Abstract
The study objective was to assess spirometric changes during resolution of acute exacerbations of COPD diagnosed and treated in primary care and their relationship to clinical features. Spirometry was carried out on 101 patients with AECOPD presenting to a primary care physician on the day of presentation, days 5, 10–14, 28, and 56 after presentation and traces were analyzed including quality and reproducibility. Eighty-three patients produced at least one technically acceptable spirometer trace at presentation and 60 patients produced acceptable traces at all time points. The increase in FEV1 and VC occurred during the first 5 days after presentation, with a median increase in postbronchodilator FEV1 of 55 ml (IQR, −63 to 128, p = 0.003) and VC of 90 ml (IQR −78 to 308 ml, p < 0.001). The improvement in prebronchodilator values related to the bronchodilator reversibility at presentation and was strongest for VC (by day 28: r = 0.522, p < 0.001). Patients presenting with purulent sputum demonstrated improvements in FEV1 and VC but this was limited to FEV1 in those with mucoid sputum. The initial dyspnoea score related to the changes in spirometry. It is possible to obtain clinically useful spirometric traces in most patients presenting with an acute exacerbation in primary care. Some patients present with changes in sputum characteristics and cough without increased breathlessness. However, exacerbations characterized by increased breathlessness are associated with increases in airflow obstruction that may be influenced by sputum characteristics and/or changes in airway reactivity.
Key words: :
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is characterized by chronic symptoms punctuated, with varying frequency, by acute exacerbations (AE). As the heterogeneity of COPD is increasingly being appreciated [Citation[1]] so are the causes and characteristics of exacerbations. Breathlessness is considered to be a key feature of such episodes and increased airflow obstruction is thought to be the cause, whether the episodes are viral or bacterial in origin, related to increased atmospheric pollution or due to other, as yet, undefined causes. Indeed, studies assessing the merit of different therapies including antibiotics, steroids and bronchodilator therapy have often used physiological measures related to airflow obstruction (usually peak flow or FEV1 measurement) to assess response to therapy [Citation[2], Citation[3], Citation[4]]. However, detailed studies of changes in lung function occurring during exacerbations through to resolution have been less well documented but are also important to determine the efficacy of treatments within future intervention studies, as well as increasing our understanding of the nature of AECOPD. Studies which have been undertaken have been performed either in hospitalized patients or from patients recruited from specialist clinics. However, most treatments for AECOPD are prescribed by primary care physicians and the results of studies performed in secondary care may not reflect these episodes.
The largest study to date that has investigated physiological changes occurring in exacerbations assessed the changes in peak expiratory flow (PEF) in a cohort of 101 patients followed through 504 exacerbations [Citation[5]]. All patients monitored their PEF and 34 supplemented these records with recordings from a handheld spirometer. Overall changes in lung function were shown to be small with a median fall in PEF of 6.8% and FEV1 of 24 ml at the start of the exacerbation. Although this study has enhanced our understanding of the natural history of COPD exacerbations it did not specifically address the nature of changes in spirometry. Other studies have used spirometry as an outcome indicator of the benefit of systemic steroids in AECOPD, in both hospital inpatients [Citation[2], Citation[3]] and outpatients [Citation[4]]. Thompson et al. studied 27 patients with COPD in an outpatient department and found an average daily increase of 50 ml in FEV1 over the first 10 days of the exacerbation treated with oral prednisolone but no change in those treated with a placebo. In a group of 209 inpatients hospitalized for AECOPD Niewoehner et al. found a mean increase in FEV1 of 274 ml over 14 days [Citation[6]] and similarly Davies et al. found that over the first 5 days of treatment for exacerbation, FEV1 increased on average by 90 ml/day in those treated with oral corticosteroids but only 30 ml/day in the placebo group [Citation[2]]. However, many patients demonstrate significant increases in FEV1 in response to steroids even when clinically stable and therefore the physiological changes described during the exacerbation may not reflect recovery from exacerbation alone. In addition, measuring lung function by spirometry while a patient is unwell presents technical problems and other measurements are not usually recorded.
Here we report the spirometric changes following presentation with exacerbation of chronic bronchitis in primary care. All patients were characterized at the start of treatment by sputum color and divided into those with mucoid or purulent sputum [Citation[7]]. The patients were then followed for a period of 2 months with regular spirometry and completed a daily diary symptom card [Citation[8]]. This enabled the relationship between the symptoms of the exacerbation and its physiological resolution to be assessed.
METHODS
The study recruited patients aged between 40 and 80 years diagnosed by their primary-care physicians with an AECOPD associated with sputum production. Symptoms of the exacerbation were documented including changes in dyspnoea, cough, sputum volume, sputum purulence, or the presence of pyrexia or malaise. Following presentation to the primary care physician, patients were assessed by the clinical research team and enrolled into the study. Patients were excluded if they had received antibiotics within the previous 4 weeks, or if the primary care physician thought that oral corticosteroid therapy or hospital admission was mandatory. The baseline characteristics of these patients has been described in detail elsewhere [Citation[1]].
Patients were seen on the day of the consultation by a respiratory research nurse. At this time FEV1 and slow vital capacity (VC) was recorded using a Fleisch pneumotachograph (Vitalograph alpha compact, Bucks, UK) both before and 20 minutes after bronchodilator therapy with 400 μg of salbutamol administered via a spacer. The best of three traces was taken as the value and each trace graded for quality: grade 1 traces were when at least two smooth, reproducible traces agreeing within 100 ml were obtained, according to ARTP criteria [Citation[9]], grade 2 traces were those in which a single observed trace using good effort producing a smooth uninterrupted trace with a terminal plateau and grade 3 traces were those in which patients were unable to produce a single complete smooth trace beyond FEV1 to FVC. A modified Borg score [Citation[10]] was obtained to assess breathlessness and a fresh sample of sputum was collected into a sterile container over a 1-hour period and graded by color with reference to a standard color chart [Citation[8]]. Patients with macroscopically purulent sputum were treated with 5–10 days of a β-lactam antibiotic whereas those with mucoid samples did not receive antibiotics.
Patients were instructed in the use of a daily diary card as described previously [Citation[8]]. As part of this card a dyspnoea score was recorded that graded breathlessness depending upon its variation from that normally experienced by the patient: 2 was the same as normal and improvement from normal was graded 1 (better than normal) through to 0 (much better than normal) and deterioration from normal was graded 3 (worse than normal) to 4 (much worse than normal) [Citation[8]]. The best of three PEF measurements was recorded both in the morning and evening and data was collected for the 2 months from presentation.
Patients were seen again by the research team 5, 10–14, and 28 days after presentation. At each review the study nurse checked the diary card completion, recorded the Borg score and repeated spirometry before and after bronchodilation with salbutamol.
Two months after the initial consultation, FEV1 and slow vital capacity were measured using a wedge bellows spirometer (Vitalograph, Buckinghamshire, UK). Short-acting bronchodilator drugs were omitted for 6 hours before spirometric measurements and long-acting bronchodilators for 12 hours. The best of three satisfactory traces agreeing within 100 ml or 5% were accepted for FEV1 and VC for analysis [Citation[9]]. The measurements were repeated 20 minutes after the inhalation of 400 μg salbutamol and again 45 minutes after inhalation of 60 μg ipratropium bromide to determine the presence and degree of any reversibility of airflow obstruction. At this time high resolution CT scans were also performed to assess for the presence of bronchiectasis using defined criteria [Citation[11]]. The study was approved by the South Birmingham Health Authority ethics committee and all subjects provided written informed consent.
Statistical analysis
Statistical analyses were performed using the SPSS statistical package (version 10.0, Chicago, USA). Normally distributed data are presented as mean and standard error and non-parametric data as median and interquartile range. Comparison between mucoid and purulent groups was assessed by student's t-test and Mann–Whitney U-test, by one-way analysis of variance (ANOVA) for multiple groups and the Jonkheere–Terpstra test as appropriate for distribution. Comparison of repeated spirometric measurements within patients was made by repeated measures analysis of variance (ANOVA). t-tests and the Wilcoxon signed rank test were used to assess changes within groups and Fisher's exact tests were used to compare categorical data between groups. Pearson's correlation coefficient was used to assess linearity of association with normally distributed data and for nonparametric data Spearman's method was employed. To correct the effect of any regression to the mean when relating changes to an initial value a correction method derived from Blomqvist was used [Citation[12]]. The level of statistical significance was taken as p < 0.05.
RESULTS
Some 130 patients were recruited to the study, 9 patients were subsequently excluded because of clinical and HRCT evidence of significant varicose or cystic bronchiectasis, 18 patients withdrew from the study and no attempts to obtain pre-bronchodilator spirometric measurements were made in 2 patients because they were too ill. The remaining 101 patients had pre-bronchodilator spirometric measurements assessed for quality. Ninety-three of these patients also attempted post-bronchodilator spirometry and these were also graded for quality.
Quality of spirometry
Table 1 shows the assessment of quality for pre- and post-bronchodilator traces in the initial 101 patients. At presentation 61 patients (60%) were able to produce at least two good quality reproducible spirometry traces (grade 1) before bronchodilator drugs were given [Citation[9]]. Of the remainder, 23 (23%) patients could produce one “acceptable” trace (grade 2) and the remaining 17 (17%) were unable to produce a single acceptable trace. Following bronchodilation there was a small increase (to 65%) in the proportion of patients able to produce two good traces. Coughing was the main reason for patients being unable to complete reproducible expiratory maneuvers. Patients with only one acceptable trace (grade 2) had a lower mean post-bronchodilator FEV1 (% predicted) compared to those with two or more (grade 1) at presentation (55.86% ± 3.1 vs. 70.38% ± 3.1, p = 0.002: 95% CI of difference: 5.48 to 23.56). When studied in the clinically stable state 2 months later all patients produced 3 acceptable traces but the difference in FEV1 between those who produced 2 or more acceptable traces at baseline persisted (67.2% ± 3.9 vs. 77.6% ± 3.0, p = 0.032: 95% CI of difference: 0.95–19.9). Those patients unable to produce even one satisfactory trace (grade 3) at presentation were, however, able to provide adequate traces in the stable state and had a mean FEV1 of 65.7% predicted (SE ± 8.02). 56 of the patients had both acceptable (grade 1 and 2) pre- and post-bronchodilator traces at days 5, 10–14, and 28 days after presentation. There was no significant difference in pre- or post-bronchodilator FEV1 or VC at presentation in the whole group (78) compared to the subset (56) in whom values were obtained at all 5 time points (data not shown).
Of the 56 patients with a complete data set, 21 were taking regular inhaled corticosteroids (ICS) and 35 were not. Those taking ICS had a lower FEV1 (% predicted) at presentation compared to those not receiving ICS (pre-bronchodilator FEV1 at presentation 50.65%, ±4.1 and 66.46%, ±3.8, respectively, p = 0.01; post-bronchodilator FEV1 at presentation 57.54%, ±4.8 and 72.42, ±3.8 respectively, p = 0.022) and in the stable clinical state (pre-bronchodilator FEV1 55.49, ±5.1 and 72.42%, ±3.5 respectively, p = 0.006; post-bronchodilator FEV1 64.02%, ±5.1 and 78.66, ±3.5, respectively, p = 0.018).
Changes in spirometry from exacerbation to resolution
The pattern of changes was analyzed for the 56 patients who had a complete set of recordings at all 5 time points. The average FEV1 and VC for the 56 patients with a complete data set is shown for each time point both before and after bronchodilation in Table 2. The FEV1 and VC values were normally distributed at all time points although the changes were non-parametric. Therefore, mean and standard error are presented for the values at each time point and median and interquartile ranges for changes in each parameter.
Between day 1 and day 5 there was a significant increase in both pre- and post-bronchodilator FEV1 (p = 0.049 and p = 0.003 respectively). The median increase in absolute pre-bronchodilator FEV1 was 20 ml (IQR −88 to 128 ml) and in post bronchodilator FEV1 was 55 ml (IQR −63 to 178 mls). The FEV1 remained stable between day 5 and day 28 but increased again between day 28 and day 56 by a median volume before bronchodilation of 45 ml (IQR −40 to 173 ml; p = 0.01) and after bronchodilation of 70 ml (IQR −100 to 198 ml; p = 0.001). There was no difference in the change between patients receiving or not receiving regular inhaled corticosteroids.
Between day 1 and day 5 there was a significant increase in both the pre- and post-bronchodilator values for VC (p = 0.006 and p < 0.001, respectively) with increases in pre-bronchodilator VC of 115 ml (IQR −52 to 320 ml) and post-bronchodilator VC of 90 ml (IQR −78 to 308 ml), respectively.
The relationship between changes in spirometry and measures of bronchodilator reversibility
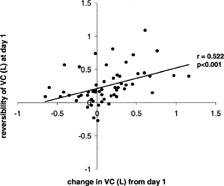
The relationship between the reversibility of FEV1 and VC on day 1 and the changes in these parameters from day 1 over the study period was studied in the 56 patients with the complete dataset. No relationship was demonstrated between the changes in post-bronchodilator FEV1 and VC and the response of these measurements to short acting bronchodilators (reversibility) at day 1. However, there was a relationship between the change in pre-bronchodilator spirometric values and the degree of reversibility at presentation (i.e., the more the FEV1 or VC increased after bronchodilation at presentation the greater the increase in pre-bronchodilator value over the recovery period). The relationship was strongest for VC and is summarized in (day 1 to 5 change: r = 0.393, p = 0.001; day 1 to 28 change: r = 0.522, p < 0.001) and weakest for FEV1 (day 1 to 5 change: r = 0.272, p = 0.015; day 1 to 28: r = 0.199, p > 0.005). However, there was no significant change in bronchodilator reversibility over the study period in any of the parameters measured. These observations could potentially represent regression to the mean and therefore an adjustment was made based upon variance of stable spirometric values reported previously [Citation[13]] by the method reported by Blomqvist [Citation[12]]. This adjustment reduced the observed correlation coefficient for VC between day 1 and day 28 to 0.478, although it still remained highly significant (p = 0.001).
Relationship of exacerbation sputum characteristics to spirometry
Of the 56 patients studied with complete data 39 had sputum graded as purulent at presentation and 17 as mucoid (patient characteristics for the two groups are given in Table 3). There was no significant difference in post bronchodilator FEV1 at any time point and sputum characteristics did not effect the variance across repeated measures.
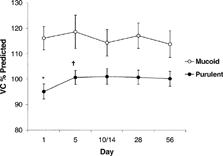
However, there was a significant between-group effect shown when sputum characteristics were introduced into the repeated measures analysis of variance (p = 0.003) (). An initial difference in mean VC of 21.0% predicted (95% CI: 10.3 to 31.7; t-test, p < 0.001) reducing to 13.6% (95% CI: −2.4 to 24.9; p = 0.018) by the end of follow up. Furthermore, independent analysis of the purulent group strengthened the observed difference between the repeated measurement of VC over the five time points (p = 0.003) while there was no difference within the mucoid group when analyzed separately.
Figure 2 The average VC (percent predicted ± SE bar lines) at the five time points is seen. Those with purulent exacerbations began with lower VC than those with mucoid exacerbations (* t-test, p = 0.002). There was a significant increase in VC by day 5 in the purulent group (†p < 0.001) but not the mucoid group. Although the difference between the groups reduced over the time course of the exacerbation a significant difference persisted by day 56 (p = 0.018).
The relationship between changes in spirometry and dyspnoea
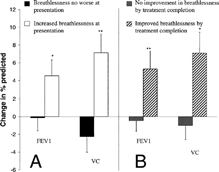
Completed diary card records were obtained from 46 of the 56 patients with a complete physiological data set (15 mucoid and 31 purulent exacerbations). The modified Borg score was recorded in 36 of these patients and related significantly to the FEV1 (after bronchodilation) at presentation (absolute value: r = −0.555, p < 0.001; and as a percent predicted: r = −0.490, p = 0.001). Twenty-eight patients reported their breathlessness on the diary card as having deteriorated as part of the exacerbation whereas 18 did not report such a change. One patient graded their breathing as 1; 17 as 2; 23 as 3; and 5 as 4. The change in spirometric measurements from day1 until all patients had completed treatment (day 10 to 14) was related to both the initial diary card perception of breathlessness and to the change in breathlessness over the same time period ( and Figure 4). Changes in post-bronchodilator spirometry between presentation and after patients had completed treatment (10–14 days later) related significantly to both the initial breathlessness score (Jonkheere–Terpstra: FEV1 p = 0.007, VC, p = 0.003) and to changes in the breathlessness score (FEV1 p = 0.002, VC p = 0.005). Thus increasing breathlessness scores at presentation and greater changes in breathlessness scores were associated with greater changes in spirometry. However, the relationship was weaker between prebronchodilator values and initial dyspnoea and change in dyspnoea score and there was no relationship to peak expiratory flow rates (data not shown).
DISCUSSION
This study is the first to report detailed data including VC on the evolution of spirometric measurements of exacerbations in a well-characterized group of patients diagnosed with COPD in primary care and helps to improve our understanding of the underlying processes involved. We have studied in detail the changes in spirometry from exacerbation through to symptom resolution. However no baseline data was obtained prior to the exacerbation and therefore we are unable to conclude on the extent of recovery to pre-exacerbation levels.
Two or more reproducible prebronchodilator traces could be obtained in 60% of cases at presentation although 1 acceptable trace was obtainable in a further 23%. The major reason for failing to produce an acceptable trace was repeated coughing during the procedure that was a presenting symptom in 79% of episodes. This improved during resolution of the illness (all patients produced 3 acceptable traces when stable) suggesting the presence of airways irritability was responsible for the poor traces at the start of the episode. The inability to produce good quality traces was shown to relate to poorer lung function where airways irritability may be more critical during forced maneuvers. Because the inclusion of patients only able to produce one acceptable trace may have influenced the results we compared the data with that from patients able to produce two or more reproducible traces. There was no difference in clinical features and the overall results of the physiological changes were not affected when the two groups were analyzed independently suggesting that even a single acceptable trace may prove useful in monitoring the type and resolution of exacerbations. However, because some patients were unable to produce adequate spirometry traces this has resulted in exclusion of approximately a third of patient records. Although the characteristics of these patients were similar to the group as a whole their exclusion may limit the extent to which the presented data can be generalized to all of the exacerbations.
Overall, resolution of the exacerbation was associated with increases in both pre- and post-bronchodilator FEV1 and post-bronchodilator VC. There was an initial increase in post-bronchodilator FEV1 and VC by day 5 and in the case of FEV1 a further increase between day 28 and day 56. Because this latter increase was in FEV1 alone we investigated the possibility that it might relate to the different equipment used (Vitalograph alpha compact Fleisch pneumotachograph versus Vitalograph wedge bellows) rather than a real change. Therefore we evaluated the devices on successive patients attending for spirometry and derived Bland–Altman plots for the two different measuring devices. We found that the higher the wedge-bellows recording of FEV1 the greater the difference from the value obtained by the Fleisch pneumotachograph, although VC recordings were not affected (data not shown). Thus the apparent increase in FEV1 occurring between day 28 and day 56 is probably instrument dependent indicating that the spirometric improvements that accompany the resolution of the episodes described here appear to occur within the first 5 days as was seen for VC.
The changes in FEV1 are less than those reported in other published studies [Citation[2], Citation[3], Citation[4]]. This may be because the episodes (diagnosed in primary care) were less severe and excluded those requiring oral corticosteroids. Not all patients presented with breathlessness and we have shown that increased airflow obstruction is only a feature of exacerbations associated with increased breathlessness. Furthermore, our patient population included some patients with chronic bronchitis but no airflow obstruction in the stable clinical state and hence would also represent a milder patient group.
Although the improvement in FEV1 was similar between those patients presenting with purulent or mucoid sputum, significant improvements in VC were observed only in the purulent group. There are two possible explanations of this difference: Firstly, purulent exacerbations are associated with a more pronounced systemic inflammatory response than mucoid exacerbations [Citation[7]], which may influence the patients' effort and ability to perform complete forced expiratory maneuvers, and secondly, the characteristics of purulent sputum may result in increased airway occlusion and thus air trapping decreasing the VC at presentation. At present the reasons remain speculative but further studies and more extensive physiological assessment may clarify this difference. Mucolytic therapies may have a role in modifying sputum characteristics to improve the rate of recovery from some exacerbations.
Although purulent exacerbations are usually associated with bacteria, mucoid exacerbations may be viral which could determine the pattern of lung function changes. Viral exacerbations may be associated with increased bronchial hyperresponsiveness [Citation[14]] and increased eosinophil infiltration to the airway mucosa and lumen [Citation[15]]. Recent studies have found increased levels of the bronchoconstrictor endothelin-1 during AECOPD [Citation[16]] and it is possible that mucoid exacerbations are associated with this change thereby having a greater effect on the FEV1 than VC. Further studies that include characterization of sputum color and volume together with viral identification will be required to investigate this possibility further.
Changes in pre-bronchodilator FEV1 and VC over the resolution period were shown to relate to the degree of reversibility at presentation. This may suggest that a proportion of exacerbations are associated with increased airway hyperresponsiveness. The fact that this was more clearly related to VC than FEV1 may relate to the site of airway hyperresponsiveness: The VC is more likely to be influenced by changes in the small airways and suggests that any hyperresponsiveness is more critical in this region. However, there was no reduction in bronchodilator reversibility over the study period for the group as a whole and the value of reversibility testing has recently been called into question in studies examining sequential spirometric measurements in the stable clinical state as there is poor reproducibility over time [Citation[17]]. Thus the observed phenomenon may simply reflect regression to the mean which would be greater for VC than for FEV1 because of the greater variability of this measure. We attempted to correct for this effect using known variance of spirometric recording [Citation[13]] and the method of Blomqvist [Citation[12]]. Using the adjusted data the relationship persisted (data not shown), indicating that the effect is not simply a reflection of regression to the mean. We would cautiously conclude from our data that the relationships shown reflect the exacerbation episodes themselves rather than being only a statistical phenomenon.
Dyspnoea was the other feature that related to the changes in spirometric measurements. It is possible that a systemic inflammatory response as part of the exacerbation could account for breathlessness without any associated increased airflow obstruction [Citation[18]]. For instance, changes in V/Q matching which may not be reflected in dynamic flow rates can result in deterioration of gas exchange and hence increased dyspnoea [Citation[19]]. However, in the study reported here, dyspnoea severity was shown to relate to the FEV1 and VC changes during resolution and the degree of improvement in dyspnoea severity was related to the degree of physiological improvement. The data therefore suggests that the symptoms of dyspnoea in these exacerbations managed in primary care are largely related to changes in airflow obstruction.
CONCLUSIONS
This study provides unique descriptive data on the natural history of exacerbations of COPD diagnosed in primary care, where the majority of such episodes are treated. It is possible to obtain more complete spirometry in patients presenting with an acute exacerbation of bronchitis. However, patients' ability to perform spirometric maneuvers was impaired during acute exacerbations with only 60% being able to reproduce two or more acceptable traces although over 80% were able to produce at least one acceptable trace. Patients who complained of worsening dyspnoea were more likely to have increased airflow obstruction. Those with a greater difference between spirometric measurements before and after bronchodilation tended to have greater increases in prebronchodilator FEV1 and VC as the episode resolved and may reflect increased bronchial hyperresponsiveness in some patients at the time of an exacerbation. Purulent exacerbations were associated with changes in FEV1, and VC whereas mucoid episodes appear to be characterized by changes in FEV1 alone. This may reflect differences in the mechanical effects of sputum and/or the site of inflammation that characterizes the episode.
GLOSSARY
AECB = Acute exacerbation of chronic bronchitis
AECOPD = Acute exacerbation of chronic obstructive pulmonary disease
ANOVA = Analysis of variance
ARTP = Association of respiratory technicians and physiologists
CI = Confidence interval
COPD = Chronic obstructive pulmonary disease
CT = Computed tomography
FEV1 = Forced expiratory volume in 1 second
FVC = Forced vital capacity
ICS = Inhaled corticosteroids
IQR = Interquartile rage
PEF = Peak expiratory flow
VC = Vital capacity
Supported by an unrestricted educational grant by GlaxoSmith Kline.
REFERENCES
- O'Brien C, Guest P J, Hill S L, Stockley R A. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000; 55: 635–642, [CSA]
- Davies L, Angus R M, Calverley P M. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999; 354: 456–460, [CSA]
- Niewoehner D E, Erbland M L, Deupree R H, Collins D, Gross N J, Light R W, Anderson P, Morgan N A. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999; 340: 1941–1947, [CSA]
- Thompson W H, Nielson C P, Carvalho P, Charan N B, Crowley J J. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996; 154: 407–412, [CSA]
- Seemungal T A, Donaldson G C, Bhowmik A, Jeffries D J, Wedzicha J A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 1608–1613, [CSA]
- Niewoehner D E, Collins D, Erbland M. Relation of FEV1 to clinical outcomes during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 161: 1201–1205, [CSA]
- Stockley R A, O'Brien C, Pye A, Hill S L. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 2001; 117: 1638–1645, [CSA]
- Woolhouse I S, Hill S L, Stockley R A. Symptom resolution assessed using a patient directed diary card during exacerbations of chronic bronchitis. Thorax 2001; 56: 947–953, [CSA]
- Guidelines for the Measurement of Respiratory Function. Recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med 1994; 88: 165–194, [CSA]
- Borg G. Simple rating methods for estimation of perceived exertion. Wenner-Gren Centre International Symposium Series. Berg, Oxford 1976; 28: 39–47
- Naidich D P, McCauley D I, Khouri N F, Stitik F P, Siegelman S S. Computed tomography of bronchiectasis. J Comput Assist Tomogr 1982; 6: 437–444, [CSA]
- Blomqvist N. On the relation between change and the initial value. J Amer Stat Asso 1977; 72: 746–749, [CSA]
- Tweeddale P M, Alexander F, McHardy G JR. Short term variability in FEV1 and bronchodilator responsiveness in patients with obstructive ventilatory defects. Thorax 1987; 42: 487–490, [CSA]
- Hegele R G, Hayashi S, Hogg J C, Pare P D. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory tract infections. Am J Respir Crit Care Med 1995; 151: 1659–1664, [CSA]
- Zhu J, Qiu Y S, Majumdar S, Gamble E, Matin D, Turato G, Fabbri L M, Barnes N, Saetta M, Jeffery P K. Exacerbations of bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Amer J Respi. Crit Care Med 2001; 164: 109–116, [CSA]
- Roland M, Bhowmik A, Sapsford R J, Seemungal T A, Jeffries D J, Warner T D, Wedzicha J A. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax 2001; 56: 30–35, [CSA]
- Calverley P MA, Burge P S, Spencer S, Anderson J A, Jones P W. for the ISOLDE study investigators. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003; 58: 659–664, [CSA]
- Bone R C. The sepsis syndrome. Definition and general approach to management. Clin Chest Med 1996; 17: 175–181, [CSA]
- Barbera J A, Roca J, Ferrer A, Felez M A, Diaz O, Roger N, Rodriguez-Roisin R. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur respir J 1997; 10: 1285–1291, [CSA]