4f-da16550b6eeb/icop_a_121792_uf0006_b.gif)
The mucin composition of airway mucus is abnormal in COPD. Mucins in sputum are less acidic than normal Citation[36], which may reflect disease-related alterations in glycosylation. MUC5AC and a low charge glycoform of MUC5B are the major mucin species in patients with COPD Citation[37-40]. Intriguingly, the low-charge glycoform appears to be proportionally increased above normal levels Citation[41]. This is a potentially significant observation, in terms of altered mucin glycosylation processing in COPD, that requires confirmation, or otherwise, in a greater number of samples. It is possible that the change in MUC5B glycoforms, coupled with the reduction mentioned above Citation[31] in gland serous cells, a rich source of antimicrobial enzymes such as lysozyme and lactoferrin Citation[42], contributes to the airway bacterial infections that are a clinical feature of many COPD patients Citation[5].
In contrast to normal airways, goblet cells in COPD contain not only MUC5AC but also MUC5B Citation[39]Citation[43] and MUC2 Citation[12]. This distribution is different to that in patients with asthma or CF, where MUC5AC and MUC5B show a similar histological distribution to normal controls Citation[44&45]. Although not found consistently Citation[27]Citation[38], there is a growing impression that MUC2 is increased in irritated airways, including COPD Citation[12]Citation[41]Citation[46].
Abnormalties in Mucociliary Clearance in COPD
Abnormal airway mucus in COPD goes in concert with abnormal ciliated cells and cilia. The number of ciliated cells and length of cilia is decreased in patients with chronic bronchitis Citation[47]. Ciliary abnormalities include compound cilia, cilia enclosed within periciliary sheaths, cilia with abnormal axonemes and cilia with intra-cytoplasmic microtubule doublets Citation[48]. These abnormalities coupled with mucus hypersecretion are associated with reduced mucus clearance and airway obstruction Citation[49]. However, differences in methodology Citation[50] and patient selection, especially ensuring exclusion of patients with asthma Citation[51], can confuse interpretation of these studies. Lung clearance is significantly reduced in heavy smokers Citation[52] and in patients with chronic bronchitis Citation[53]. However, it should be noted that forced expirations and cough compensate relatively effectively for decreased mucociliary clearance in patients with chronic bronchitis, although they are much less effective in patients with emphysema where lung elastic recoil is impaired Citation[54&55].
Epidemiology of Mucus Hypersecretion in COPD
The perception of the role of airway mucus hypersecretion in pathophysiology and clinical symptoms in COPD has shifted from being a condition independent of disease progression to now being positively associated with morbidity and mortality Citation[22]Citation[56]. Epidemiological studies sampling many hundreds of subjects in the late 1970s to early 1990s found scant evidence for the involvement of mucus in either the mortality or accelerated age-related decline in lung function associated with COPD Citation[1&2]Citation[57-60]. In all studies, sputum production, assessed by standardised questionnaire, was the index of mucus hypersecretion. However, the relationship between sputum production and mucus hypersecretion, particularly in the small airways, the main site of airflow obstruction in COPD Citation[61], is unclear. It is also noteworthy that these studies were primarily in occupational cohorts, and were exclusively in men. Nevertheless, the consensus of these studies was that airflow obstruction and mucus hypersecretion were largely independent disease processes.
In contrast, a number of studies over the last 18 years, again with large sample number, and with an emphasis on general population samples rather than occupational cohorts, found positive associations between sputum production and decline in lung function, hospitalisation and death Citation[62-70]. Some of these reports were re-examinations of the same patients, now older, reported previously Citation[62]. Of note is the observation that incidence of death was related to increased risk in patients with phlegm production to die of respiratory infection Citation[8]. Additionally, the association between chronic mucus hypersecretion and frequency of lower respiratory illness extends to an association with an accelerated decline in lung function with successive bouts of pulmonary infection Citation[71]. In summary, although not associated with disease progression in all cases, mucus hypersecretion contributes to morbidity and mortality in many patients with COPD, particularly those prone to infection, those with low lung function Citation[63] and, possibly, as patients age. This highlights the importance of developing drugs that inhibit mucus hypersecretion in these patients.
Pharmacotherapy of Mucus Hypersecretion in COPD
As discussed before, airway mucus contributes to morbidity and mortality in patients in whom the mucus hypersecretory phenotype impacts significantly on pathophysiology and clinical status. Consequently, drugs affecting the bronchitic component of COPD should be beneficial in these patients. However, COPD has specific trigger factors, profile of pulmonary inflammation and mucus hypersecretory phenotype (), and specific drugs may be required to fulfil the theoretical requirements for treatment of hypersecretion in COPD (). The following sections give a condensed summary of different approaches to inhibition of mucus hypersecretion in COPD, starting with conventional pharmacotherapy and followed by consideration of the wide range of potential novel pharmacotherapeutic approaches. It should be noted that drugs that suppress lung inflammation should also indirectly suppress mucus hypersecretion and are, therefore, possibly the most beneficial therapy overall ().
Table 1. Theoretical objectives for pharmacotherapy of mucus hypersecretory pathophysiology in COPD
Figure 7 Relationship between neutrophilic inflammation in COPD and generation of a hypersecretory phenotype (e.g., goblet cell hyperplasia). Interactions between inhaled pollutants (e.g., cigarette smoke), macrophages and epithelial cells generate neutrophil chemoattractants, with resultant release of factors that induce mucin synthesis, goblet cell hyperplasia and mucus hypersecretion. The sequence of initiating events can be inhibited at different levels of the inflammatory pathway. PDE, phosphodiesterase.
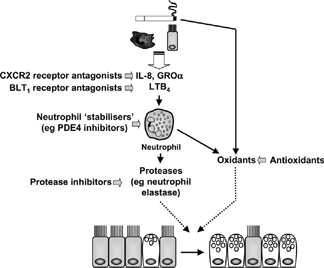
Conventional Pharmacotherapy
The medications currently used in clinical management of COPD, namely bronchodilators (anticholinergics, β2-adrenoceptor agonists and methylxanthines) and anti-inflammatories, primarily glucocorticosteroids Citation[5], are not administered necessarily to target airway hypersecretion, but may nevertheless exert some of their beneficial effects via actions on mucus. The activity of these drugs on mucociliary dysfunction in COPD has been recently reviewed in detail Citation[72]. Theoretically, anticholinergics are likely to have beneficial effects on mucociliary function, but clinically these effects have been difficult to demonstrate. Long-acting β2-agonists (LABAs), rather than short-acting β2-agonists, might improve the mucociliary component of COPD, in addition to providing symptomatic treatment by their bronchodilator action.
Suppression of inflammation should indirectly suppress mucus hypersecretion. Anti-inflammatory drugs also directly affect the mucus hypersecretory phenotype. For example, in experimental systems, glucocorticosteroids inhibit mucus secretion, MUC gene expression, mucus synthesis and goblet cell hyperplasia Citation[73]. However, in contrast to asthma where they are clinically effective Citation[74], in part due to an anti-hypersecretory action, glucocorticoids have limited effectiveness in stable COPD Citation[5]. This limited effectiveness appears to be due to a relative lack of effect of corticosteroids on the pulmonary inflammation in COPD Citation[75&76]. A limited effect on pulmonary inflammation will, therefore, hinder suppression of the component of hypersecretion that is inflammation driven (for example, by neutrophil elastase). In addition, it may be that the COPD-specific aspects of mucus hypersecretion (for example, increased proportion of gland mucous cells) are similarly limited in their response to corticosteroids. However, it should be noted that combination therapy with a LABA and an inhaled glucocorticosteroid may address the multifactorial nature of COPD by providing not only bronchodilation but also anti-inflammatory activity, which may indirectly further improve mucociliary clearance.
Mucolytics and/or Anti-Oxidants
Decreasing the viscosity of, or “thinning”, viscous airway mucus with mucolytic drugs should be a way of improving mucus clearance, both by mucociliary transport and by cough. This last could be aided by use of expectorant drugs, some of which may in fact increase secretion temporarily to aid the effectiveness of cough in dislodging and expelling mucus. However, although numerous mucolytic drugs are available worldwide, their effectiveness in treatment of stable COPD has not been established Citation[77]. In addition, there are safety issues with a number of mucolytic preparations, for example iodinated glycerol. Consequently, mucolytics are not generally recommended in current guidelines on clinical management of COPD Citation[5]. However, last year's NICE guideline recommended that mucolytic therapy should be considered in patients with a chronic productive cough, and that mucolytic therapy be continued if there was symptomatic improvement in cough and sputum production Citation[6].
These recommendations were based upon data from 3 rigorous meta-analyses that found that treatment for at least 2 months with certain mucolytic drugs, with a heavy bias towards N-acetylcysteine, reduced the number of exacerbations and days of illness Citation[78-80]. The reduction in exacerbations leads to reductions in both direct hospital costs and indirect costs due to sick leave from work. It has been calculated that, in the Swiss health care system, when cost of treatment is balanced against direct and indirect costs, treatment becomes cost effective in patients who would be expected to have 1 exacerbation during the winter months (approximately 6-month interval), and increases proportionally with severity of disease Citation[81].
However, it is not clear whether the beneficial effects of a number of these drugs, in particular N-acetylcysteine Citation[82], are due to their mucolytic or anti-oxidant properties (or both). Nevertheless, there is continuing interest in mucolytic-anti-oxidant compounds for treatment of COPD. For example, a recent clinical trial of the thiol derivative erdosteine Citation[83] found that 8 months' treatment of patients with moderate COPD reduced exacerbation and hospitalization rates and improved health status. The authors suggested that erdosteine is likely to make an important contribution to therapy of patients with symptomatic COPD.
Oxidant stress is considered a pathophysiological feature of COPD Citation[84] and nitric oxide (NO) is elevated in COPD Citation[85]. Oxidants and NO have marked effects on airway mucus and goblet cells Citation[86&87]. Consequently, anti-oxidants and inhibitors of inducible NO synthase (iNOS) may have therapeutic benefit for mucus hypersecretion in COPD. However, at present, the NICE guidelines do not recommend use of the anti-oxidants alpha-tocopherol or beta-carotene in clinical management of COPD Citation[6]. Clearly, suitable clinical investigations with more potent anti-oxidants are warranted.
Macrolide Antibiotics
Although antibiotics are recommended in clinical management of exacerbations of COPD, there is no evidence to recommend prophylactic antibiotic treatment in management of stable COPD Citation[5&6]. However, erythromycin, clarithromycin and flurythromycin are macrolide antibiotics that have a variety of beneficial effects on airway mucus, for example inhibition of mucin secretion in a variety of experimental preparations including human airways in vitro Citation[22]Citation[88]. Anecdotally, erythromycin reduces excessive sputum production in patients with airway mucus hypersecretion Citation[89&90]. The mechanism of action of erythromycin is relatively unexplored, but may involve anti-inflammatory effects Citation[91&92] as well as direct inhibitory effects on MUC gene expression, mucin synthesis and mucin secretion Citation[93]. Formal clinical studies of its effects on the pathophysiology of mucus hypersecretion, for example sputum production and lung function, in COPD would be of interest.
Novel Pharmacotherapy
The clinical symptoms of cough and sputum production, coupled with a perception of the importance of mucus hypersecretion in the pathophysiology of a number of severe lung conditions, including COPD, has prompted renewed interest in research into airway hypersecretion and, in concert, in development of drugs targeting mucus and the hypersecretory phenotype in COPD. These drugs and their purported targets have been recently discussed in detail Citation[22]Citation[94&95], and will be only briefly highlighted herein (). It should be noted that the activity of many of these compounds is not as selective for the target as may be thought and, in any event, whether or not any beneficial activity of the drug is due to activity at the target is, for the most part, substantially unproven.
Table 2. Novel targets for inhibition of the mucus hypersecretory phenotype in COPD
Inhibition of Nerve Activity
There are novel options for inhibiting the effects of cholinergic nerves Citation[100], none of which are used clinically. These include inhibition of neurotransmitter (acetylcholine) release by activation of prejunctional receptors, for example opioid μ and δ receptors, cannabinoid CB2 receptors or vasoactive intestinal peptide VPAC1 receptors, and activation of large conductance calcium-activated potassium (BKCa) channels (). There is also the possibility that sensory nerve activation may mediate mucus output in COPD Citation[101]. These nerves can also be inhibited in a similar fashion to cholinergic nerves (see above), and also by inhibition of the effects of their tachykinin neurotransmitters, including substance P and neurokinin A, by tachykinin receptor antagonists Citation[102]. The vanilloid VR-1 receptor mediates activation of sensory nerves and selective VR-1 antagonists, such as capsazepine, are in development Citation[103].
Figure 8 Pharmacotherapy of airway mucus hypersecretion in COPD. The pathophysiological ‘cascade’ from initiating factors to clinical symptoms can be accessed at different levels by ‘antihypersecretory’ pharmacotherapeutic compounds. The precise site(s) of action of many compounds is unclear, and some compounds may act at more than one site. hCLCA, human calcium-activated chloride channel; COX, cyclooxygenase; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; MARCKS, myristoylated alanine-rich C kinase substrate; MEK, mitogen-activated protein kinase kinase; MUC, mucin (gene); NKCC, Na+-K+-Cl− cotransporter; PI-3K, phosphatidylinositol 3-kinase; RAR, retinoic acid receptor.
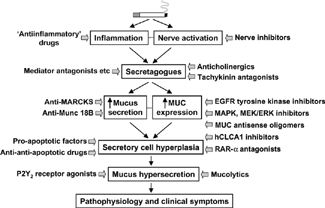
Epoxygenase Inducers
Epoxygenases (cytochrome P-450 enzymes) metabolise arachidonic acid and regulate inflammation Citation[104]. Benzafibrate, an inducer of epoxygenase, inhibits airway goblet cell hyperplasia in a rat model of chronic bronchitis Citation[105]. The mechanisms underlying the inhibition include production of anti-inflammatory mediators and reduction in amount of available arachidonic acid. Epoxygenase inducers, or selective eopxyeicosanoids, would be potential therapy for both the inflammation and mucus hypersecretion of COPD.
Inhibitors of Mucin Exocytosis
Inhibition of mucin exocytosis is a therapeutic option for mucus hypersecretion in COPD. However, it should be noted that inhibition of secretion could lead to excessive accumulation of intracellular mucins, with unknown, and potentially detrimental, effects on secretory cell function. Myristoylated alanine-rich C kinase substrate (MARCKS) protein is a key intracellular molecule involved in intracellular movement and exocytosis of mucin granules Citation[106]. Inhibition of MARCKS production by an antisense oligonucleotide down-regulated both mRNA and protein levels of MARCKS and attenuated mucin secretion. Blockade of MARCKS by a synthetic peptide to its N-terminal region (MANS peptide) inhibited mucin secretion by normal human bronchial epithelial cells in vitro Citation[106] and by mouse airway epithelium in vivo Citation[107]. Similar to MARCKS, the Sec1/Munc18 family are critical to exocytosis in airway goblet cells. Experimental induction of Munc18B induces a marked airway hypersecretory phenotype Citation[108]. Inhibition of Munc18B using antisense technology is under investigation.
Inhibitors of Goblet Cell Hyperplasia
Increased MUC gene expression, mucin synthesis and goblet cell hyperplasia appear to be linked processes that are regulated by a number of inflammatory mechanisms. For example, airway epidermal growth factor receptor (EGF-R) expression is induced by experimental procedures pertinent to COPD Citation[87]. It appears that long-term cigarette smoking induces enhanced expression of EGF-R, as well as ErbB3 (another member of the EGF-R family) and MUC5AC Citation[109]. In experimental systems, EGF-R upregulation and signalling via EGF-R tyrosine kinase is a signalling event for induction of mucin synthesis and goblet cell hyperplasia. Inhibitors of EGF-R tyrosine kinase block these responses. One of these, gefitinib (ZD1839, also known as Iressa), is in clinical trial for cancer, but not yet for COPD or similar respiratory diseases.
Unsurprisingly, the p38 mitogen activated protein (MAP) kinase pathway, the MEK/ERK pathway, and the phosphatidylinositol 3-kinase pathway are all involved, to a greater or lesser extent, in intracellular events leading to mucin synthesis and goblet cell hyperplasia Citation[110-113]. Inhibitors of these pathways inhibit mucus hypersecretory endpoints in experimental systems.
Calcium-activated chloride (CLCA) channels also appear to be critically involved in development of an airway hypersecretory phenotype Citation[114]. In mice, suppression of mCLCA3 inhibits goblet cell hyperplasia, whilst overexpression increases goblet cell number Citation[115]. Talniflumate (MSI 1956 or ‘Lomucin’) is a small molecule putative inhibitor of hCLCA1 originally developed by Laboratorios Bago which is currently being developed by Genaera as a mucoregulatory treatment for asthma, CF and COPD Citation[116]. Phase I clinical trials were completed in 2001 and phase II trials in CF are underway in Ireland. The results of these trials are awaited with great interest.
Retinoic acid (vitamin A) is perceived to be of clinical benefit in a variety of clinical conditions. Agonists at the retinoic acid RAR-γ are currently being intensely investigated as inhibitors and ‘reversers’ of alveolar destruction in emphysema Citation[117]. In contrast, the RAR-α receptor appears to be involved in mucin expression Citation[118-120] and in the development and maintenance of a hypersecretory phenotype Citation[120]. RAR-α antagonists such as RO-41-5253 inhibit a number of these activities Citation[121]. Consequently, there is interest in development of selective RAR-α antagonists for mucus hypersecretion in a number of respiratory diseases, including COPD Citation[117].
Finally, antisense technology is also being explored as a new approach to inhibition of goblet cell hyperplasia. For example, an 18-mer MUC antisense oligomer suppressed mucin gene expression and in wood smoke-induced epithelial metaplasia in rabbit airways Citation[122].
Inducers of Goblet Cell Apoptosis
Hyperplastic airway goblet cells in COPD models express the anti-apoptotic factor Bcl-2 Citation[123]. Conversely, the pro-apoptotic factor Bax is crucial for resolution of hyperplasia. Thus, the balance between Bcl-2 and Bax may affect the persistence of goblet cell hyperplasia. Reduction of Bcl-2 expression by antisense oligonucleotides induces a dose-dependent resolution of hyperplasia.
Mucus Inhibition vs. Mucus Hydration
The purine nucleotides, adenosine 5′-triphosphate (ATP) and uridine triphosphate (UTP), increase airway mucin and water secretion via interaction with P2Y2 purinoceptors Citation[124&125]. Consequently, P2Y2 antagonists might be effective in inhibiting airway hypersecretion Citation[126]. However, mucus hydration is associated with improvements in mucociliary clearance and stimulation of water secretion may have greater therapeutic potential than inhibition of P2Y2-mediated mucin secretion Citation[9]. Consequently, there is considerable interest in development of P2Y2 agonists. In phase I clinical trial, a second generation P2Y2 agonist, INS365, was safe, well tolerated and significantly enhanced sputum expectoration Citation[124]. However, uncontrolled thinning of airway mucus may have adverse clinical effects.
Summary and Conclusions
Airway mucus hypersecretion and the pathophysiological changes that accompany it, for example goblet cell hyperplasia, are features of many patients with COPD. The impact of airway hypersecretion on morbidity and mortality is now more fully understood, albeit that it may be limited to certain groups of patients, particularly those who are prone to respiratory tract infection. Nevertheless, it is important to develop drugs that inhibit mucus hypersecretion in these susceptible patients. However, before addressing these issues in a rational manner, considerably more information is required on basic mucus physiology and, in particular, mucus pathophysiology. For example, more detail is required concerning the biochemical and biophysical nature of airway mucins in normal healthy subjects. Answers to the questions of whether or not there is an intrinsic abnormality of mucus in COPD, and whether any abnormality is specific for COPD are urgently required. In addition, the factors that regulate MUC gene expression in health and disease, and the relationship between this regulation and development of a hypersecretory phenotype that appears to be specific to the bronchitic component of COPD, need to be determined. The above information could then be used in delineation of therapeutic targets which, in turn, should lead to rational design of anti-hypersecretory drugs for specific treatment of airway mucus hypersecretion in COPD. At present, drugs that suppress lung inflammation should also suppress mucus hypersecretion and may be the most beneficial therapy overall. Of the more selective molecules currently under investigation, it would appear that the EGF signalling cascade mediates generation of the hypersecretory phenotype in a wide variety of experimental preparations and is upregulated in COPD. Consequently, inhibitors of EGF signalling may be prime contenders as antihypersecretory pharmacotherapy. However, considerably more information is required concerning which specific EGF receptors are involved in airway mucus hypersecretion, and whether selective single or dual (or multiple) inhibitors will be most effective.
References
- Fletcher C, Peto R. The natural history of chronic airflow obstruction.Br Med J 1977; 1:1645–1648. [PUBMED], [INFOTRIEVE], [CSA]
- Peto R, Speizer F E, Cochrane A L, Moore F, Fletcher C M, Tinker C M, Higgins I T, Gray R G, Richards S M, Gilliland J, Norman-Smith B. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation.Am Rev Respir Dis 1983; 128:491–500. [PUBMED], [INFOTRIEVE], [CSA]
- Fletcher C M, Pride N B. Definitions of emphysema, chronic bronchitis, asthma, and airflow obstruction: 25 years on from the Ciba symposium.Thorax 1984; 39:81–85. [PUBMED], [INFOTRIEVE], [CSA]
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma.Am Rev Respir Dis 1987; 136:225–244. [CSA]
- National Heart Lung and Blood Institute/WHO. Global Initiative for Chronic Obstructive Lung Disease. Publication No. 2701, National Institutes of Health, 2001.
- National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax 2004; 59. [CSA]
- Hogg J C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease.Lancet 2004; 364:709–721. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection.Eur Respir J 1995; 8:1333–1338. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Knowles M R, Boucher R C. Mucus clearance as a primary innate defense mechanism for mammalian airways.J Clin Invest 2002; 109:571–577. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Morgenroth K, Bolz J. Morphological features of the interaction between mucus and surfactant on the bronchial mucosa.Respiration 1985; 47:225–231. [PUBMED], [INFOTRIEVE], [CSA]
- Schurch S, Gehr P, Im Hof V, Geiser M, Green F. Surfactant displaces particles toward the epithelium in airways and alveoli.Respir Physiol 1990; 80:17–32. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Davies J R, Herrmann A, Russell W, Svitacheva N, Wickström C, Carlstedt I. Respiratory tract mucins: structure and expression patterns. In: Mucus Hypersecretion in Respiratory Disease. Chichester: John Wiley & Sons, 2002:76–93.
- Rogers D F. The airway goblet cell.Int.J.Biochem.Cell Biol. 2003; 35:1–6. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Finkbeiner W E. Physiology and pathology of tracheobronchial glands.Respir Physiol 1999; 118:77–83. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Verdugo P. Molecular biophysics of mucin secretion. In: Airway Secretion. Takishima T, Shimura S, eds. New York: Marcel Dekker, 1994:101–121.
- Dekker J, Rossen J W, Buller H A, Einerhand A W. The MUC family: an obituary.Trends Biochem Sci 2002; 27:126–131. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gum J R Jr, Crawley S C, Hicks J W, Szymkowski D E, Kim Y S. MUC17, a novel membrane-tethered mucin.Biochem Biophys Res Commun 2002; 291:466–475. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Moniaux N, Escande F, Porchet N, Aubert J P, Batra S K. Structural organization and classification of the human mucin genes.Front Biosci 2001; 6:D1192–D1206. [PUBMED], [INFOTRIEVE], [CSA]
- Wu G J, Wu M W, Wang S W, Liu Z, Qu P, Peng Q, Yang H, Varma V A, Sun Q C, Petros J A, Lim S D, Amin M B. Isolation and characterization of the major form of human MUC18 cDNA gene and correlation of MUC18 over-expression in prostate cancer cell lines and tissues with malignant progression.Gene 2001; 279:17–31. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chen Y, Zhao Y H, Kalaslavadi T B, Hamati E, Nehrke K, Le A D, Ann D K, Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues.Am J Respir Cell Mol Biol 2004; 30:155–165. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, Sun H W, Falk R J, Jennette J C, Alcorta D A, Li H, Yamamoto T, Saito Y, Nakamura M. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney.J Biol Chem 2004; 279:1968–1979. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rogers D F. Mucus pathophysiology in COPD: differences to asthma, and pharmacotherapy.Monaldi Arch Chest Dis 2000; 55:324–332. [PUBMED], [INFOTRIEVE], [CSA]
- Jeffery P K. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture.Chest 2000; 117:251S–260S. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Barnes P J. Mediators of chronic obstructive pulmonary disease.Pharmacol Rev 2004; 56:515–548. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Reid L. Pathology of chronic bronchitis.Lancet 1954; i:275–278. [CSA], [CROSSREF]
- Aikawa T, Shimura S, Sasaki H, Takishima T, Yaegashi H, Takahashi T. Morphometric analysis of intraluminal mucus in airways in chronic obstructive pulmonary disease.Am Rev Respir Dis 1989; 140:477–482. [PUBMED], [INFOTRIEVE], [CSA]
- Steiger D, Fahy J, Boushey H, Finkbeiner W E, Basbaum C. Use of mucin antibodies and cDNA probes to quantify hypersecretion in vivo in human airways.Am J Respir Cell Mol Biol 1994; 10:538–545. [PUBMED], [INFOTRIEVE], [CSA]
- Mullen J B, Wright J L, Wiggs B R, Pare P D, Hogg J C. Structure of central airways in current smokers and ex-smokers with and without mucus hypersecretion: relationship to lung function.Thorax 1987; 42:843–848. [PUBMED], [INFOTRIEVE], [CSA]
- Reid L. Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis.Thorax 1960; 15:132–141. [PUBMED], [INFOTRIEVE], [CSA]
- Restrepo G, Heard B E. The size of the bronchial glands in chronic bronchitis.J Pathol Bacteriol 1963; 85:305–310. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Glynn A A, Michaels L. Bronchial biopsy in chronic bronchitis and asthma.Thorax 1960; 15:142–153. [CSA]
- Jamal K, Cooney T P, Fleetham J A, Thurlbeck W M. Chronic bronchitis. Correlation of morphologic findings to sputum production and flow rates.Am Rev Respir Dis 1984; 129:719–722. [PUBMED], [INFOTRIEVE], [CSA]
- Hayes J A. Distribution of bronchial gland measurement in a Jamaican population.Thorax 1960; 24:619–622. [CSA]
- Thurlbeck W M, Angus C W, Paré J AP. Mucous gland hypertrophy in chronic bronchitis, and its occurrence in smokers.Br J Dis Chest 1963; 57:73–78. [PUBMED], [INFOTRIEVE], [CSA]
- Thurlbeck W M, Angus G E. A distribution curve for chronic bronchitis.Thorax 1964; 19:436–442. [PUBMED], [INFOTRIEVE], [CSA]
- Davies J R, Hovenberg H W, Linden C J, Howard R, Richardson P S, Sheehan J K, Carlstedt I. Mucins in airway secretions from healthy and chronic bronchitic subjects.Biochem J 1996; 313:431–439. [PUBMED], [INFOTRIEVE], [CSA]
- Thornton D J, Carlstedt I, Howard M, Devine P L, Price M R, Sheehan J K. Respiratory mucins: identification of core proteins and glycoforms.Biochem J 1996; 316:967–975. [PUBMED], [INFOTRIEVE], [CSA]
- Hovenberg H W, Davies J R, Herrmann A, Linden C J, Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions.Glycoconj J 1996; 13:839–847. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wickstrom C, Davies J R, Eriksen G V, Veerman E C, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage.Biochem J 1998; 334:685–693. [PUBMED], [INFOTRIEVE], [CSA]
- Sheehan J K, Howard M, Richardson P S, Longwill T, Thornton D J. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug.Biochem J 1999; 338:507–513. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kirkham S, Sheehan J K, Knight D, Richardson P S, Thornton D J. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B.Biochem J 2002; 361:537–546. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Basbaum C B, Jany B, Finkbeiner W E. The serous cell.Annu Rev Physiol 1990; 52:97–113. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chen Y, Zhao Y H, Di Y P, Wu R. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino-terminal and 5′-flanking region.Am J Respir Cell Mol Biol 2001; 25:542–553. [PUBMED], [INFOTRIEVE], [CSA]
- Groneberg D A, Eynott P R, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson A G, Harrison B D, Chung K F. Expression of respiratory mucins in fatal status asthmaticus and mild asthma.Histopathology 2002; 40:367–373. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Groneberg D A, Eynott P R, Oates T, Lim S, Wu R, Carlstedt I, Nicholson A G, Chung K F. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung.Respir Med 2002; 96:81–86. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Davies J R, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions.Biochem J 1999; 344(Pt 2):321–330. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wanner A. Clinical aspects of mucociliary transport.Am Rev Respir Dis 1977; 116:73–125. [PUBMED], [INFOTRIEVE], [CSA]
- McDowell E M, Barrett L A, Harris C C, Trump B F. Abnormal cilia in human bronchial epithelium.Arch Pathol Lab Med 1976; 100:429–436. [PUBMED], [INFOTRIEVE], [CSA]
- Wanner A, Salathe M, O'Riordan T G. Mucociliary clearance in the airways.Am J Respir Crit Care Med 1996; 154:1868–1902. [PUBMED], [INFOTRIEVE], [CSA]
- Pavia D, Agnew J E, Glassman J M, Sutton P P, Lopez-Vidriero M T, Soyka J P, Clarke S W. Effects of iodopropylidene glycerol on tracheobronchial clearance in stable, chronic bronchitic patients.Eur J Respir Dis 1985; 67:177–184. [PUBMED], [INFOTRIEVE], [CSA]
- Moretti M, Lopez-Vidriero M T, Pavia D, Clarke S W. Relationship between bronchial reversibility and tracheobronchial clearance in patients with chronic bronchitis.Thorax 1997; 52:176–180. [PUBMED], [INFOTRIEVE], [CSA]
- Goodman R M, Yergin B M, Landa J F, Golivanux M H, Sackner M A. Relationship of smoking history and pulmonary function tests to tracheal mucous velocity in nonsmokers, young smokers, ex-smokers, and patients with chronic bronchitis.Am Rev Respir Dis 1978; 117:205–214. [PUBMED], [INFOTRIEVE], [CSA]
- Agnew J E, Little F, Pavia D, Clarke S W. Mucus clearance from the airways in chronic bronchitis—smokers and ex-smokers.Bull Eur Physiopathol Respir 1982; 18:473–484. [PUBMED], [INFOTRIEVE], [CSA]
- van der Schans C P, Piers D A, Beekhuis H, Koeter G H, van der Mark T W, Postma D S. Effect of forced expirations on mucus clearance in patients with chronic airflow obstruction: effect of lung recoil pressure.Thorax 1990; 45:623–627. [PUBMED], [INFOTRIEVE], [CSA]
- Ericsson C H, Svartengren K, Svartengren M, Mossberg B, Philipson K, Blomquist M, Camner P. Repeatability of airway deposition and tracheobronchial clearance rate over three days in chronic bronchitis.Eur Respir J 1995; 8:1886–1893. [PUBMED], [INFOTRIEVE], [CSA]
- Vestbo J. Epidemiological studies in mucus hypersecretion.Novartis Found Symp 2002; 248:3–12. [PUBMED], [INFOTRIEVE], [CSA]
- Kauffmann F, Drouet D, Lellouch J, Brille D. Twelve years spirometric changes among Paris area workers.Int J Epidemiol 1979; 8:201–212. [PUBMED], [INFOTRIEVE], [CSA]
- Higgins M W, Keller J B, Becker M, Howatt W, Landis J R, Rotman H, Weg J G, Higgins I. An index of risk for obstructive airways disease.Am Rev Respir Dis 1982; 125:144–151. [PUBMED], [INFOTRIEVE], [CSA]
- Ebi-Kryston K L. Respiratory symptoms and pulmonary function as predictors of 10-year mortality from respiratory disease, cardiovascular disease, and all causes in the Whitehall Study.J Clin Epidemiol 1988; 41:251–260. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wiles F J, Hnizdo E. Relevance of airflow obstruction and mucus hypersecretion to mortality.Respir Med 1991; 85:27–35. [PUBMED], [INFOTRIEVE], [CSA]
- Hogg J C, Macklem P T, Thurlbeck W M. Site and nature of airway obstruction in chronic obstructive lung disease.N Engl J Med 1968; 278:1355–1360. [PUBMED], [INFOTRIEVE], [CSA]
- Annesi I, Kauffmann F. Is respiratory mucus hypersecretion really an innocent disorder? A 22-year mortality survey of 1,061 working men.Am Rev Respir Dis 1986; 134:688–693. [PUBMED], [INFOTRIEVE], [CSA]
- Vollmer W M, McCamant L E, Johnson L R, Buist A S. Respiratory symptoms, lung function, and mortality in a screening center cohort.Am J Epidemiol 1989; 129:1157–1169. [PUBMED], [INFOTRIEVE], [CSA]
- Speizer F E, Fay M E, Dockery D W, Ferris B G Jr. Chronic obstructive pulmonary disease mortality in six U.S. cities.Am Rev Respir Dis 1989; 140:S49–S55. [PUBMED], [INFOTRIEVE], [CSA]
- Vestbo J, Rasmussen F V. Respiratory symptoms and FEV1 as predictors of hospitalization and medication in the following 12 years due to respiratory disease.Eur Respir J 1989; 2:710–715. [PUBMED], [INFOTRIEVE], [CSA]
- Vestbo J, Knudsen K M, Rasmussen F V. The value of mucus hypersecretion as a predictor of mortality and hospitalization. An 11-year register based follow-up study of a random population sample of 876 men.Respir Med 1989; 83:207–211. [PUBMED], [INFOTRIEVE], [CSA]
- Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Relation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causes.Thorax 1990; 45:579–585. [PUBMED], [INFOTRIEVE], [CSA]
- Sherman C B, Xu X, Speizer F E, Ferris B G Jr, Weiss S T, Dockery D W. Longitudinal lung function decline in subjects with respiratory symptoms.Am Rev Respir Dis 1992; 146:855–859. [PUBMED], [INFOTRIEVE], [CSA]
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group.Am J Respir Crit Care Med 1996; 153:1530–1535. [PUBMED], [INFOTRIEVE], [CSA]
- Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F, Segu J L. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group.Respiration 2000; 67:495–501. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kanner R E, Anthonisen N R, Connett J E. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study.Am J Respir Crit Care Med 2001; 164:358–364. [PUBMED], [INFOTRIEVE], [CSA]
- Rogers D F. Mucociliary dysfunction in COPD: effect of current pharmacotherapeutic options.Pulm Pharmacol Ther 2005; 18:1–8. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rogers D F. Airway goblet cells: responsive and adaptable front-line defenders.Eur Respir J 1994; 7:1690–1706. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- National Institutes of Health. Global Initiative for Asthma: Pocket Guide for Asthma Management and Prevention. Bethesda: National Institutes of Health, National Heart, Lung and Blood Institute, 2002, vol. Publication no. 02-3659.
- Culpitt S V, Rogers D F. Evaluation of current pharmacotherapy of chronic obstructive pulmonary disease.Expert Opin Pharmacother 2000; 1:1007–1020. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Barnes P J, Ito K, Adcock I M. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase.Lancet 2004; 363:731–733. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rogers D F. Mucoactive drugs for asthma and COPD: any place in therapy?Expert Opin Investig Drugs 2002; 11:15–35. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Grandjean E M, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials.Clin Ther 2000; 22:209–221. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Stey C, Steurer J, Bachmann S, Medici T C, Tramer M R. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review.Eur Respir J 2000; 16:253–262. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Poole P J, Black P N. Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: systematic review.Br Med J 2001; 322:1271–1274. [CSA]
- Grandjean E M, Berthet P H, Ruffmann R, Leuenberger P. Cost-effectiveness analysis of oral N-acetylcysteine as a preventive treatment in chronic bronchitis.Pharmacol Res 2000; 42:39–50. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Dekhuijzen P N. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease.Eur Respir J 2004; 23:629–636. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Moretti M, Bottrighi P, Dallari R, Da Porto R, Dolcetti A, Grandi P, Garuti G, Guffanti E, Roversi P, De Gugliemo M, Potena A. The effect of long-term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE Study.Drugs Exp Clin Res 2004; 30:143–152. [PUBMED], [INFOTRIEVE], [CSA]
- MacNee W. Oxidative stress and lung inflammation in airways disease.Eur J Pharmacol 2001; 429:195–207. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Montuschi P, Kharitonov S A, Barnes P J. Exhaled carbon monoxide and nitric oxide in COPD.Chest 2001; 120:496–501. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wright D T, Fischer B M, Li C, Rochelle L G, Akley N J, Adler K B. Oxidant stress stimulates mucin secretion and PLC in airway epithelium via a nitric oxide-dependent mechanism.Am J Physiol 1996; 271:L854–L861. [PUBMED], [INFOTRIEVE], [CSA]
- Burgel P R, Nadel J A. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium.Thorax 2004; 59:992–996. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rhee C S, Majima Y, Arima S, Jung H W, Jinn T H, Min Y G, Sakakura Y. Effects of clarithromycin on rheological properties of nasal mucus in patients with chronic sinusitis.Ann Otol Rhinol Laryngol 2000; 109:484–487. [PUBMED], [INFOTRIEVE], [CSA]
- Suez D, Szefler S J. Excessive accumulation of mucus in children with asthma: a potential role for erythromycin? A case discussion.J Allergy Clin Immunol 1986; 77:330–334. [PUBMED], [INFOTRIEVE], [CSA]
- Marom Z M, Goswami S K. Respiratory mucus hypersecretion (bronchorrhea): a case discussion—possible mechanisms(s) and treatment.J Allergy Clin Immunol 1991; 87:1050–1055. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Shibuya Y, Wills P J, Cole P J. The effect of erythromycin on mucociliary transportability and rheology of cystic fibrosis and bronchiectasis sputum.Respiration 2001; 68:615–619. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gorrini M, Lupi A, Viglio S, Pamparana F, Cetta G, Iadarola P, Powers J C, Luisetti M. Inhibition of human neutrophil elastase by erythromycin and flurythromycin, two macrolide antibiotics.Am J Respir Cell Mol Biol 2001; 25:492–499. [PUBMED], [INFOTRIEVE], [CSA]
- Shimizu T, Shimizu S, Hattori R, Gabazza E C, Majima Y. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells.Am J Respir Crit Care Med 2003; 168:581–587. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Barnes P J. Current and future therapies for airway mucus hypersecretion.Novartis Found Symp 2002; 248:237–249. [PUBMED], [INFOTRIEVE], [CSA]
- Donnelly L E, Rogers D F. Therapy for chronic obstructive pulmonary disease in the 21st century.Drugs 2003; 63:1973–1998. [PUBMED], [INFOTRIEVE], [CSA]
- Tesfaigzi Y, Fischer M J, Daheshia M, Green F H, De Sanctis G T, Wilder J A. Bax is crucial for IFN-gamma-induced resolution of allergen-induced mucus cell metaplasia.J Immunol 2002; 169:5919–5925. [PUBMED], [INFOTRIEVE], [CSA]
- Herbst R S, Frankel S R. Oblimersen sodium (Genasense bcl-2 antisense oligonucleotide): a rational therapeutic to enhance apoptosis in therapy of lung cancer.Clin Cancer Res 2004; 10:4245s–4248s. [PUBMED], [INFOTRIEVE], [CSA]
- Bonomi P. Clinical studies with non-iressa EGFR tyrosine kinase inhibitors.Lung Cancer 2003; 41(suppl 1):S43–S48. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Adler K B, Holden-Stauffer W J, Repine J E. Oxygen metabolites stimulate release of high-molecular-weight glycoconjugates by cell and organ cultures of rodent respiratory epithelium via an arachidonic acid-dependent mechanism.J Clin Invest 1990; 85:75–85. [PUBMED], [INFOTRIEVE], [CSA]
- Rogers D F. Motor control of airway goblet cells and glands.Respir Physiol 2001; 125:129–144. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rogers D F. Pharmacological regulation of the neuronal control of airway mucus secretion.Curr Opin Pharmacol 2002; 2:249–255. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rogers D F. Tachykinin receptor antagonists for asthma and COPD.Expert Opin Ther Pat 2001; 11:1097–1121. [CSA], [CROSSREF]
- Caterina M J, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway.Annu Rev Neurosci 2001; 24:487–517. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zeldin D C. Epoxygenase pathways of arachidonic acid metabolism.J Biol Chem 2001; 276:36059–36062. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tesfaigzi Y, Kluger M, Kozak W. Clinical and cellular effects of cytochrome P-450 modulators.Respir Physiol 2001; 128:79–87. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Li Y, Martin L D, Spizz G, Adler K B. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro.J Biol Chem 2001; 276:40982–40990. [PUBMED], [INFOTRIEVE], [CSA]
- Singer M, Martin L D, Vargaftig B B, Park J, Gruber A D, Li Y, Adler K B. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma.Nat Med 2004; 10:193–196. [PUBMED], [INFOTRIEVE], [CSA]
- Evans C, Kheradmand F, Corry D, Tuvim M, Densmore C, Waldrep C, Knight V, Dickey B. Gene therapy of mucus hypersecretion in experimental asthma.Chest 2002; 121:90S–91S. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- O'Donnell R A, Richter A, Ward J, Angco G, Mehta A, Rousseau K, Swallow D M, Holgate S T, Djukanovic R, Davies D E, Wilson S J. Expression of ErbB receptors and mucins in the airways of long term current smokers.Thorax 2004; 59:1032–1040. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Longphre M, Li D, Li J, Matovinovic E, Gallup M, Samet J M, Basbaum C B. Lung mucin production is stimulated by the air pollutant residual oil fly ash.Toxicol Appl Pharmacol 2000; 162:86–92. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Shim J J, Dabbagh K, Takeyama K, Burgel P R, Dao-Pick T P, Ueki I F, Nadel J A. Suplatast tosilate inhibits goblet-cell metaplasia of airway epithelium in sensitized mice.J Allergy Clin Immunol 2000; 105:739–745. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wang B, Lim D J, Han J, Kim Y S, Basbaum C B, Li J D. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway.J Biol Chem 2002; 277:949–957. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Atherton H C, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation.Am J Physiol, Lung Cell Mol Physiol 2003; 285:L730–L739. [CSA]
- Zhou Y, Shapiro M, Dong Q, Louahed J, Weiss C, Wan S, Chen Q, Dragwa C, Savio D, Huang M, Fuller C, Tomer Y, Nicolaides N C, McLane M, Levitt R. A calcium-activated chloride channel blocker inhibits goblet cell metaplasia and mucus overproduction. In: Mucus Hypersecretion in Respiratory Disease. Novartis Foundation Symposium. Chichester: Wiley, 2002; 248:150–165.
- Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma.Proc Natl Acad Sci U S A 2001; 98:5175–5180. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Knight D. Talniflumate (Genaera).Curr Opin Investig Drugs 2004; 5:557–562. [PUBMED], [INFOTRIEVE], [CSA]
- Donnelly L E, Rogers D F. Antiproteases and retinoids for treatment of chronic obstructive pulmonary disease.Expert Opin Ther Pat 2003; 13:1345–1372. [CSA], [CROSSREF]
- Guzman K, Gray T E, Yoon J H, Nettesheim P. Quantitation of mucin RNA by PCR reveals induction of both MUC2 and MUC5AC mRNA levels by retinoids.Am J Physiol 1996; 271:L1023–L1028. [PUBMED], [INFOTRIEVE], [CSA]
- Yoon J H, Gray T, Guzman K, Koo J S, Nettesheim P. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix.Am J Respir Cell Mol Biol 1997; 16:724–731. [PUBMED], [INFOTRIEVE], [CSA]
- Koo J S, Jetten A M, Belloni P, Yoon J H, Kim Y D, Nettesheim P. Role of retinoid receptors in the regulation of mucin gene expression by retinoic acid in human tracheobronchial epithelial cells.Biochem J 1999; 338:351–357. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Apfel C, Bauer F, Crettaz M, Forni L, Kamber M, Kaufmann F, LeMotte P, Pirson W, Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects.Proc Natl Acad Sci U S A 1992; 89:7129–7133. [PUBMED], [INFOTRIEVE], [CSA]
- Bhattacharyya S N, Manna B, Smiley R, Ashbaugh P, Coutinho R, Kaufman B. Smoke-induced inhalation injury: effects of retinoic acid and antisense oligodeoxynucleotide on stability and differentiated state of the mucociliary epithelium.Inflammation 1998; 22:203–214. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tesfaigzi Y, Fischer M J, Martin A J, Seagrave J. Bcl-2 in LPS- and allergen-induced hyperplastic mucous cells in airway epithelia of Brown Norway rats.Am J Physiol, Lung Cell Mol Physiol 2000; 279:L1210–L1217. [CSA]
- Kellerman D J. P2Y(2) receptor agonists: a new class of medication targeted at improved mucociliary clearance.Chest 2002; 121:201S–205S. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Roger P, Gascard J P, Bara J, de Montpreville V T, Yeadon M, Brink C. ATP induced MUC5AC release from human airways in vitro.Mediat Inflamm 2000; 9:277–284. [CSA], [CROSSREF]
- Jackson A D. Airway goblet-cell mucus secretion.Trends Pharmacol Sci 2001; 22:39–45. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]