Abstract
Many patients with chronic obstructive pulmonary disease (COPD) have increased resting energy expenditure (REE) and may have an increased need for energy during activity. However, in terms of total energy balance, the influence of differences in REE may be compensated for by differences in daily energy expenditure. Energy needs may therefore be difficult to predict by measuring REE. The aim of this study was to predict the energy intake necessary for weight gain following dietary counselling. We studied 42 COPD patients (n = 27 underweight, n = 15 normal-weight) who were being considered for lung transplantation and had completed an intervention lasting a mean of 22 weeks. In the underweight patients, the dietary intervention consisted of dietary counselling for weight gain, while, in the normal-weight patients it focused on weight maintenance. It has been shown that a weight gain of over 2 kg in patients with COPD improves the prognosis and this was obtained in 52% of our patients. The mean (SD) increase in energy intake in the responders was 3448 (1310) kJ, while it was 635 (2454) kJ, p < 0.01 in the non-responders. Patients who used or had free access to nutritional supplements did not show greater success than patients who only used ordinary foods. Based on the relationship between the dependent variable (kg weight change) and the independent variable (energy intake), we can use linear regression to predict that an energy intake of 180% of REE predicted or 186 kJ/kg (44 kcal/kg) is necessary to obtain a weight gain of 2 kg.
INTRODUCTION
Involuntary weight loss is common among patients with chronic obstructive pulmonary disease (COPD), is associated with impaired muscle function [Citation[1]] and negatively influences morbidity [Citation[2]] and survival [Citation[3]]. On the other hand, dietary intervention studies in COPD patients have shown that it is possible to reverse weight loss and improve the prognosis [Citation[4]]. Energy requirements cannot solely be calculated from prediction equations [Citation[5]]. Many patients with chronic obstructive pulmonary disease have increased resting energy expenditure and may have an increased need for energy during activity [Citation[6]]. However, in terms of total energy balance, the influence of differences in resting energy expenditure may be compensated for by differences in daily energy expenditure [Citation[7]]. Energy needs may therefore be difficult to predict by measuring REE.
We have previously reported on dietary intervention in patients with advanced pulmonary disease [Citation[8]]. The aim of this study is to investigate COPD patients in order to predict the energy intake necessary for weight gain after dietary intervention.
Patients and methods
Patients and Study Design
Forty-six patients who were underweight (n = 29) and normal-weight (n = 17) with advanced COPD, who were being considered for lung transplantation, were included in our dietary intervention study [Citation[8]]. The patients were consecutively admitted to the Department of Thoracic Medicine, Rikshospitalet, to be assessed for lung transplantation between August 1993 and August 1998. None of the patients was a current smoker. Being free from smoking for at least six months prior to assessment for lung transplantation is one of our criteria for acceptance.
The dietary intervention study consisted of inpatient dietary support [Citation[9]], followed by outpatient dietary intervention [Citation[8]]. Following arrival at the hospital, the underweight patients were randomly allocated to two groups (Group A and Group B). Group A received intensified dietary support; while they were in hospital, they were offered an energy-rich diet with the addition of oral ready-made liquid nutritional supplements if they wanted them. Group B and C (normal-weight patients) received the normal diet and regular support in hospital. Both groups A and B received outpatient dietary counselling, including meal planning and individual dietary suggestions. As outpatients, Group A received oral liquid nutritional supplements free of charge, while Group B received no supplements free of charge. The normal-weight patients received dietary counselling for weight maintenance. The patients were contacted by telephone in their homes by a dietician 3–4 weeks prior to their first admission to hospital. After obtaining the patient's informed consent, a scale and a booklet for recording food were mailed to the patient. The patients recorded their food intake 3 to 4 weeks prior to their first admission to hospital. Food records were also kept during hospitalisation and at the end of the intervention period, three to four weeks prior to the second visit to the hospital.
Forty-two patients (n = 27 underweight, n = 15 normal-weight) completed the inpatient and outpatient intervention. Those who did not complete either died or were transplanted. After intervention the underweight patients were divided into two groups: responders (weight gain > 2 kg) and non-responders (weight gain ≤2 kg). Nutritional assessments were performed after arrival at hospital when entering the study and on the second visit.
On the second visit, at the end of the study, the patients were asked if they had had any infection that was treated with antibiotics during the intervention period. After the patients had been assessed for lung transplantation, they were put on a waiting list and readmitted to hospital every 4 to 5 months for medical controls. All measurements were performed before transplantation. The regional ethics committee approved the study.
Measurements
Anthropometrical Measurements
The body mass index (BMI) was calculated as the ratio of the body weight to the height squared and expressed in kg/m2. Patients with a BMI of below 19 or between 19 and 25 with a weight loss greater than 10% from their usual weight were defined as underweight, while normal-weight patients had a BMI of between 19 and 25 and a weight loss equal to or less than 10%. Fat mass (FM) was determined by four skinfold measurements (bicipital, tricipital, subscapular and suprailiac) using the Durnin tables [Citation[10]]. The fat-free mass (FFM) was assessed by subtracting the FM from the body weight. The fat-mass index and fat-free mass index was computed by dividing FM and FFM, respectively, by the height squared.
Blood Samples and Lung Function
Venous blood samples were taken by the hospital staff after the patients had fasted overnight. The samples were then stored in the refrigerator and processed within 1 hour after collection. Concentrations in serum of C-reactive protein (CRP) were analysed using routine laboratory methods at the Clinical Chemistry Department, Rikshospitalet. Transthretin was measured with antiserum in an immunochemical reaction using nephelometry on a Behring Nephelometer 2. Immunological parameters were measured in serum samples, which had been stored at −70°C. Soluble TNF receptors (sTNF-α RI, sTNF-α RII) were measured by ELISA technique, using kits from R&D Systems, Inc., Minneapolis, MN, USA. Measurements of soluble TNF receptors were performed at the Institute of Immunology, Rikshospitalet, Oslo. Arterial blood samples were obtained from the radial artery for measurements of arterial O2 (PaO2), arterial CO2 (PaCO2) tension and arterial O2 saturation, with the patients resting and breathing room air. They were analysed on a Ciba Corning Blood Gas Analyser 845.
An automated pulmonary function unit (Gould 2400, Sensormedics, Bilthoven, the Netherlands) was used to measure spirometry variables; forced expiratory volume in one second (FEV1). The reference values were those recommended by the European Respiratory Society [Citation[11]].
Dietary Record
For dietary assessment in outpatients, the patients recorded their food intake for seven consecutive days prior to hospital admission. During the visit to the clinic, the same dietician reviewed the records with the patients to ensure completeness. Patients who were accepted for pre-lung transplant assessment at short notice and were not contacted before admission were asked to participate in the study after arriving at hospital. They were interviewed by the same trained dietician about their food habits using the dietary-history method with a cross-check [Citation[12]]. The eating pattern during the preceding three weeks was defined as the usual eating pattern. To check for the potential influence of different dietary assessment methods on energy intake at entry it was previously performed a two-way ANOVA with dietary method and group as factors and ln(total energy intake) as the dependent variable [Citation[8]]. This analysis confirmed that there were no significant differences in energy intake between the groups at entry. In the selection in the present study, dietary-history method at entry was performed in 3 of the 27 underweight patients. The patients recorded their food intake for 3 consecutive days during their hospital stay by using a self-administered form adapted to the hospital menu, previously described and validated [Citation[13]]. Dietary intake evaluation was generally done on the fourth day, occasionally on the third and fifth day of in-hospital stay. When the records were collected on the fourth day, they were checked for any omission of food items to ensure completeness. We chose to record dietary intake for 3 days. In general, increasing the length of dietary records increases the risk of interfering with the intake.
Statistical Analysis
The data were analysed using the SPSS program (SPSS for Windows, Release 9.0, SPSS Inc., Chicago, IL, 1999). Energy intake in relation to predicted resting energy expenditure (REE) was calculated using the Harris and Benedict equation (REE predicted) [Citation[14]]. An independent-samples t-test was used to compare outcome measures in responders and non-responders (). Correlations were performed with Pearson's correlation coefficient. Linear regression was used to predict the energy intake necessary for weight gain. Then 2 kg was set as the cut-off point for weight gain. p-values of ≤ 0.05 were regarded as significant.
Table 3 Characteristics of the underweight COPD patients in hospital and after outpatient intervention divided into those with a total weight gain of more than 2 kg and those with a total weight gain of 2 kg or less
RESULTS
In are given results for the original Groups A, B and C. After intervention, the energy intake/REE predicted was mean (SD) 198 (55%) in all the underweight patients and 133 (26%) in the normal-weight ones. A weight gain of mean 2.8 (SD 2.5) kg occurred in the underweight patients compared with 0.6 (2.0) kg in the normal-weight ones. Patient characteristics at baseline for the responders and non-responders are shown in . The mean intervention time was inpatients 1.8 weeks (range 0.4–6.0) and 1.6 (1.3–2.4) for responders and non-responders, respectively and outpatients 20 weeks (range 14–30 weeks) and 20 (range 12–36), respectively and for normal-weight 1.7 weeks (range 1.4–2.6) inpatients and 19 (range 13–26) outpatients. A weight gain of over 2 kg was obtained by about half of the underweight patients (). They had a larger weight gain (borderline significance, p = 0.065) during hospitalisation than the underweight patients, who had a smaller weight gain. There was a significant positive association between weight gain in hospital and total weight gain (r = 0.55, p < 0.001). There was no significant difference in the use of ready-made liquid nutritional supplements between the groups (). The mean (SD) intake from supplements was 793 (1402) kJ in the responders and 436 (846) kJ in the non-responders. Most of the increase in energy intake in the responders was provided by ordinary food (77%), while 31% came from ordinary food in the non-responders. In the underweight outpatients, the energy intake in those patients who had infections was a mean (SD) of 9.1 (2.7) MJ, while it was 11.5 (2.6) MJ (p = 0.03) among those without infections.
Table 1 Dietary data after intervention
Table 2 Characteristics at baseline for underweight COPD patients divided into those with a total weight gain of more than 2 kg and those with a total weight gain of 2 kg or less
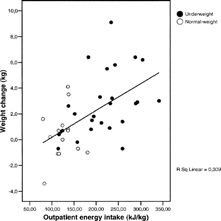
Based on the relationship between the dependent variable (kg total weight change) and the independent variable (outpatient energy intake/ REE predicted or kJ per kg body weight), we used linear regression to predict that an energy intake of 180% of REE predicted or 186 kJ/kg (44 kcal/kg) was necessary for a weight gain of 2 kg (, r = 0.56, p < 0.001).
DISCUSSION
For underweight COPD patients, the energy that is needed for weight gain is difficult to calculate by measuring resting energy expenditure and using prediction equations [Citation[5], Citation[7]]. Assessing physical activity and daily energy expenditure is time consuming and rarely feasible in clinical practice. We therefore wanted to predict the energy intake necessary for weight gain after dietary intervention had been completed. We used 2 kg as a cut off point, as a weight gain of more than 2 kg has been shown to improve the prognosis for COPD patients [Citation[4]]. Short-term intervention over 8 weeks, resulting in a weight gain of more than 2 kg, was shown to increase survival during the following four years in patients with COPD [Citation[4]]. To identify differences between our COPD patients who succeeded in gaining more than 2 kg and those who did not, the underweight patients were divided into two groups. There was a markedly higher energy intake in those who succeeded with a weight gain of more than 2 kg. This is an observational study and difficulties are connected with observing covariance. The groups (responders/non-responders) may show systematic differences in other variables that are related to the response variable weight change. For example, the responders could have been more physical active, which could have contributed to the increase in FFM in this group.
Additional assumptions must also hold if the adjustment is to remove all bias. Dietary intake must be measured without error. To get a true measure of intake is connected with difficulties. The validation of the self-administered form used by patients to record their food intake in the hospital revealed an acceptable validity for most patients and could be a tool for estimating intake in groups of patients [Citation[13]]. For measurements of dietary intake in free-living individuals none of the current methods provides an objective or “true” picture of the habitual intake. In the present study, the method of 7-day records by weighing was chosen for dietary assessment in outpatients. This method has often been used as the standard to validate the accuracy of other methods [Citation[15]]. Weighed records may have a drawback relating to underestimation [Citation[16]]. Even if the dietary record is acceptable for the dietary intake for the recording period, they may not be representative for the whole intervention period. But still, they may be acceptable on group level. In fact, our result showed a significant association between recorded energy intake and weight change. The predicted energy intake of 180% of REE predicted or 186 kJ/kg for a weight gain of 2 kg in our COPD patients is about the same or less as that previously reported when groups of patients succeeded in obtaining a mean weight gain of about 2 kg or more, ranging from 4.2 (range 0.7–8.2) kg [Citation[17]], 2.6 kg (50% > 2 kg) (4;18), 2.4 (range −1.0–4.7) kg [Citation[19]] and 2.1 (SD 2.1) kg [Citation[20]]. This supports our view that the mean intake in a group should be at least more than 180% of REE predicted or 186 kJ/kg for more patients to obtain a weight gain of over 2 kg. The energy intake/REE predicted in all the underweight patients was about the same level (200%) as reported by Schols et al. [Citation[18]] with a similar weight gain and response rate as in all our underweight patients. Unlike our patients, their patients participated in physical training. This might indicate a higher need in our more impaired patients, judged by lung function and blood gases, but is difficult to compare because of the age differences.
Results from Groups A and B showed that there were not more responders in Group A than Group B. However, there was a significant association between weight gain in the hospital and total weight gain and the responders had a somewhat higher weight gain during hospitalisation, with a more favourable weight gain by an increase in FFM after outpatient intervention. In the responders the energy intake at hospital was on a level at which a weight gain would be expected and was higher than in the non-responders, even if the difference was not significant. Dietary data in the hospital were missing for seven patients. This may explain, at least to some extent, the lack of significant difference. Some of the underweight patients in the control group (Group B) at hospital might have improved the intake already at the hospital. Being a participant in the study might have created awareness in some of the underweight control patients in the hospital. The responders already gained 1.3 kg during hospitalisation with a lower energy intake than during outpatients. Probably, energy expenditure during outpatient period would be higher and, therefore, further gain in body weight would require higher energy intake. Because of the short hospital stay a clear improvement in FFM could not be expected; we thus did not measure other outcome parameters than diet and body weight. During hospitalisation, in the absence of exercise, you would expect the weight gain mostly be due to increase in fat mass.
Most of the dietary intervention studies in COPD patients have used oral liquid nutritional supplements for the extra energy supply [Citation[17], Citation[18], Citation[20]]. It appears to be more difficult to succeed with outpatient dietary support than inpatient support [Citation[21]]. After outpatient intervention Group B succeeded with weight gain predominantly by improving their habitual diet. There was no significant difference in the use of supplements between those who gained more than 2 kg and those who gained less. For those who gained more than 2 kg, 23% of the increase in energy intake came from nutritional supplements while, in the case of those with a smaller weight gain, 69% of the smaller increase in energy intake was provided by supplements. The protein intake in the responders increased from 1.4 g/kg to 1.9 g/kg, while in the non-responders it remained unchanged. The protein intake of 1.4 g/kg should be more than sufficient for nitrogen balance and not cause loss of FFM. The normal-weight patients had a protein intake of 1.1 g/kg and maintained their FFM. The tendency for a decrease in FFM and increase in FM in the non-responders could have been due to less physical activity. There was no difference in age between the groups. Age did not appear to play a part in weight change in our study, contrary to what has been reported in a group of older patients [Citation[22]].
More than 50% of the patients who did not increase their weight by more than 2 kg had infections during intervention, while this applied to about one-third of those who gained more than 2 kg. We did not record the frequency of infections or infections that were not treated with antibiotics, but some of the patients who had infections could have been more ill throughout the intervention period and could consequently have experienced a prolonged negative effect on energy intake and weight gain. Patients often lose weight during infections and acute exacerbations [Citation[23], Citation[24]]. One study has examined the effect of nutritional support in patients with COPD during hospitalisation for an acute exacerbation, to prevent the consequences of nutritional deterioration [Citation[25]]. In this study, the group on extra nutritional support consumed an additional 10 kcal/kg/day compared with subjects receiving regular hospital care. The authors concluded that it was difficult to prevent muscle wasting, but some small gains were observed as a result of increased dietary intake. In dietary intervention studies, a substantial number of patients failed to respond to nutritional support [Citation[17], Citation[18], Citation[19], Citation[20], Citation[21]]. In the present study, it appears that those who failed to gain weight were those who failed to increase their energy intake. However, in the case of subgroups, this might deviate. In addition to acute exacerbations [Citation[25]], it has been suggested that an elevated systemic inflammation characterises non-response to high-energy therapy [Citation[22]]. From our results it was not possible to explain insufficient increase in energy intake in the non-responder. They did not seem to be more disabled than the responders or had elevated systemic inflammation.
In conclusion, we predicted that an energy intake of 180% of REE predicted or 186 kJ/kg (44 kcal/kg) was necessary for a weight gain of 2 kg. Patients who used nutritional supplements for extra energy supply or had access to ready-made liquid nutritional supplements free of charge did not appear to have a greater chance of succeeding with weight gain than the patients who only used ordinary food to increase their energy intake.
REFERENCES
- Arora N S, Rochester D F. Respiratory Muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis 1982; 126: 5–8, [CSA]
- Hoch D, Murray D, Blalock J, Levin D, Lorance L. Nutritional status as an index of morbidity in chronic airflow limitation. Chest 1984; 85: 66S–67S, [CSA]
- Wilson D O, Rogers R M, Wright E C, Anthonisen N R. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis 1989; 139: 1435–1438, [CSA]
- Schols A M, Slangen J, Volovics L, Wouters E F. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1791–1797, [CSA]
- Slinde F, Ellegard L, Gronberg A M, Larsson S, Rossander-Hulthen L. Total energy expenditure in underweight patients with severe chronic obstructive pulmonary disease living at home. Clin Nutr 2003; 22: 159–165, [CSA]
- Creutzberg E C, Schols A M, Bothmer-Quaedvlieg F C, Wouters E F. Prevalence of an elevated resting energy expenditure in patients with chronic obstructive pulmonary disease in relation to body composition and lung function. Eur J Clin Nutr 1998; 52: 396–401, [CSA]
- Baarends E M, Schols A M, Westerterp K R, Wouters E F. Total daily energy expenditure relative to resting energy expenditure in clinically stable patients with COPD. Thorax 1997; 52: 780–785, [CSA]
- Førli L, Bjørtuft Ø, Vatn M, Kofstad J, Boe J. A study of intensified dietary support in underweight candidates for lung transplantation. Ann Nutr Metab 2001; 45: 159–169, [CSA]
- Førli L, Pedersen J I, Bjortuft O, Vatn M, Boe J. Dietary support to underweight patients with end-stage pulmonary disease assessed for lung transplantation. Respiration 2001; 68: 51–57, [CSA]
- Durnin J V, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br. J Nutr 1974; 32: 77–97, [CSA]
- Quanjer P H, Tammeling G J, Cotes J E, Pedersen O F, Peslin R, Yernault J C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 5–40, [CSA]
- Burke B S. The dietary history as a tool in research. J Am Diet Assoc 1947; 23: 1041–1046, [CSA]
- Førli L, Oppedal B, Skjelle K, Vatn M. Validation of a self-administered form for recording food intake in hospital patients. Eur J Clin Nutr 1998; 52: 929–933, [CSA]
- Harris J A, Benedict E G. A Biometric Study of Basal Metabolism. Carnegie Institution of Washington, Washington 1919
- Stuff J E, Garza C, Smith E O, Nichols B L, Montandon C M. A comparison of dietary methods in nutritional studies. Am J Clin Nutr 1983; 37: 300–306, [CSA]
- Black A E, Goldberg G R, Jebb S A, Livingstone M B, Cole T J, Prentice A M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. (Review. 75 refs). Eur J Clin Nutr 1991; 45: 583–599, [CSA]
- Efthimiou J, Fleming J, Gomes C, Spiro S G. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988; 137: 1075–1082, [CSA]
- Schols A M, Soeters P B, Mostert R, Pluymers R J, Wouters E F. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med 1995; 152: 1268–1274, [CSA]
- Whittaker J S, Ryan C F, Buckley P A, Road J D. The effects of refeeding on peripheral and respiratory muscle function in malnourished chronic obstructive pulmonary disease patients. Am Rev Respir Dis 1990; 142: 283–288, [CSA]
- Creutzberg E C, Wouters E F, Mostert R, Weling-Scheepers C A, Schols A M. Efficacy of nutritional aupplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition 2003; 19: 120–127, [CSA]
- Rogers R M, Donahoe M, Costantino J. Physiologic effects of oral supplemental feeding in malnourished patients with chronic obstructive pulmonary disease. A randomized control study. Am Rev Respir Dis 1992; 146: 1511–1517, [CSA]
- Creutzberg E C, Schols A M, Weling-Scheepers C A, Buurman W A, Wouters E F. Characterization of nonresponse to high caloric oral nutritional therapy in depleted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 745–752, [CSA]
- Vermeeren M A, Schols A M, Wouters E F. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J 1997; 10: 2264–2269, [CSA]
- Creutzberg E C, Wouters E FM, Vanderhoven-Augustin I ML, Dentener M A, Schols A MWJ. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 162: 1239–1245, [CSA]
- SaudnyUnterberger H, Martin J G, Gray Donald K. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. Am. J Respir Crit Care Med 1997; 156: 794–799, [CSA]